Abstract
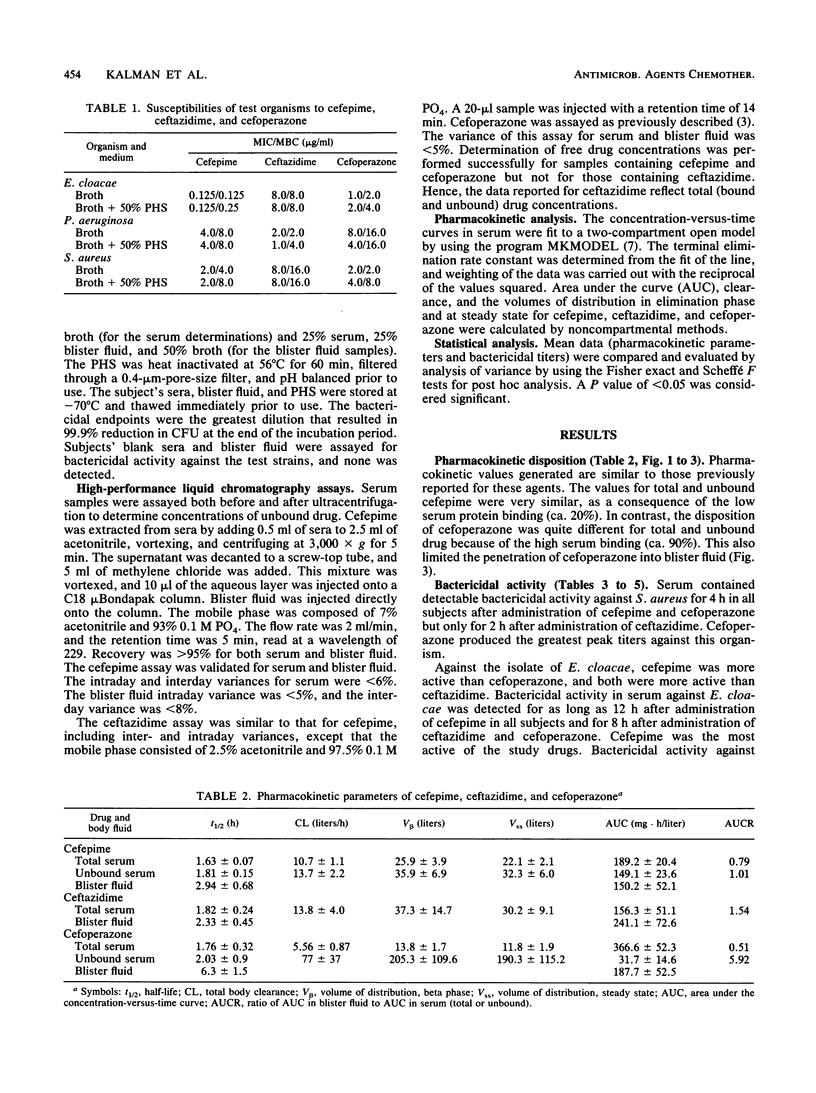
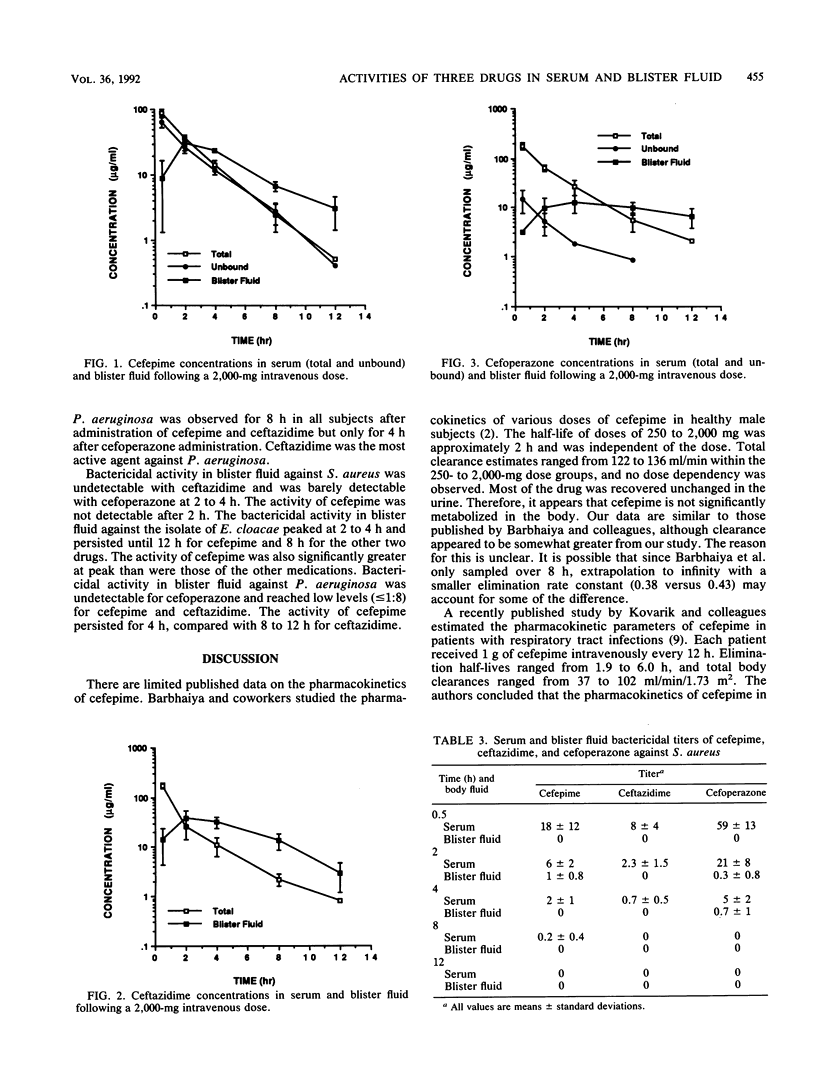
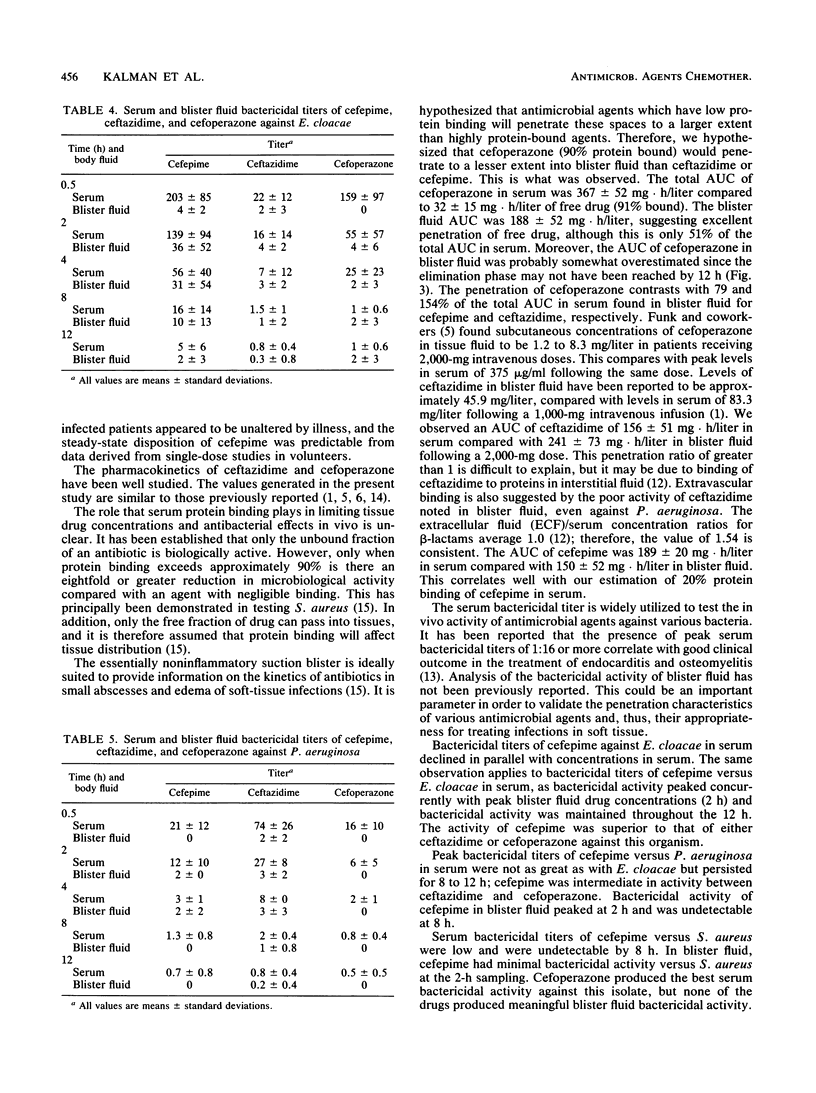
Cefepime is a new broad-spectrum cephalosporin with excellent gram-positive and gram-negative activity including activity against Staphylococcus aureus, Pseudomonas aeruginosa, and Enterobacter cloacae. The pharmacokinetic disposition of cefepime is similar to that of ceftazidime. We compared the pharmacokinetic characteristics and the extent and duration of bactericidal activity in serum and suction-induced blister fluid after single 2-g intravenous doses of cefepime, ceftazidime, and cefoperazone given to healthy subjects. One clinical isolate each of E. cloacae, P. aeruginosa, and S. aureus was used to assess bactericidal activity. Results of the pharmacokinetic analysis were similar to previously reported data for these drugs. The high serum protein binding of cefoperazone (approximately 90%) contributed to poor blister fluid penetration. The other drugs penetrated well into this fluid compartment. Cefepime showed significantly greater bactericidal activity in serum and blister fluid against E. cloacae than the other study drugs, ceftazidime was significantly better in serum and blister fluid against P. aeruginosa, and cefoperazone was significantly better against S. aureus only in serum. None of the study drugs had significant bactericidal activity in blister fluid against S. aureus. Cefepime is a promising antimicrobial agent for the treatment of infections due to E. cloacae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. C., Wise R., Brown R. M., Hancox J. Comparison of ceftazidime and cefamandole pharmacokinetics and blister fluid concentrations. Antimicrob Agents Chemother. 1981 Sep;20(3):356–358. doi: 10.1128/aac.20.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Gleason C. R., Knupp C. A., Pittman K. A., Weidler D. J., Martin R. R. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990 Jun;34(6):1118–1122. doi: 10.1128/aac.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawdon R. E., Hemsell D. L., Guss S. P. Comparison of cefoperazone and cefoxitin concentrations in serum and pelvic tissue of abdominal hysterectomy patients. Antimicrob Agents Chemother. 1982 Dec;22(6):999–1003. doi: 10.1128/aac.22.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Tomc J., Dougherty T. J., DeOrio F. J., Simich-Jacobson V., Kessler R. E. Activity of cefepime against ceftazidime- and cefotaxime-resistant gram-negative bacteria and its relationship to beta-lactamase levels. Antimicrob Agents Chemother. 1989 Apr;33(4):498–502. doi: 10.1128/aac.33.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk E. A., Strausbaugh L. J. Antimicrobial activity, pharmacokinetics, adverse reactions, and therapeutic indications of cefoperazone. Pharmacotherapy. 1982 Jul-Aug;2(4):185–196. doi: 10.1002/j.1875-9114.1982.tb03186.x. [DOI] [PubMed] [Google Scholar]
- Kovarik J. M., ter Maaten J. C., Rademaker C. M., Deenstra M., Hoepelman I. M., Hart H. C., Matzke G. R., Verhoef J. Pharmacokinetics of cefepime in patients with respiratory tract infections. Antimicrob Agents Chemother. 1990 Oct;34(10):1885–1888. doi: 10.1128/aac.34.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel M., Vallée F., Bergeron M. G. Tissue penetration of ciprofloxacin after single and multiple doses. Antimicrob Agents Chemother. 1986 Mar;29(3):501–505. doi: 10.1128/aac.29.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix D. E., Goodwin S. D., Peloquin C. A., Rotella D. L., Schentag J. J. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob Agents Chemother. 1991 Oct;35(10):1953–1959. doi: 10.1128/aac.35.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers D. K., Walters L., Van Wyk M., Harding S. M., Paton A. M., Ayrton J. Pharmacokinetics of ceftazidime in male and female volunteers. Antimicrob Agents Chemother. 1983 Jun;23(6):892–896. doi: 10.1128/aac.23.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. The clinical relevance of protein binding and tissue concentrations in antimicrobial therapy. Clin Pharmacokinet. 1986 Nov-Dec;11(6):470–482. doi: 10.2165/00003088-198611060-00004. [DOI] [PubMed] [Google Scholar]


