Abstract
Aim
This open-label, multiple-dose trial investigated the effect of concurrent administration of donepezil HCl with risperidone on the pharmacokinetics (PK) and safety profiles of both drugs.
Methods
Sixteen male patients with schizophrenia, who were receiving stable, physician-optimized risperidone (1–4 mg twice daily), and 15 healthy age- and weight-matched male controls, received donepezil HCl 5 mg daily for 7 days. Patients with schizophrenia remained on their physician-optimized dose of risperidone throughout the study. Pharmacokinetic parameters (Cmax, tmax and AUC) were assessed from plasma drug concentrations measured in blood collected before, during and after administration (for 12 h after risperidone on days 0 and 7, and for 24 h after donepezil HCl on day 7).
Results
The mean age of all the subjects was 38.5 years. Donepezil PK parameters were similar between patients taking donepezil HCl + risperidone (AUC0–24 h = 329.0 ± 17.2 ng·h ml−1) and controls taking donepezil HCl alone (AUC0–24 h = 354.7 ± 28.2 ng·h ml−1). Pharmacokinetic parameters for risperidone and 9-OH risperidone were not altered in patients with schizophrenia after 7 days of donepezil HCl administration (AUC0–12 h standardized by dose: risperidone = 59.6 ± 16.3 ng·h ml−1 at day 0, 56.0 ± 15.8 ng·h ml−1 at day 7; 9-OH risperidone = 162.1 ± 19.2 ng·h ml−1 at day 0, 163.3 ± 15.0 ng·h ml−1 at day 7). The most common adverse event in both treatment groups was diarrhoea (6/16 risperidone + donepezil HCl patients and 9/16 donepezil HCl only subjects). There were no significant changes in physical examination, ECG, vital signs or treatment-emergent abnormal laboratory values associated with either of the treatment regimens. No subject developed extrapyramidal side-effects following donepezil administration.
Conclusions
These results suggest that once-daily dosing of 5 mg donepezil HCl does not alter the PK of risperidone in patients with schizophrenia. The combination of risperidone and donepezil HCl was well tolerated.
Keywords: acetylcholinesterase inhibitor, donepezil hydrochloride, PK interaction, risperidone, schizophrenia
Introduction
The cholinergic hypothesis of Alzheimer's disease (AD) [1–3] has led to the development of agents designed to increase cholinergic function in the central nervous system, such as inhibitors of acetylcholinesterase (AChE), the centrally active enzyme responsible for the catabolism of the neurotransmitter acetylcholine. Donepezil HCl (Aricept®) is a chemically unique, piperidine-based AChE inhibitor that has been shown in pre-clinical studies to be a potent and relatively specific inhibitor of AChE [4]. Donepezil has been shown to be effective in randomized, controlled clinical trials of up to 1 year in duration, showing significant benefits on cognition, global function and ability to perform activities of daily living [5–9].
The pharmacokinetics (PK) of donepezil are linear [10], and similar in young and elderly healthy subjects [11], as well as in patients with AD [12]. Due to its slow clearance (elimination half-life of ∼70 h) [13], donepezil is effective as a once-daily dose. It undergoes metabolism in the liver by the cytochrome P450 isoenzymes CYP 3A4 and CYP 2D6. However, in vitro studies have suggested that the inhibitory effects of donepezil on these enzymes are minimal, due to the low rate of binding relative to the therapeutic plasma concentrations of donepezil [14]. The majority of metabolites are excreted in the urine (79% of recovered dose), suggesting elimination via the renal rather than the biliary route [11]. One metabolite, 6-O-desmethyldonepezil, possesses a pharmacological activity similar to donepezil, but has a negligible pharmacodynamic (PD) effect due to its low concentration in plasma. As a cholinomimetic agent, donepezil treatment can result in pharmacologically mediated adverse events (AEs), most notably in the gastrointestinal tract. However, the incidence of cholinergic side-effects is low at the clinically effective starting dose (5 mg day−1) [5, 6], and is further minimized at the higher dose using the established dose schedule (5 mg day−1 for 4–6 weeks, then 10 mg day−1 according to tolerability) [6, 7].
Risperidone (Risperdal®), a benzisoxazole derivative, is an ‘atypical’ anti-psychotic whose main pharmacological actions are serotonin type-2 blockade and dopamine D2 antagonism. Risperidone is indicated for the management of manifestations of schizophrenia, including improvement of both positive and negative symptoms, and is considered to be associated with a relatively low incidence of extrapyramidal symptoms compared with typical agents [15]. Risperidone is metabolized primarily in the liver by CYP 2D6 to a major active metabolite, 9-OH risperidone, which itself undergoes clearance by CYP 3A4 [16]. Risperidone and 9-OH risperidone together make up the active moiety. A steady state is usually achieved within 24 h for the parent compound, and within approximately 5 days for the metabolite [17]. The renal and total oral clearance of the active fraction (risperidone + 9-OH risperidone) has been found to be significantly reduced in the elderly [19] (the target population of donepezil), requiring a reduction in dose and careful dose titration.
A syndrome consisting of irreversible, involuntary, dyskinetic movements, known as tardive dyskinesia, may develop in some patients treated with anti-psychotic drugs, for which the risk appears to be related to both dose and treatment duration. The potentially fatal Neuroleptic Malignant Syndrome (NMS) has also been reported in patients receiving neuroleptic drugs. Since there are no reliable predictors for the potentially serious side-effects associated with risperidone treatment, it has been recommended that 1 mg twice daily be the starting dose of risperidone (lower in elderly patients with dementia), with slow increases in dose to avoid the emergence of serious side-effects.
A study evaluating the PK interaction of risperidone and donepezil will be invaluable, since risperidone is often used in dementia patients for the treatment of agitation or psychosis, increasing the likelihood that the two drugs may be administered concomitantly. The objectives of this study were to examine the effect of donepezil on the PK of risperidone following the administration of multiple doses of donepezil HCl during steady-state risperidone in a group of patients with schizophrenia, and the effect of risperidone on the PK of donepezil using a healthy comparator group receiving only donepezil HCl. The potential for a (PD) interaction between donepezil and risperidone was also investigated by assessing the emergence of side-effects after the addition of donepezil HCl to steady-state risperidone, and comparing the incidence with the healthy comparator group receiving only donepezil HCl.
Methods
This study was conducted in accordance with the principles stated in the Declaration of Helsinki, and conformed to all local laws and regulations, whichever afforded the greater protection to the individual. Prior to initiation of the study, the protocol and informed consent form were reviewed and approved by the Via Christi Regional Medical Center Institutional Review Board, Wichita, KS, USA.
Protocol
This was a single-centre, multiple-dose, open-label study conducted in male patients diagnosed with schizophrenia, and healthy age- and weight-matched male controls. The study design for the schizophrenia cohort followed a typical design for establishing steady state in a drug interaction study: patients were previously stabilized on physician-optimized twice-daily doses of risperidone (1–4 mg twice daily, as provided by the Physicians’ Desk Reference) and remained on this dose during the trial [18]. The healthy cohort was included to provide a baseline for donepezil PK. All subjects received a 5 mg film-coated tablet of donepezil HCl once daily for 7 days.
Patients with schizophrenia assigned to the donepezil HCl + risperidone regimen were admitted to the site on the evening prior to the first study day (day −1). On day 0, blood samples were collected prior to the morning dose of risperidone, and at 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12 h post-dose, after which the evening dose of risperidone was administered. On day 1 (after an 8 h fast), a pre-donepezil blood sample was collected, after which the patients received their morning dose of risperidone and a single dose of 5 mg donepezil HCl. Patients continued to take one 5 mg dose of donepezil HCl with their morning dose of risperidone each day until day 7, with blood samples collected on days 2 and 6, prior to the donepezil HCl dose. Further samples for the measurement of both donepezil and risperidone plasma concentrations were collected on the morning of day 7 (after an 8 h fast) prior to dose administration, and at 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12 h post-dose. Patients were discharged from the study on day 8, after a 24 h, post-donepezil HCl dose blood sample had been collected.
Healthy matched subjects, assigned to receive donepezil HCl only, reported to the site on the morning of day 1 following an 8 h fast the night before. A pre-donepezil HCl blood sample was drawn, after which the subjects received a single 5 mg dose of donepezil HCl. They returned to the site daily for the next 5 days (days 2–6) to receive daily doses of donepezil HCl, with further blood samples collected prior to donepezil HCl dosing on days 2 and 6. On the evening of day 6, the subjects were admitted to the site. On day 7 (after an 8 h fast) a pre-donepezil HCl blood sample was drawn, and post-donepezil HCl blood samples were taken at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 18 h after the last donepezil HCl dose. The subjects were discharged from the study on day 8, after a 24 h post-donepezil HCl dose blood sample had been collected.
Subjects
Male volunteers of any race, aged 19–65 years, who were willing and able to abide by the requirements and restrictions of the study and who met all entry criteria were enrolled. Prior to screening, the nature, purpose and potential hazards of the study were explained to each subject, after which voluntary, written informed consent was obtained. Subjects were free to refuse to enter the study or to withdraw at any time.
For entry into the risperidone + donepezil HCl treatment group, the patients were required to have documented evidence of schizophrenia (based on DSM-IV criteria [20], medical history and physical examination) and otherwise be in good health, as determined by medical history and laboratory evaluations. Patients should have been taking physician-optimized, stable daily doses of not less than 2 mg day−1 risperidone given in two equal doses for at least 2 months prior to screening. Patients were to weigh no more than 20% below or 35% above their ideal weight, as specified in the 1983 Metropolitan Life Insurance Height and Weight Tables. Entry was not permitted if the patient had experienced an acute exacerbation of schizophrenia within 60 days prior to study, had evidence of clinically significant extrapyramidal symptoms at screening or baseline, or was taking medication to control motor symptoms.
Enrolment to the donepezil HCl only treatment group required subjects to be ambulatory and in good health, as determined by medical history and laboratory evaluations. Each healthy subject was required to be within 7 years of age of the schizophrenic patient to whom they were to be matched, and to weigh within 20% of both the schizophrenic patient's and their own ideal weight.
Entry to the study was not permitted for any subject with documentation of diagnosed AD, or evidence of clinically significant abnormalities at screening. Subjects with a history indicating current alcohol or drug abuse were excluded, as were those with a positive urine drug screen for a prohibited medication or drug of abuse. Subjects were also excluded if they had any condition that, in the opinion of the investigator or sponsor, would have made them unsuitable for the study.
No subject was permitted to take any investigational medication within 30 days of donepezil HCl administration. Subjects could receive only those medications mutually agreed as acceptable by the investigator and sponsor prior to the patient's enrolment in the study. All medications, including over-the-counter preparations, were recorded during the risperidone administration (and within 30 days of screening) for the schizophrenic cohort, and within 21 days of donepezil HCl administration for the healthy cohort. Patients with schizophrenia were allowed to take amantadine, if needed, to control any motor symptoms that emerged during the study.
Sample collection and analysis
At each blood collection, the required amount of blood (7 ml from the donepezil HCl only group and a total of 14 ml from the donepezil HCl + risperidone group) was collected into one or two 7 ml evacuated heparinized tubes. Immediately after collection, all blood samples were centrifuged (2000 g) for 15 min at 4 °C. At least 2 ml of plasma from each tube was transferred into 10 ml polypropylene storage tubes, and stored in an upright position at −20 °C or colder until analysis.
Plasma samples were analysed for donepezil, 6-O-desmethyldonepezil, risperidone and 9-OH risperidone concentrations using a sensitive and specific high-performance liquid chromatographic system. The assay method for the determination of donepezil and 6-O-desmethyldonepezil plasma concentrations was validated over a range from 0.200 to 60.0 ng ml−1. The assay method for the determination of plasma levels of risperidone and 9-OH risperidone was validated over a range from 0.100 to 60.0 ng ml−1 (inter-assay coefficients of variation: donepezil = 3.57–6.37%, 6-O-desmethyldonepezil = 2.29%, risperidone = 2.9–5.8%, 9-OH risperidone = 4.2–6.6%).
Donepezil and 6-O-desmethyldonepezil PK parameters were calculated in all subjects, from drug plasma concentrations obtained from the blood samples. In addition, risperidone alone and 9-OH risperidone PK parameters were calculated for the cohort of patients with schizophrenia who both received risperidone alone and in combination with donepezil HCl. The PK parameters calculated included the area under the concentration–time curve using the trapezoidal rule from 0 to 24 h for donepezil (AUC0–24 h) and 0–12 h for risperidone (AUC0–12 h); the maximum measured donepezil and risperidone plasma concentration over the entire study (Cmax); and the time taken to attain the maximum concentration of donepezil and risperidone (tmax).
Safety assessments
Adverse events (AEs), defined as any untoward medical occurrence in a subject who received donepezil HCl or risperidone, were monitored throughout the trial and recorded by the investigator, together with an assessment of their severity and possible relationship to donepezil. A complete physical examination was performed at screening and at the end of the study, and a routine examination was performed prior to the first dose of donepezil HCl (day −1 for the donepezil HCl + risperidone group, and day 1 for the donepezil HCl only group). Vital signs, including blood pressure and pulse rate, were measured at screening, days 0 (donepezil HCl + risperidone group only), 1 and 7, and at the end of the study. A complete, standardized 12-lead electrocardiogram (ECG) was performed at screening and on days 0 (donepezil HCl + risperidone group only) and 7. Clinical laboratory tests, including haematology, clinical chemistry and routine urinalysis, were performed at screening and on days 1 and 8.
Statistical analysis
Data from 14 completed patients with schizophrenia and 14 completed healthy subjects were analysed to determine the differences between the donepezil and risperidone PK parameters. All statistical tests were two-tailed and significance was set at the 0.05 level. Differences in the mean values of donepezil PK parameters between subjects receiving donepezil HCl only and those receiving donepezil HCl in combination with risperidone were compared using a generalized linear model. Risperidone PK parameters measured on days 0 and 7 were compared using a paired t-test.
Results
Subjects
Thirty-one of 45 subjects screened met the entry criteria for the study, of whom 16 had schizophrenia and entered the risperidone + donepezil HCl treatment group, and 15 were healthy, normal volunteers and received donepezil only. Mean age of the patients with schizophrenia was 39.4 ± 2.5 years (range 19–57), and of healthy subjects was 37.5 ± 2.6 years (range 20–52). The mean weight of the patients with schizophrenia was 91.8 ± 4.4 kg (62–120), and of the healthy subjects was 85.8 ± 2.8 kg (66–108). Fourteen subjects in each group completed the study; one healthy subject was discontinued at the request of the subject/investigator, and two patients with schizophrenia were withdrawn due to protocol violations.
Pharmacokinetics of donepezil
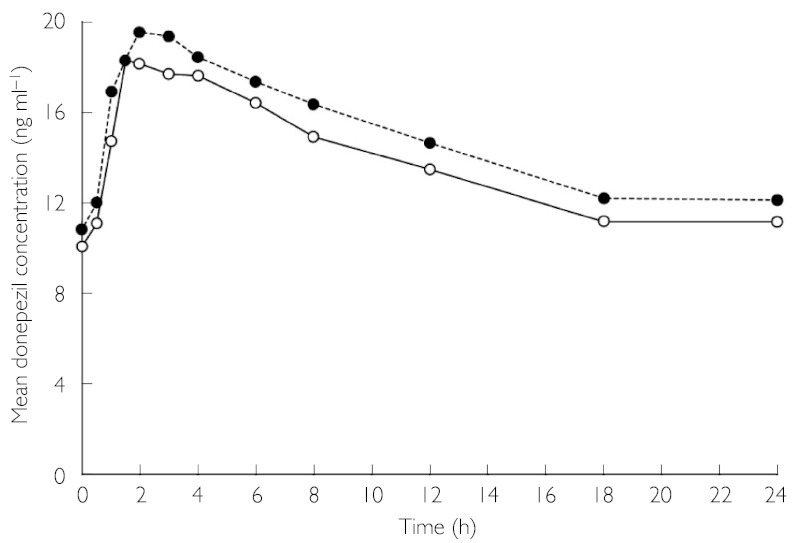
Mean plasma donepezil concentrations for each sample collection time point on day 7 for healthy subjects receiving donepezil HCl only and for patients with schizophrenia receiving donepezil HCl + risperidone are presented in Figure 1. At day 7, there were no significant differences in the values for AUC0–24 h, Cmax and tmax between the subjects receiving donepezil HCl + risperidone and those receiving donepezil HCl only (Table 1). Plasma concentrations of the active metabolite 6-O-desmethyldonepezil were also quantified. However, only one sample had a concentration above the lower limit of quantification (0.2 ng ml−1).
Figure 1.
Comparison of mean plasma donepezil concentration–time profiles of healthy subjects receiving donepezil HCl (5 mg day−1) alone and patients with schizophrenia receiving donepezil HCl (5 mg day−1) in combination with stable doses of risperidone. Donepezil HCI + risperidone (○—○), donepezil HCI only (•---•)
Table 1.
Donepezil pharmacokinetics (PK) parameters at day 7 in patients with schizophrenia and healthy controls
| Mean ± s.e. (range) | ||
|---|---|---|
| PK parameter | Donepezil HCl + risperidone (n = 14) | Donepezil HCl only (n = 14) |
| AUC0–24 h (ng·h ml−1)* | 329.0 ± 17.2 | 354.7 ± 28.2 |
| (235.1–421.2) | (213.9–536.2) | |
| Cmax (ng ml−1)* | 20.2 ± 1.2 | 21.2 ± 1.6 |
| (13.7–28.3) | (13.0–33.7) | |
| tmax (h)* | 2.5 ± 0.4 | 2.3 ± 0.3 |
| (1.0–6.0) | (1.0–4.0) | |
Differences between treatment groups were not significant.
Pharmacokinetics of risperidone
A paired t-test was used to evaluate the differences in AUC0–12 h, Cmax and tmax for each individual schizophrenic patient on day 0 and day 7. Although patients with schizophrenia were taking a range of doses of risperidone, there were no significant differences between the risperidone and 9-OH risperidone PK parameters on day 0 and after 7 days of once-daily donepezil HCl administration (Table 2). The ratio of risperidone/9-OH risperidone concentrations was similar on days 0 and 7. When the PK parameters were stratified by risperidone dose, mean values for AUC0–12 h, Cmax and tmax were similar on days 0 and 7. However, no statistical analyses were performed within each dose group, due to the small number of patients receiving each individual dose.
Table 2.
Risperidone and 9-OH risperidone pharmacokinetics (PK) parameters at day 0 and day 7 in patients with schizophrenia. PK parameter, mean ± s.e. (range)
| AUC0–12 h (ng.h/ml) | Cmax (ng/ml) | tmax (h) | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | Day 0 | Day 7 | |
| Risperidone | ||||||
| All patients (n = 14) | 120.7 ± 34.1(16.7–518.1) | 115.6 ± 33.3(19.9–500.8) | 24.2 ± 4.6(5.0–62.8) | 23.8 ± 4.4(5.7–60.3) | 1.1 ± 0.2(0.5–3.0) | 1.0 ± 0.2(0.5–4.0) |
| Dose-adjusted (n = 14)* | 59.6 ± 16.3(15.6–259.0) | 56.0 ± 15.8(10.6–250.4) | 12.0 ± 1.9(5.0–31.4) | 11.5 ± 1.7(4.1–30.2) | –– | –– |
| 9-OH risperidone | ||||||
| All patients (n = 14) | 348.0 ± 72.5(98.7–1092.0) | 348.2 ± 61.8(111.7–832.6) | 36.0 ± 7.9(9.8–119.0) | 35.1 ± 6.1(10.5–80.2) | 1.8 ± 0.2(0.5–3.0) | 2.1 ± 0.3(0.5–4.0) |
| Dose-adjusted (n = 14)* | 162.1 ± 19.2(63.9–364.1) | 163.3 ± 15.0(57.2–277.5) | 16.8 ± 2.2(6.3–39.7) | 16.4 ± 1.5(5.5–26.7) | –– | –– |
Doses of risperidone: 1 mg twice daily (n = 3), 1.5 mg twice daily (n = 4), 2 mg twice daily (n = 2), 3 mg twice daily (n = 4), 4 mg twice daily (n = 1).
Safety
No deaths or serious AEs were reported during the study, and no subjects discontinued the study prematurely as a result of AEs. The incidence of AEs was similar between subjects receiving donepezil HCl + risperidone and those receiving donepezil HCl only, with no significant differences between the two treatment groups. Fourteen patients receiving donepezil HCl + risperidone experienced a total of 33 AEs, and 13 subjects receiving donepezil HCl only experienced a total of 28 AEs. The highest incidence of AEs occurred in the digestive system (reported in 10/16 donepezil HCl + risperidone patients and 9/15 donepezil HCl only subjects), with diarrhoea the most common (6/16 donepezil HCl + risperidone patients and 9/15 donepezil HCl only subjects). Table 3 summarizes the overall incidence of AEs for each treatment group by preferred term. In patients receiving donepezil HCl + risperidone, 9/33 AEs were considered possibly or probably related to donepezil, compared with 22/28 AEs in subjects receiving donepezil HCl only, of which the majority for both groups were rated mild in intensity. No subject developed extrapyramidal side-effects following the addition of donepezil, and none required treatment with the study-approved medication, amantadine. No clinically significant abnormal physical examination findings, ECGs, laboratory values or vital signs were observed during the study.
Table 3.
Total incidence of treatment-emergent adverse events (AEs)
| Subjects experiencing AE (n) | ||
|---|---|---|
| Preferred term | Donepezil HCl + risperidone (n = 16) | Donepezil HCl only (n = 15) |
| Subjects with ≥1 AE | 14 | 13 |
| Diarrhoea | 6 | 9 |
| Dyspepsia | 3 | 1 |
| Asthenia | 1 | 3 |
| Cramp | 1 | 3 |
| Abnormal dreams | 3 | 0 |
| Insomnia | 2 | 1 |
| Syncope | 1 | 2 |
| Nausea | 2 | 0 |
| Pain | 2 | 0 |
| Somnolence | 0 | 2 |
| Anxiety | 1 | 0 |
| Application site reaction | 1 | 0 |
| Myalgia | 1 | 0 |
| Postural hypotension | 1 | 0 |
| Tachycardia | 1 | 0 |
| Vomiting | 1 | 0 |
| Accidental injury | 0 | 1 |
| Headache | 0 | 1 |
| Photosensitivity reaction | 0 | 1 |
Discussion
In addition to the treatment of schizophrenia, risperidone may be prescribed in elderly patients for the treatment of some of the neuropsychiatric symptoms of dementia, e.g. apathy, agitation and delusions, increasing the possibility of its concurrent administration with donepezil HCl. The objective of this study was to investigate the possibility of a pharmacological interaction between donepezil and risperidone, by examining whether either drug's pharmacokinetic or side-effect profile was altered following the administration of donepezil HCl to patients with schizophrenia who were already receiving stable doses of risperidone.
The traditional crossover study design used to evaluate the potential for drug–drug interaction was not considered appropriate for this particular study. The inclusion of healthy volunteers in such a design would have exposed them to the possible side-effects of risperidone, such as orthostatic hypotension and the onset of extrapyramidal symptoms. In addition, a crossover design using patients with schizophrenia would have required the cessation of risperidone treatment during the washout period. While the effect of risperidone administration on the PK profile of steady-state donepezil cannot be completely characterized with this design, the use of a control group receiving only donepezil HCl provided a good comparator for those receiving donepezil in combination with risperidone. Like donepezil, risperidone and 9-OH risperidone are mainly metabolized by the liver, via the CYP 2D6 and CYP 3A4 pathways, respectively. However, in vitro studies have indicated that risperidone does not significantly inhibit P450 isozymes, and the results of drug interaction studies with risperidone suggest that clinically significant interactions are unlikely [21]. It was therefore anticipated that, despite the design limitations, the donepezil PK parameters would not be significantly different between the two treatment groups. Indeed, the results of the study show that donepezil AUC0–24 h, Cmax and tmax were similar between the two groups. Whilst the study was not powered to detect a difference between the treatment groups, a difference of <20% between groups was considered not clinically significant.
Previous clinical studies have shown that steady-state Cmax concentrations for the 5 mg dose of donepezil HCl are more than 500-fold lower than the lowest Ki value obtained with CYP 2D6, and more than 1400-fold lower than the Ki observed with CYP 3A4 [14]. Furthermore, the concomitant administration of risperidone with medications that inhibit the CYP 2D6 metabolic pathway has not resulted in increased levels of combined risperidone and 9-OH risperidone [22]. Therefore, the possibility that donepezil would inhibit the metabolism of risperidone was considered unlikely. In this study, risperidone PK parameters in the patients with schizophrenia were similar at day 0 when they were receiving stable risperidone doses, and at day 7 after receiving seven daily doses of donepezil HCl. This apparent absence of interaction, together with data from previous studies, provides evidence that donepezil does not produce measurable inhibition or induction of CYP 2D6, 3A, 1A2 [23, 24] or 2C [23, 24], and does not affect P-glycoprotein function [25].
Substantial inter-individual variability of risperidone plasma concentrations to dose ratios was observed in this study. Risperidone Cmax values ranged from 5.0 ng ml−1 to 62.8 ng ml−1 on day 0, and remained similar at day 7, even when patients were further stratified by risperidone dose groups (Table 2). This observation is consistent with previous findings [26, 27], for which concentrations have been observed to vary by as much as a factor of 70 in a single patient group receiving a controlled dose of risperidone [28].
The primary metabolite 9-OH risperidone possesses a similar bioactivity to the parent compound (particularly with regard to the blockade of specific biogenic amine receptors and prolactin release) [29]. Mean PK parameters of 9-OH risperidone were also unchanged after seven daily doses of donepezil HCl, indicating that donepezil at the levels used in this study did not alter the activity of CYP 3A4. The rate of formation of 9-OH risperidone is dependent on the debrisoquine hydroxylation type genetic polymorphism, resulting in a longer time to peak concentrations of the metabolite in ‘poor’ metabolizers. Lower concentrations of risperidone in ‘extensive’ metabolizers have been found to be compensated by increased levels of 9-OH risperidone, with the result that all subjects have similar bioactivity for combined risperidone and 9-OH risperidone [30]. This study was unable to confirm that metabolic status was unimportant for the bioactivity of combined risperidone and 9-OH risperidone, since only one schizophrenic patient was a ‘poor’ metabolizer (established by genotyping blood samples of completers).
In general, the co-administration of donepezil HCl and risperidone was well tolerated by all patients with schizophrenia. There was no significant difference in the incidence of AEs between the treatment groups, indicating that the addition of donepezil HCl to a stable therapeutic risperidone regimen does not result in an increased incidence of AEs. The highest incidence of AEs in both study populations was reported in the digestive system, which is consistent with findings from previous studies using donepezil [5–7], and is a predictable consequence of its cholinomimetic action. Some of the common side-effects of risperidone, e.g. agitation, constipation and insomnia, were not reported (or were reported with a low incidence) by the patients with schizophrenia. These patients were somewhat self-selected to be tolerant of risperidone by virtue of the fact that they had been on the drug at a stable dose for at least 2 months.
Orthostatic hypotension has been associated with the use of risperidone [31], and is most likely linked to its α-adrenergic antagonist properties. It has also been reported as an AE during donepezil Phase II and III trials (albeit infrequently). In the current study, one schizophrenic patient experienced a drop in systolic blood pressure of 14 points on day 4. However, this single episode was not considered clinically significant since the subject was asymptomatic, no intervention or follow-up was required, and the subject completed the study. In addition, the American Academy of Neurology has defined orthostatic hypotension as ‘a reduction of systolic blood pressure of at least 20 mmHg or diastolic blood pressure of at least 10 mmHg within 3 min of standing’[32]. Although the patients in this study were younger than would generally be prescribed donepezil, the mean difference between supine and standing systolic and diastolic blood pressure was well within these limits. No physical examination, vital sign, ECG or laboratory abnormalities were associated with the combination of donepezil HCl and risperidone.
Risperidone at doses up to 4 mg twice daily and donepezil HCl at a dose of 5 mg day−1 did not interact pharmacokinetically, or pharmacodynamically in terms of a change in frequency, severity or duration of AEs. These results show that donepezil HCl (5 mg day−1) can safely be administered to young patients (mean age 38 years) with schizophrenia who are otherwise healthy and able to tolerate risperidone. Elderly patients with AD and particularly those with co-morbid medical conditions and their attendant compromised organ function might be more susceptible to the side-effects of the combination of donepezil and risperidone than younger patients with schizophrenia. However, since the most common dose of risperidone taken by elderly patients is <1 mg twice daily, which is up to fourfold lower than the dose taken by some subjects in this study, these data suggest that risperidone and donepezil HCl are likely to be well tolerated in elderly AD patients.
Acknowledgments
The analytical work for this study was performed at PPD Development, 8500 Research Way, Middleton, WI, USA.
References
- 1.Davies P, Maloney AJF. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 2.Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- 3.Bowen DM, Benton JS, Spillane JA, Smith CC, Allen SJ. Choline acetyltransferase activity and histopathology of frontal neocortex from biopsies of demented patients. J Neurol Sci. 1982;57:191–202. doi: 10.1016/0022-510x(82)90026-0. [DOI] [PubMed] [Google Scholar]
- 4.Rogers SL, Yamanishi Y, Yamatsu K. E2020: the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 314–20. [Google Scholar]
- 5.Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer's disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–31. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50:136–45. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 7.Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Moller HJ, Rogers SL, Friedhoff LT. The effects of donepezil in Alzheimer's disease – results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10:237–44. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- 8.Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P Donepezil Nordic Study Group. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–95. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 9.Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, Pratt RD “312” Study Group. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001;57:481–8. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 10.Rogers SL, Cooper NM, Sukovaty R, Pederson JE, Lee JN, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following multiple oral doses. Br J Clin Pharmacol. 1998;46(Suppl. 1):7–12. doi: 10.1046/j.1365-2125.1998.0460s1007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiseo PJ, Perdomo CA, Friedhoff LT. Metabolism and elimination of 14C-donepezil in healthy volunteers: a single-dose study. Br J Clin Pharmacol. 1998;46(Suppl. 1):19–24. doi: 10.1046/j.1365-2125.1998.0460s1019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers SL, Friedhoff LT the Donepezil Study Group. The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US multileft, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M, Ohnishi A, Tomono Y, Hasegawa J, Shimamura Y, Yamazaki K, Morishita N. Pharmacokinetics of E2020 – a new compound for Alzheimer's disease, in healthy male volunteers. Int J Clin Pharmacol Ther Toxicol. 1993;31:223–9. [PubMed] [Google Scholar]
- 14.ARICEPT® (donepezil hydrochloride tablets) Teaneck, NJ: Eisai Inc; 2000. US Package Insert December. [Google Scholar]
- 15.Song F. Risperidone in the treatment of schizophrenia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 1997;11:65–71. doi: 10.1177/026988119701100116. [DOI] [PubMed] [Google Scholar]
- 16.Heykants J, Huang ML, Mannens G, Meuldermans W, Snoeck E, Van Beijsterveldt L, Van Peer A, Woestenborghs R. The pharmacokinetics of risperidone in humans: a summary. J Clin Psychiatry. 1994;55(Suppl.):13–17. [PubMed] [Google Scholar]
- 17.Cohen LJ. Risperidone. Pharmacotherapy. 1994;14:253–65. [PubMed] [Google Scholar]
- 18.Physician's Desk Reference. 57. Montvale: Thomson Healthcare; 2003. [Google Scholar]
- 19.Snoek E, Van Peer A, Sack M, Horton M, Mannens G, Woestenborghs R, Meibach R, Heykants J. Influence of age, renal, and liver impairment in the PK of risperidone in man. Psychopharmacology. 1995;122:223–9. doi: 10.1007/BF02246543. [DOI] [PubMed] [Google Scholar]
- 20.DSM-IV™. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Ereshefsky L. Pharmacokinetics and drug interactions: update for new antipsychotics. J Clin Psychiatry. 1996;57(Suppl. 11):12–25. [PubMed] [Google Scholar]
- 22.Olesen OV, Licht RW, Thomsen E, Bruun T, Viftrup JE, Linnet K. Serum concentrations and side effects in psychiatric patients during risperidone therapy. Ther Drug Monit. 1998;20:380–4. doi: 10.1097/00007691-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Tiseo PJ, Foley K, Friedhoff LT. Concurrent administration of donepezil HCl and theophylline: assessment of pharmacokinetic changes following multiple-dose administration in healthy volunteers. Br J Clin Pharmacol. 1998;46(Suppl. 1):35–40. doi: 10.1046/j.1365-2125.1998.0460s1035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiseo PJ, Foley K, Friedhoff LT. The effect of multiple doses of donepezil HCl on the pharmacokinetic and pharmacodynamic profile of warfarin. Br J Clin Pharmacol. 1998;46(Suppl. 1):45–50. doi: 10.1046/j.1365-2125.1998.0460s1045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiseo PJ, Perdomo CA, Friedhoff LT. Concurrent administration of donepezil HCl and digoxin: assessment of pharmacokinetic changes. Br J Clin Pharmacol. 1998;46(Suppl. 1):40–4. doi: 10.1046/j.1365-2125.1998.0460s1040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darby JK, Pasta DJ, Elfand L, Daribi L, Clark L, Herbert J. Risperidone dose and blood level variability: accumulation effects and interindividual and intraindividual variability in the nonresponder patient in the clinical practice setting. J Clin Psychopharmacol. 1997;17:478–84. doi: 10.1097/00004714-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Aravagiri M, Marder SR, Wirshing D, Wirshing WC. Plasma concentrations of risperidone and its 9-OH metabolite and their relationship to dose in schizophrenic patients: simultaneous determination by a high performance liquid chromatography with electrical detection. Pharmacopsychiatry. 1998;31:102–9. doi: 10.1055/s-2007-979308. [DOI] [PubMed] [Google Scholar]
- 28.DeVane CL. Brief comparison of the pharmacokinetics and pharmacodynamics of the traditional and new antipsychotic drugs. Am J Health Syst Pharm. 1995;52(Suppl. 1):S15–S18. doi: 10.1093/ajhp/52.3_Suppl_1.S15. [DOI] [PubMed] [Google Scholar]
- 29.Mannens G, Huang ML, Meuldermans W, Hendrikx J, Woestenborghs R, Heykants J. Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Disposition. 1993;21:1134–41. [PubMed] [Google Scholar]
- 30.Huang ML, Van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen AA, Zylicz Z, Visscher HW, Jonkman JH. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther. 1993;54:257–68. doi: 10.1038/clpt.1993.146. [DOI] [PubMed] [Google Scholar]
- 31.Borison RL, Diamond B, Pathiraja A, Meibach RG. Pharmacokinetics of risperidone in chronic schizophrenic patients. Psychopharmacol Bull. 1994;30:193–7. [PubMed] [Google Scholar]
- 32.Carlson JE. Assessment of orthostatic blood pressure: measurement technique and clinical applications. South Med J. 1999;92:167–73. doi: 10.1097/00007611-199902000-00002. [DOI] [PubMed] [Google Scholar]