Abstract
Aims
To characterize the pharmacokinetic, pharmacodynamic and safety profiles of donepezil in subjects with moderate renal impairment and matched healthy controls during single-dose and multiple-dose phases.
Methods
This open-label study enrolled subjects with moderate renal impairment (creatinine clearance [CLCr] 17–33 ml min−1 1.73 m−2 body surface area) and age, weight and sex-matched healthy controls. A single-dose (5 mg donepezil) phase was followed by a 23-day multiple dose (5 mg day−1 donepezil) steady-state phase. The pharmacokinetic and pharmacodynamic parameters of donepezil were determined for up to 144 h after the first dose and 168 h after the last dose.
Results
Thirty-six subjects were enrolled, 19 renally impaired and 17 healthy controls. All pharmacokinetic and pharmacodynamic parameters were similar between groups after a single dose of donepezil (Cmax 5.17 ± 0.36 and 6.07 ± 0.49 ng ml−1; AUC0–24 76.05 ± 5.54 and 77.45 ± 4.49 ng·h ml−1; mean maximum percentage inhibition [Imax] red blood cell (RBC) AChE activity 32.07 ± 2.00 and 31.69 ± 2.45%; for subjects with renal impairment and healthy subjects, respectively). Pharmacokinetic parameters under steady-state conditions did not differ between renally impaired and healthy subjects (CSS 20.83 ± 1.78 and 18.38 ± 1.52 ng ml−1; AUC0–24 500.0 ± 42.8 and 441.1 ± 36.4 ng·h ml−1; degree of accumulation [RA] 6.98 ± 0.59 and 5.94 ± 0.53; for subjects with renal impairment and healthy subjects, respectively). Main pharmacodynamic parameters were also similar in renally impaired and healthy subjects at steady state (average percentage inhibition [ISS] RBC AChE activity 65.11 ± 2.52 and 60.62 ± 2.95, respectively). Protein binding was also similar between groups (% free donepezil 23.54 ± 1.96 and 20.23 ± 0.64, respectively). Donepezil was well tolerated by both groups.
Conclusions
These results indicate that the pharmacokinetics of donepezil are not altered after dosing to steady state, and that donepezil can be administered safely to subjects with moderate renal impairment.
Keywords: acetylcholinesterase inhibitor, Alzheimer's disease, donepezil, pharmacokinetics, renal impairment
Introduction
Alzheimer's disease (AD) is associated with a deficit of the neurotransmitter acetylcholine in specific areas of the brain [1]. This deficit can be reduced by preventing the breakdown of acetylcholine by cholinesterases, and cholinesterase inhibitors are currently the only approved agents for the symptomatic relief of AD. Donepezil HCl (Aricept®) is a unique, piperidine-class compound that selectively inhibits brain acetylcholinesterase [2]. Donepezil has been shown to produce significant benefits on cognition, global function and ability to perform activities of daily living in randomized, controlled clinical trials of up to 1 year in duration [3–6].
Donepezil undergoes first-pass metabolism in the liver by the hepatic enzymes CYP 3A4 and CYP 2D6. However, the primary route of elimination of donepezil is renal (57% of the administered dose is recovered in the urine), where it is excreted both intact and as multiple metabolites. The parent compound is the predominant elimination product in urine, accounting for 17% of the recovered dose. Hydroxylation metabolites (principally conjugates) account for 10%, the hydrolysis product accounts for 6% and the remainder includes oxidation products and unknown compounds [7]. Pharmacokinetic studies in healthy volunteers have shown that donepezil has a long half-life of approximately 70 h [8], facilitating a once-daily dosing schedule.
The target population for donepezil treatment is predominantly elderly patients, who are likely to have some degree of renal impairment, as this becomes more common with increasing age [9]. The pharmacokinetics (PK) of a single dose of donepezil have been shown to be similar in patients with moderate to severe renal impairment and in healthy volunteers [10]. However, donepezil treatment is long-term, and steady-state levels are only reached after 14–21 days of once-daily dosing [11]. This study aimed to establish the PK, pharmacodynamics (PD) and safety of donepezil in subjects with moderate renal impairment and matched healthy controls, following multiple daily dosing to steady state.
Methods
Subjects
Male or female volunteers of any race, aged 21–70 years, who were willing and able to provide written informed consent, were eligible for inclusion in the study. Women were required to be at least 2 years post-menopausal, surgically sterile, or using suitable birth control. Pregnant or lactating women were excluded. All subjects with known or suspected history of drug or alcohol abuse, significant blood loss or donation (>450 ml) within 30 days of the study, or who had taken other investigational medication within 21 days of study medication, were excluded from entry to the study.
Subjects with a creatinine clearance (CLCr) ≤ 34 ml min−1 1.73 m−2 body surface area, and a documented history of renal impairment were considered for inclusion as subjects with impaired renal function. Subjects with renal impairment were required to have a weight range within 30%of ideal weight [12]. They were excluded if they were on renal dialysis or had a transplanted kidney. Serum albumin levels of ≤ 2.0 g dl−1 or the presence of active acute or chronic hepatitis in the subjects rendered them ineligible. Subjects with controlled hypertension were permitted, and other medical conditions were acceptable, if they had been stable for at least 3 months prior to study entry.
Healthy volunteers with normal renal function (CLCr≥ 75 ml min−1 1.73 m−2 body surface area) were matched to subjects with impaired renal function by sex, age (within 7 years) and weight (within 25%). Weight range was required to be within 20% of ideal weight [12]. Subjects were ambulatory, and had no clinically significant hepatic, gastrointestinal, neurological, respiratory, haematological or cardiovascular disease.
Protocol
This non-randomised, open-label study was carried out at four sites in the USA and involved two study phases – an initial single-dose phase and a subsequent 23-day multiple-dose phase. Subjects with renal impairment and healthy matched controls received single daily doses of donepezil HCl on day 1 and days 7–29. Blood samples for PK and PD assessments were collected on days 1–7 and days 29–36.
During the 2-week screening period, the subjects underwent a complete physical examination, in conjunction with laboratory evaluations. Those who were eligible for the study arrived at the study site on day 0, where they remained for 2 nights and 1 day. After a light evening meal, the subjects fasted for at least 8 h before a single 5 mg dose of donepezil HCl was administered the next morning (day 1). Food and fluids, including water, were not permitted for 1 h before or 4 h after this dose, and subjects remained upright for 4 h post-dose. The consumption of alcohol and caffeine was not allowed for 48 h before patient admission and while at the study site. Foods likely to induce or inhibit hepatic enzymes, such as grapefruit juice, were prohibited for 48 h before and throughout the study.
The steady-state phase of the study commenced on day 7 following the completion of assessments for the single-dose phase. Once-daily oral doses of 5 mg donepezil HCl were administered for 23 consecutive days on days 7–29. Doses on days 7, 8, 15, 22, 24, 26, 27, 28 and 29 were administered at the study site. The dose on day 29 was administered under the same conditions as on day 1.
The study was conducted according to the guidelines of Good Clinical Practice (GCP), and in full compliance with the World Medical Assembly Declaration of Helsinki and its most recent amendments. All subjects provided written informed consent and had the right to withdraw from the study at any time.
Sample collection and analysis
Blood samples for PK and PD assessments relating to the day 1 and day 29 doses were collected 1 h before dosing and at 1, 2, 3, 4, 6, 8, 12, 18, 24, 48, 72, 96, 120, 144 and 168 (steady-state phase only) h post-dose. Additional pre-dose samples were collected on days 22, 24, 26, 27 and 28.
Plasma concentrations of donepezil and its active metabolite, 6-OH donepezil, were measured using high performance liquid chromatography (HPLC) with detection by mass spectrometry against an internal standard. The assay was linear between 0.2 and 60.0 ng ml−1, and the lower limit of detection for donepezil and 6-OH donepezil was 0.2 ng ml−1.
Pharmacokinetic assessments
Pharmacokinetic parameters were calculated for both healthy subjects and renally impaired subjects, using non-compartmental methods. Assessments included the plasma concentration of donepezil at each sampling time, peak donepezil plasma concentration (Cmax), time taken to attain Cmax (tmax), time taken for donepezil plasma concentration to decline by 50% (terminal disposition half-life, t½), area under the plasma concentration–time curve from 0 to 24 h after a single dose (AUC0–24, day 1) and at steady state (AUC0–24, SS), average steady-state concentration (CSS; CSS = AUC0–24, day 29/24 h), degree of accumulation (RA; RA = AUC0–24, day 29/AUC0–24, day 1), and time-averaged total clearance at steady state adjusted for systemic bioavailability (CLss/F; CLss/F = dose/AUC0–24, day 29).
Pharmacodynamic assessments
The degree of inhibition of red blood cell (RBC) AChE was assessed by radioenzyme assay, and used to calculate pharmacodynamic parameters at days 1 and 29 for both renally impaired and healthy subjects. These included the area under the AChE inhibition–time curve within 24 h of dosing (AUE0–24), maximum AChE inhibition (Imax), time taken to attain Imax (tImax), and average percentage AChE inhibition at steady state (ISS; ISS = AUE0–24, day 29/24 h).
Protein binding
The extent of donepezil plasma protein binding was determined on days 28 and 29 (pre-dose) and 4 h post-dose on day 29 by equilibrium dialysis. Donepezil concentrations were measured using HPLC with mass spectrometry detection, and the relative percentages of free and bound donepezil were calculated. The lower detection limit for donepezil in this assay was 0.04 ng ml−1.
Safety
Safety assessments included the incidence of adverse events (AEs), clinical laboratory test results, vital sign measurements, electrocardiogram (ECG), changes in physical examination assessments from baseline, and the monitoring of concomitant medications.
Subjects were continually monitored for AEs throughout the study. All AEs experienced during the study were recorded and evaluated by the investigator for severity and possible relationship to the study drug.
Complete physical examinations, including orthostatic vital sign assessments (blood pressure and pulse rate while supine and standing), weight, temperature and respiration rate were carried out at screening and at discharge. Routine physical examinations (including head and eye, ear, nose and throat [EENT]) and weight measurements were carried out on days 0, 2, 28 and 30. Routine vital signs (blood pressure and pulse rate while sitting) were measured at screening and on days 0–8, 15, 22 and 28–30. Additional orthostatic vital sign assessments were conducted pre-dose and 6 h post-dose on days 1 and 29. A complete, standardized 12-lead ECG was carried out at screening, on day 30 and at discharge. Urine and blood samples for laboratory evaluations (haematology, clinical chemistry, and routine urinalysis) were collected after an 8-h fast, at screening, on days 1, 2, 8, 15, 22, 29 and 30, and at discharge.
Statistical analysis
Sixteen renally impaired and 16 healthy volunteers were planned to be enrolled, with 12 in each group considered sufficient to detect a 40% difference in mean AUC (after a single dose) between cohorts with 80% power at the 0.05 significance level.
Analysis of variance was used to test for intergroup differences in PK parameters, PD parameters, and percentage of unbound donepezil. Analyses were carried out on the completed subject population for these parameters. Demographic characteristics for each treatment group were compared by Fisher's exact test for categorical variables and by analysis of variance for quantitative variables. These tests were also used to compare changes from baseline to last visit in vital signs, physical examination and laboratory results between treatment groups. Fisher's exact test was used to test for differences between groups in AE incidence. All tests were two-tailed and carried out at the 0.05 significance level.
Results
Demographics and disposition
Thirty-six subjects were enrolled for the study, 19 with renal impairment and 17 matched healthy controls. The CLCr values for enrolled renally impaired subjects were 17–33 ml min−1 1.73 m−2 body surface area, and those for healthy matched subjects were 75–200 ml min−1 1.73 m−2 body surface area.
The two cohorts were similar with respect to baseline subject demographic characteristics, other than race (Table 1). The study was completed by 16/19 renally impaired subjects and 15/17 healthy subjects. Withdrawals were due to AEs (two renally impaired subjects and one healthy subject), protocol violation (one renally impaired subject), and voluntary withdrawal (one healthy subject).
Table 1.
Baseline subject characteristics
| Renally impaired (n = 19) | Healthy (n = 17) | ||
|---|---|---|---|
| Race (n) | Black | 9 | 2 |
| White | 9 | 12 | |
| Other | 1 | 3 | |
| Sex (n) | Male | 8 | 8 |
| Female | 11 | 9 | |
| Age (years) | Mean (range) | 54.2 (30–75) | 52.9 (26–72) |
| Weight (kg) | Mean (range) | 81.7 (51.8–113.6) | 80.9 (63.6–104.5) |
Pharmacokinetics
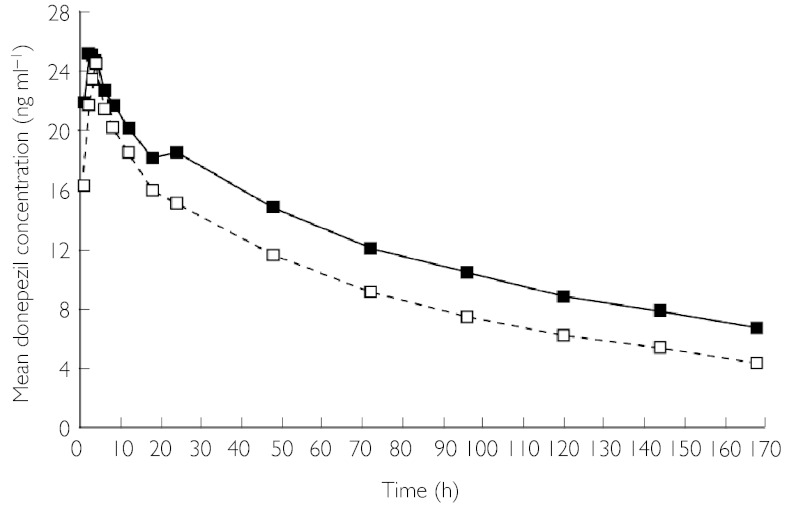
No significant differences were found between subjects with renal impairment and healthy subjects in any of the PK parameters evaluated, either after a single dose (Table 2) or at steady state (Table 3). There were no significant differences between renally impaired and healthy subjects in donepezil plasma concentrations over time during the single-dose phase (data not shown) or the multiple-dose phase (Figure 1). The plasma concentration of the active metabolite of donepezil, 6-OH donepezil, was close to or below the lower detection limit of the assay (0.2 ng ml−1) in all samples.
Table 2.
Pharmacokinetics (mean ± s.e. [range]) of donepezil at day 1
| Renally impaired (n = 16) | Healthy (n = 15) | |
|---|---|---|
| AUC0–24 (ng·h ml−1) | 76.05 ± 5.54 | 77.45 ± 4.49 |
| (37.9–105.1) | (49.7–114.7) | |
| CMax (ng ml−1 | 5.17±0.36 | 6.07±0.49 |
| (2.7–7.3) | (3.7–10.9) | |
| tmax (h) | 4.2 ± 0.4 | 3.5 ± 0.3 |
| (2–8) | (2–6) | |
| t½ (h) | 99.04 (14.36)* | 78.76 (6.32)* |
| 50.9–258.1 | 47.5–113.5 |
values are approximations only due to insufficient PK sampling during the terminal elimination phase.
Table 3.
Pharmacokinetics (mean ± s.e. [range]) of donepezil at steady state (day 29)
| Renally impaired (n = 15)* | Healthy (n = 14)* | |
|---|---|---|
| AUC0–24 (ng·h ml−1) | 500.0 ± 42.8 | 441.1 ± 36.4 |
| (243.2–844.2) | (256.1–702.7) | |
| Cmax (ng ml−1) | 26.49 ± 2.28 | 24.84 ± 1.99 |
| (12.4–46.3) | (13.5–39.1) | |
| tmax (h) | 3.6 ± 0.4 | 3.7 ± 0.3 |
| (1–6) | (2–6) | |
| t½ (h) | 100.12 ± 12.09† | 78.34 ± 5.88† |
| (46.1–213.0) | (49.5–120.5) | |
| CSS (ng ml−1) | 20.83 ± 1.78 | 18.38 ± 1.52 |
| (10.13–35.17) | (10.67–29.28) | |
| RA | 6.98 ± 0.59 | 5.94 ± 0.53 |
| (4.3–12.3) | (3.4–11.2) | |
| CLss/F(l h−1·kg) | 0.121 ± 0.009 | 0.141 ± 0.012 |
| (0.063–0.172) | (0.074–0.232) |
Although the study was completed by 16 renally impaired and 15 healthy subjects, one subject in each group was not included in the day 29 PK parameter calculations because of missed doses during the study.
t½values are approximations only due to insufficient PK sampling during the terminal elimination phase.
Figure 1.
Plasma donepezil concentration over time after final dose of steady-state dosing phase (day 29), in renally impaired subjects and healthy controls. Renally impaired (▪), healthy (□)
Pharmacodynamics
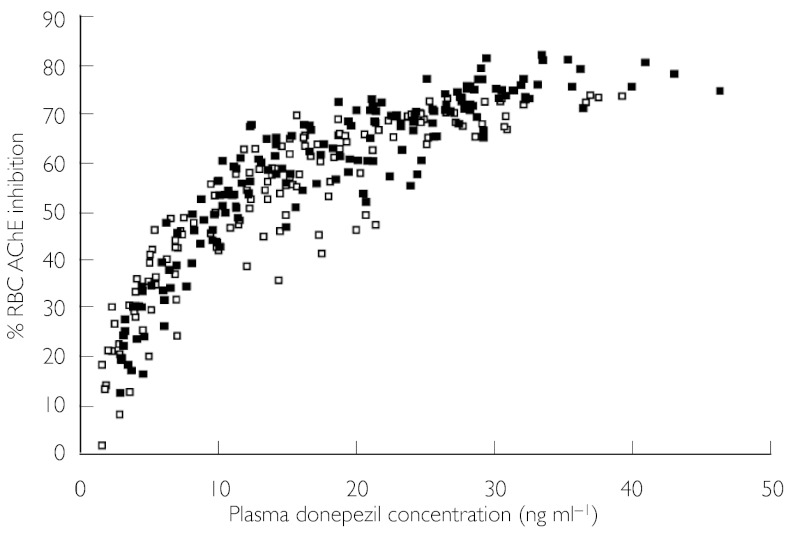
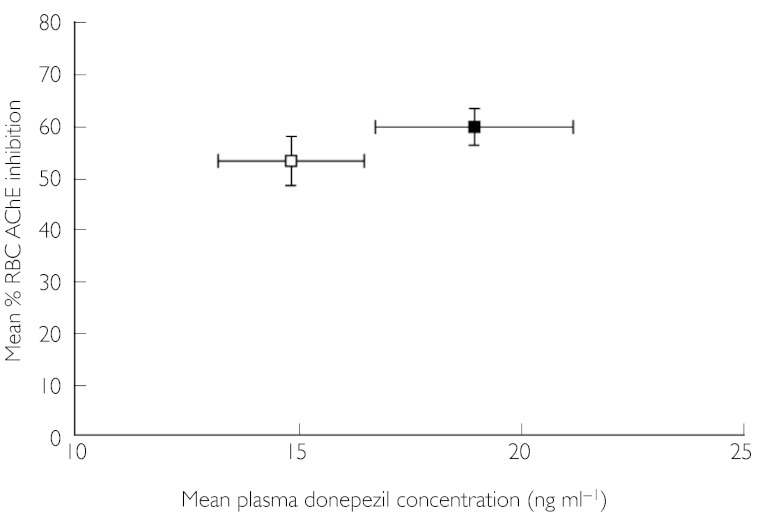
The mean maximum percentage inhibition (Imax) of RBC AChE activity after a single dose (day 1) did not differ significantly between cohorts (Table 4). On day 29, a significant increase in Imax was observed in the renally impaired group compared with the healthy group (73.97% vs. 66.81%, respectively; P = 0.02) (Table 4). No other PD parameters, including the time-averaged measure of inhibition AUE0–24, differed between the two groups at either day 1 or day 29. The average percentage RBC AChE inhibition at steady state (Iss) was not significantly different between renally impaired and healthy subjects (Table 4, Figures 2 and 3).
Table 4.
Pharmacodynamics (mean ± s.e. [range]) of donepezil at day 1 and at steady state (day 29)
| Day 1 | Day 29 | |||
|---|---|---|---|---|
| Renally impaired (n = 12) | Healthy (n = 11) | Renally impaired (n = 12) | Healthy (n = 10) | |
| AUE0–24 (t·% inhibition) | 475.9 ± 39.8 | 477.1 ± 34.4 | 1563 ± 60.4 | 1455 ± 70.7 |
| (298.5–730.5) | (231.6–648.1) | (1266–1938) | (1076–1715) | |
| Imax (% inhibition) | 32.07 ± 2.00 | 31.69 ± 2.45 | 73.97 ± 1.45* | 66.81 ± 2.63 |
| (23.2–43.1) | (17.3–42.4) | (65.2–82.4) | (49.4–74.5) | |
| tImax (h) | 5.6 ± 0.5 | 4.5 ± 0.5 | 4.1 ± 0.9 | 5.2 ± 1.0 |
| 3–8 | 2–6 | 2–12 | 3–12 | |
| ISS (% inhibition) | – | – | 65.11 ± 2.52 | 60.62 ± 2.95 |
| – | – | (52.7–80.7) | (44.8–71.4) | |
P < 0.03, renally impaired vs. healthy subjects.
Figure 2.
Relationship of percentage RBC AChE inhibition to plasma donepezil concentration at steady state (day 29), in renally impaired subjects and healthy controls. Renally impaired (▪), healthy (□)
Figure 3.
Plasma donepezil concentration (mean ± s.e.) at steady state (pre-dose, day 29) vs. percentage RBC AChE inhibition (mean ± s.e.) at steady state (pre-dose, day 29), in renally impaired subjects and healthy controls. Renally impaired (▪), healthy (□)
Protein binding
The proportion of unbound donepezil in plasma did not differ between cohorts after multiple dosing with donepezil (renally impaired mean = 23.54 ± 1.96%; healthy mean = 20.23 ± 0.64%).
Safety
A total of three subjects withdrew from the study due to AEs: two from the renally impaired group (one with a viral syndrome considered not related to the study drug, one with diarrhoea considered possibly related to the study drug), and one from the healthy group (with moderate nausea considered definitely related to the study drug).
Four patients with renal impairment reported seven serious AEs, none of which was considered to be related to the study drug. Of these patients, three were hospitalized (one with a viral infection with headache, nausea and neck rigidity; one with a hypoglycaemic episode; and one with a fractured leg) and one experienced a hypertensive episode that was thought to be medically significant. All remained in the study, with the exception of the patient with the viral syndrome.
There was no significant difference in AE incidence between the two groups; 15/19 (79%) renally impaired and 12/17 (71%) healthy subjects reported at least one AE. The main AEs that were considered possibly or definitely related to the study drug were headache (two renally impaired subjects and six healthy subjects), diarrhoea (five renally impaired subjects and two healthy subjects) and nausea (four renally impaired subjects and one healthy subject). Most AEs [63/86 events (73%)] were reported as mild in intensity.
No clinically relevant abnormalities in vital signs were observed during the study. Although some subjects in both groups (nine renally impaired and four healthy subjects) had abnormal ECGs, none of these was considered clinically important. There were no significant changes in heart rate in either group during the study [e.g. mean ± s.e. change from baseline (standing minus supine) heart rate at steady state: renally impaired, −1.5 ± 1.3; healthy, 2.5 ± 3.0]. No subjects discontinued due to treatment-emergent abnormal laboratory values.
Discussion
The data reported here are in good agreement with previous studies on donepezil PK [8, 11], including a single-dose study of donepezil in renally impaired subjects that showed no significant alterations in donepezil's PK compared with healthy controls [10]. As much as 79% of an administered dose of donepezil may be excreted in the urine, both intact (17%) and as metabolites [7]. However, it is likely that the long half-life and linear kinetics of donepezil mean the renal clearance system does not become saturated, and this may explain the lack of significant change in the PK of donepezil in patients with moderate renal impairment. Alternatively, in patients with renal insufficiency, the non-renal clearance of donepezil may increase to offset the reduction in clearance by the renal route, similar to the findings with digitoxin [13]. Since the clearance of donepezil is similar in elderly and younger subjects [14], the results of this study in younger subjects suggest that there would be no significant changes in the PK or PD of donepezil in its target treatment population.
The dose of donepezil administered (5 mg day−1) is the clinically effective starting dose [3]. It was not necessary to expose subjects to acute AChE inhibition and potential adverse reactions by using the 10 mg day−1 dose, since the PK of donepezil are linear and can be extrapolated from the 5 mg day−1 data [8].
It was not possible to analyse the pharmacokinetics of the active metabolite, 6-OH donepezil, as its plasma levels were below the assay detection limit. A ratio of metabolite to parent drug of 23% has been reported in a study in which plasma samples were pooled before analysis, and a different assay method was used [7]. The different methodological approach used in the current study may explain our inability to measure detectable levels of 6-OH donepezil. However, the comparison of groups within each study is valid for both approaches.
Pharmacodynamic parameters were similar between patient groups, with the exception of the mean maximum percentage RBC AChE inhibition at steady state. The small increase in this value observed in the renally impaired group relative to the healthy group was statistically significant, but it was not considered to be clinically relevant. All other parameters relating to RBC AChE inhibition were similar between the groups, following dosing of donepezil to steady state. The fraction of free donepezil in plasma did not differ between renally impaired and healthy subjects in the present study. Differences between methodologies used in the present and previous studies make it difficult to compare the protein binding results with those obtained previously [15]. However, based on in vitro protein-binding data [16], it is unlikely that the drug would be significantly removed by haemodialysis.
The high completion rates indicate that donepezil was well tolerated by both healthy and renally impaired subjects. Most AEs were mild in intensity, transient in nature, and consistent with donepezil's cholinomimetic effect.
In summary, a rise in donepezil concentrations is to be expected in renally impaired patients, as it is predominantly renally cleared. However, this study was powered to detect clinically significant differences between renally impaired and matched healthy subjects, and no clinically relevant differences were found in any PK or PD parameters. Previous studies have indicated that renal impairment does not significantly alter the PK of donepezil given as a single oral dose [10], and this study extends these findings to steady-state conditions. The results of this study indicate that donepezil can be administered safely to patients with moderate renal insufficiency (CLCr 17–33 ml min−1 1.73 m−2 body surface area).
Acknowledgments
This work was funded by Eisai Inc. and Pfizer Inc. The analytical work for this study was performed at PPD Development, 8500 Research Way, Middleton, WI 53562-3581, USA and at MDS Pharma Services, 621 Rose Street, PO Box 80837, Lincoln NE 68501, USA.
References
- 1.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;ii:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 2.Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-yl) methyl]piperidine hydrochloride and related compounds. J Med Chem. 1995;38:4821–4829. doi: 10.1021/jm00024a009. [DOI] [PubMed] [Google Scholar]
- 3.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 4.Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Moller HJ, Rogers SL, Friedhoff LT. The effects of donepezil in Alzheimer's disease – results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10:237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- 5.Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P Donepezil Nordic Study Group. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 6.Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, Pratt RD “312” Study Group. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001;57:481–488. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 7.Tiseo PJ, Perdomo CA, Friedhoff LT. Metabolism and elimination of 14C-donepezil in healthy volunteers: a single-dose study. Br J Clin Pharmacol. 1998;46(Suppl. 1):19–24. doi: 10.1046/j.1365-2125.1998.0460s1019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br J Clin Pharmacol. 1998;46(Suppl. 1):1–6. doi: 10.1046/j.1365-2125.1998.0460s1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abreu PF, Ramos LR, Sesso R. Abnormalities of renal function in the elderly. Geriatr Nephrol Urol. 1999;9:141–145. doi: 10.1023/a:1008308213377. [DOI] [PubMed] [Google Scholar]
- 10.Tiseo PJ, Foley K, Friedhoff LT. An evaluation of the pharmacokinetics of donepezil HCl in patients with moderately to severely impaired renal function. Br J Clin Pharmacol. 1998;46(Suppl. 1):56–60. doi: 10.1046/j.1365-2125.1998.0460s1056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers SL, Cooper NM, Sukovaty R, Pederson JE, Lee JN, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following multiple oral doses. Br J Clin Pharmacol. 1998;46(Suppl. 1):7–12. doi: 10.1046/j.1365-2125.1998.0460s1007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Height and Weight Standards. New York: Metropolitan Life Insurance Company; 1983. [Google Scholar]
- 13.Voehringer H-F, Rietbrock N. Digitalis therapy in renal failure with special regard to digitoxin. Intern J Clin Pharmacol Toxicol. 1981;19:175–184. [PubMed] [Google Scholar]
- 14.Ohnishi A, Mihara M, Kamakura H, Tomono Y, Hasegawa J, Yamazaki K, Morishita N, Tanaka T. Comparison of the pharmacokinetics of E2020, a new compound for Alzheimer's disease, in healthy young and elderly subjects. J Clin Pharmacol. 1993;33:1086–1091. doi: 10.1002/j.1552-4604.1993.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 15.Tiseo PJ, Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following evening administration. Br J Clin Pharmacol. 1998;46(Suppl. 1):13–18. doi: 10.1046/j.1365-2125.1998.0460s1013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ARICEPT® (donepezil hydrochloride tablets) Teaneck, NJ: Eisai Inc; 2000. US package insert December. [Google Scholar]