Abstract
Aims
The coadministration of subantinociceptive doses of oxycodone with morphine has recently been shown to result in a synergistic antinociceptive effect in rats. The present study was aimed to investigate the possibility that coadministration of morphine and oxycodone can produce a similar synergistic effect in humans exposed to an experimental model of cold pressor test (CPT).
Methods
The enriched enrolment design was used to exclude ‘stoic’ and ‘placebo responders’ in a single-blind fashion. ‘Nonstoic’, placebo ‘nonresponder’ female volunteers (n = 30) were randomly assigned to receive 0.5 mg kg−1 oral morphine sulphate, 0.5 mg kg−1 oral oxycodone hydrochloride, and the combination of 0.25 mg kg−1 morphine sulphate with 0.25 mg kg−1 oxycodone hydrochloride, 1 week apart from each other, in a double-blind crossover design. Latency to pain onset (threshold), pain intensity (VAS), and pain tolerance (time until removal of the hand from the water) were measured six times over a 3-h period, subsequent to the administration of each medication, and were used to assess their antinociceptive effect.
Results
The combination produced a significantly higher effect on latency to pain onset than that of morphine alone [difference in mean postbaseline value 2.2; 95% confidence interval (CI) 0.48, 3.9; P = 0.01] but the effect was nonsignificantly smaller that that of oxycodone alone. Similarly, the effect of the combination on pain tolerance was significantly larger than that of morphine alone (combination difference 8.4; 95% CI 2.5, 14.3; P = 0.007), whereas oxycodone alone caused a nonsignificantly larger effect than that of the combination treatment. Comparisons of pain magnitude failed to show any significant differences between the three treatments.
Conclusions
These results indicate that at the doses tested, morphine and oxycodone do not produce synergistic antinociceptive effects in healthy humans exposed to the CPT.
Keywords: κ-opioid agonist, µ-opioid agonist, cold pressor test, pain magnitude, pain threshold, pain tolerance
Introduction
Morphine and oxycodone are opioids with a similar structure that are used for the treatment of moderate to severe pain [1], but their usage is often limited by inadequate analgesia, excessive adverse effects, or both [2]. New approaches aimed to improve analgesia and reduce adverse effects are therefore required.
In a recent study, Ross et al.[3] have shown that the administration of subantinociceptive doses of morphine or oxycodone alone produced almost no effect, whereas the coadministration of the same doses of the two drugs markedly increased the levels of antinociception, and significantly reduced central nervous system (CNS) side-effects in rats. Based on their earlier findings, which suggested that oxycodone is a putative κ-opioid agonist [4], while morphine is a well known µ-opioid agonist, they raised the possibility that this synergistic antinociceptive effect results from coactivation of both κ and µ receptors simultaneously. The present study was aimed to investigate the possibility that the coadministration of morphine and oxycodone can produce a similar synergistic effect in humans exposed to an experimental model of cold-induced pain.
Methods
Subjects
Healthy paid female volunteers were enrolled in the study. The volunteers were recruited mostly from the student body of the medical and nursing schools, and enrolled in the study after meeting the following criteria: (i) freedom from chronic pain of any type; (ii) no medication use (except for oral contraceptives); (iii) ability to understand the purpose and the instructions of the study. Subjects were excluded from the study if they had a history of substance abuse or were known to suffer from Raynaud's syndrome. The volunteers were not allowed to consume alcohol or any drugs except for the study medication and were asked not to eat for at least 6 h before starting the trial. Menstrual cycle data were not taken into account upon scheduling of subjects for the experimental sessions. The study protocol was approved by the hospital's Helsinki Committee and by the National Board of Health in Israel, and written informed consent was obtained from all subjects.
Study design
The study was a double-blind crossover trial with a modification of the enriched enrolment strategy [5, 6]. According to this approach, patients are screened for response to a drug to be studied, and only then are enrolled into a double-blind, controlled trial. This approach can improve the ability of such a study to detect treatment effects. In the present study subjects were first screened for their response to placebo in a single-blinded fashion, after which only placebo nonresponders (see conduct of the study) were enrolled to the three-arm, randomized, double-blind, crossover part of the trial.
Apparatus
The cold pressor test (CPT) apparatus (Heto CBN 8–30 Laboratory equipment, Allerod, Denmark) is a temperature-controlled water bath of 1.0 °C with a maximum temperature variance of ±0.5 °C that is continuously stirred in order to keep the temperature even throughout the bath.
Cold pain measures
Three pain measures were used in this study: (i) latency to pain onset: subjects were instructed to indicate the exact point in time when the cold sensation began to hurt, and they first felt pain. This was defined as the latency to pain onset; (ii) pain magnitude: immediately after withdrawing their hand, subjects were asked to mark the maximal pain intensity they experienced during the cold stimulus on a 100-mm visual analogue scale (VAS). The VAS was defined a priori to the subjects as a continuum with one anchor representing ‘no pain at all’, and the other representing the ‘worst imaginable pain’. This mark was defined as the pain magnitude; (iii) pain tolerance: the time until spontaneous hand withdrawal was defined as pain tolerance. A cut-off time of 3 min was set for safety reasons. Tolerance for subjects who did not withdraw their hand for the entire 180 s was recorded as 180 s.
Study medications
Study medications consisted of two opioids, namely, morphine sulphate and oxycodone hydrochloride (Rafa Laboratories Ltd., Jerusalem, Israel). An active placebo, chlorpheniramine maleate was employed in an attempt to mimic opioids’ adverse effects, and thus to reduce the risk of unblinding the study medications. All study medications were administered orally in the form of solutions and were diluted with 50 ml tap water and 5 ml grape-flavoured syrup. Dosages were calculated according to the body weight of each individual subject.
Conduct of the study
A detailed explanation of the study design, the CPT technique, the pain measures, and the fact that in each session one of four possible study medications would be administered in a blind fashion was given to all subjects. Each subject was told that she was scheduled to participate in one to four sessions, 1 week apart. No explanation was given to the subjects about how the number of sessions would be determined. After giving written informed consent, subjects were asked to place their nondominant hand in the cold pressor test bath, according to the standard protocol (for example, see [7]), in a still position with the fingers wide apart for as long as they could (the dominant hand was used to mark pain intensity on the VAS). The first immersion was considered a training immersion and its results were not used in the statistical analysis. Thirty minutes later a second CPT immersion was conducted and regarded as the baseline measurement. Subjects with baseline latency of ≥60 s, VAS <40, or tolerance of ≥180 s were regarded as ‘stoic’ (or relatively ‘insensitive to pain’) and were removed from the study. Each subject then received the oral active placebo chlorpheniramine maleate 0.033 mg kg−1 in a single-blind fashion (the subject was not aware of which medication she was taking). Six additional immersions were conducted thereafter, 30 min apart, and pain measures of each immersion were recorded. From the six measures that were taken after drug administration, the highest value of latency to pain onset and pain tolerance and the lowest value of pain magnitude were independently chosen. Every parameter was compared with its own baseline value. Based on these comparisons, subjects were classified as either placebo responders or placebo nonresponders. A subject was defined as a placebo responder if following placebo administration her latency increased by ≥6 s compared with baseline, her VAS decreased by ≥10 points in two or more immersions, or if her tolerance increased by ≥10 s. These values were based on preliminary (unpublished) data from our laboratory. Subjects who were defined as placebo responders were withdrawn from the study. Placebo nonresponders were randomly assigned to receive 0.5 mg kg−1 oral morphine sulphate, 0.5 mg kg−1 oral oxycodone hydrochloride and the combination of 0.25 mg kg−1 morphine sulphate and 0.25 mg kg−1 oxycodone hydrochloride, in a double-blind fashion during the remaining three sessions. It should be noted that all four sessions were conducted in a similar fashion and that each consisted of a training immersion, a baseline immersion 30 min later, oral drug administration, and six additional immersions 30 min apart. This dosage regimen was based on preliminary results from our laboratory, as well from others [8], which showed that the dose of 0.25 mg kg−1 of morphine is subantinociceptive in the CPT model. Equal doses of morphine and oxycodone were used – as commonly done in clinical practice – regardless of the differences in the bioavailability of the two drugs [9, 10]. For us to be able to demonstrate a synergistic effect of the two drugs, rather that simply an additive effect, the coadministration of 0.25 mg of morphine sulphate with 0.25 mg kg−1 oxycodone hydrochloride was compared with the effect of 0.5 mg kg−1 of each drug when administered alone.
Adverse events
At the end of each session subjects were requested to record any adverse effect and to grade them on a 0–3 scale, where 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
Statistical analysis
Based on previous CPT studies, a 20–25% difference in efficacy between treatments was considered to have a significant relevance. A sample size of 30 subjects was found to be large enough to provide 80% power for detecting such a difference. Differences in postbaseline time profiles (and marginal means) between the three treatment groups were evaluated by means of anova with repeated measures of each parameter (latency, magnitude and tolerance). The model in-cluded two within-subject factors: time, drug and their interactions. Bonferroni correction was used for multiple comparisons. Excel (Microsoft Corp., Seattle, WA, USA) and SAS (SPSS Inc., Chicago, IL, USA) were employed in the statistical analyses. P was considered significant at the 0.05 level. Results are presented as means ± SEM.
Results
Subjects
Of the 47 healthy female paid volunteers, four ‘stoic’ subjects were removed from the study after the baseline immersion because their tolerance reached 180 s. Eight were defined as ‘placebo responders’ and were also withdrawn. Five additional subjects stopped their participation prematurely: two complained of symptoms of upper respiratory infection, which they attributed to the first (placebo) treatment, one left the study after the first session for no clear reason, and two others declined to participate because of adverse effects (nausea, vomiting and weakness) subsequent to the second treatment, found retrospectively to be due to oxycodone. Thus, 30 subjects with age range 18–34 years (mean 23.8 ± 4.9) were included in the final analysis (Table 1).
Table 1.
Study participants
| Recruited | 47 |
| ‘Stoic’ | −4 |
| ‘Placebo responders’ | −8 |
| Withdrawn during the double-blind phase | −5 |
| Completed the study | 30 |
Effect on latency to pain onset
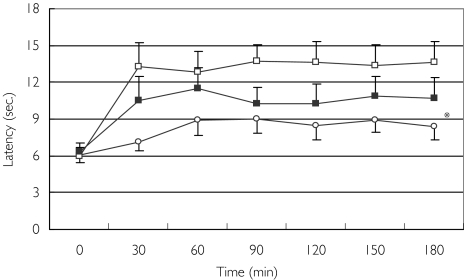
Mean baseline latency to pain onset of all three treatments ranged from 6.0 ± 0.6 s to 6.4 ± 0.7 s (Figure 1). Morphine produced its maximal effect at 90 min and increased the latency from 6.1 ± 0.6 s to 9.0 ± 1.2 s (47% change). The peak effect of oxycodone was recorded at 90 min with an increase from a mean of 6.0 ± 0.6 s to 13.8 ± 3.0 s (130%), and of the combination treatment at 60 min, from 6.4 ± 0.7 s at baseline to 11.5 ± 1.7 s (80% change). Comparisons of these three groups failed to show a synergistic effect of morphine and oxycodone compared with each treatment alone. While the combination treatment caused a significantly larger effect than that of morphine alone [difference in mean postbaseline value 2.2; 95% confidence interval (CI) 0.48, 3.9; P = 0.01], it exerted a nonsignificantly smaller effect than that produced by oxycodone alone (difference −2.8; 95% CI −6.2, 0.7; P = 0.12).
Figure 1.
Effect of morphine (○), oxycodone (□), and the combination of morphine and oxycodone (▪) on latency to pain onset. Data are presented as means ± SEM. *Significant difference in mean postbaseline value of the combination treatment from that of morphine alone (P= 0.01)
Effect on pain magnitude
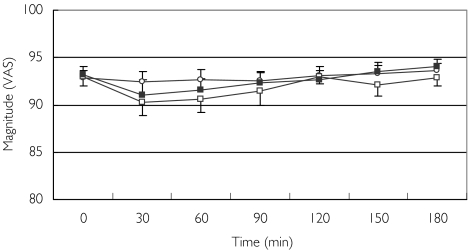
Mean baseline pain magnitude ranged from 92 ± 1 mm to 93 ± 1 mm on the 0–100 mm VAS. All three treatments produced only a minimal effect on pain magnitude. The average maximal pain reduction effect was 0.4 mm in response to morphine treatment, 2.6 mm in response to oxycodone, and 2.1 mm when morphine and oxycodone where coadministrated (Figure 2). The repeated measures analysis failed to show any significant differences between the three treatments in their effects on pain magnitude.
Figure 2.
Effect of morphine (○), oxycodone (□), and the combination of morphine and oxycodone (▪) on pain magnitude. Data are presented as means ± SEM
Effect on tolerance
Tolerance for subjects who did not withdraw their hand for the entire 180 s was recorded as 180 s. None of the subjects exhibited a tolerance of 180 s at any of the baseline measurements or following morphine treatment. Two subjects reached the 180-s cut-off time following oxycodone treatment and two following the combination treatment.
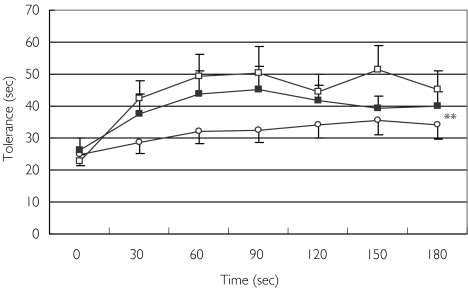
Average baseline tolerance at all four treatments ranged from 23 ± 2 s to 26 ± 3 s. As shown in Figure 3, the peak effect of morphine was recorded at 150 min with an increase from a mean of 24 ± 4 s at baseline to 35 ± 4 s (45% change). Oxycodone also produced its maximal effect at 150 min with an increase from a mean 23 ± 2 s at baseline to 51 ± 5 s (122% change), and the combination treatment caused an increase from a mean of 26 ± 4 s at baseline to 45 ± 7 s at 90 min (73% change). The combination of oxycodone and morphine, and oxycodone alone, both produced significantly larger effects than that of morphine alone (combination vs. morphine difference in mean postbaseline values 8.4; 95% CI 2.5, 14.3; P = 0.007; oxycodone vs. morphine difference 14.4; 95% CI 4.6, 24.2; P = 0.006). Oxycodone alone caused a nonsignificantly larger effect than that of the combination treatment. Thus, no synergistic effect of the combination treatment on tolerance could be demonstrated.
Figure 3.
Effect of morphine (○), oxycodone (□), and the combination of morphine and oxycodone (▪) on pain tolerance. Data are presented as means ± SEM. **Significant difference in mean postbaseline values of the combination treatment and of oxycodone treatment from that of morphine alone (P = 0.006 and 0.007, respectively)
Adverse effects
Seven out of the 30 subjects (23%) experienced side-effects following the administration of the placebo, none of which was defined as severe. Twenty-four (80%) reported having a side-effect after receiving morphine, with two severe events (7%). All subjects reported at least one side-effect in response to the administration of oxycodone, and 14 (47%) of them were defined as severe. All subjects but one (97%) reported at least one side-effect after coadministration of morphine and oxycodone, but only eight (27%) of them were defined as severe (Table 2).
Table 2.
Summary of adverse events, % (n)
| Event/drug | Placebo | Morphine | Oxycodone | Morphine + oxycodone |
|---|---|---|---|---|
| Somnolence | ||||
| Mild | 7 (2) | 50 (15) | 53 (16) | 73 (22) |
| Moderate | 10 (3) | 63 (19) | 43 (13) | |
| Severe | 20 (6) | 13 (4) | ||
| Headache | ||||
| Mild | 7 (2) | 10 (3) | 13 (4) | 7 (2) |
| Moderate | 3 (1) | 10 (3) | 13 (4) | |
| Severe | ||||
| Dizziness | ||||
| Mild | 7 (2) | 27 (8) | 73 (22) | 77 (23) |
| Moderate | 57 (17) | 43 (13) | ||
| Severe | 3 (1) | 20 (6) | 3 (1) | |
| Nausea | ||||
| Mild | 23 (7) | 47 (14) | 37 (11) | |
| Moderate | 13 (4) | 33 (10) | 7 (2) | |
| Severe | 3 (1) | 20 (6) | 3 (1) | |
| Euphoria | ||||
| Mild | 3 (1) | 10 (3) | 23 (7) | 23 (7) |
| Moderate | 3 (1) | 7 (2) | ||
| Severe | ||||
| Restlessness | ||||
| Mild | 7 (2) | 33 (10) | 47 (14) | 53 (16) |
| Moderate | 3 (1) | 7 (2) | 27 (8) | 23 (7) |
| Severe | 13 (4) | 3 (1) | ||
| Itch | ||||
| Mild | 3 (1) | 20 (6) | 43 (13) | 50 (15) |
| Moderate | 7 (2) | 43 (13) | 33 (10) | |
| Severe | 10 (3) | 10 (3) | ||
| Flush | ||||
| Mild | 3 (1) | 7 (2) | 10 (3) | 10 (3) |
| Moderate | 3 (1) | 3 (1) | 7 (2) | |
| Severe | ||||
| Dry mouth | ||||
| Mild | 37 (11) | 53 (16) | 60 (18) | |
| Moderate | 3 (1) | 20 (6) | 33 (10) | 30 (9) |
| Severe | 3 (1) | 10 (3) | ||
| Visual disturbances | ||||
| Mild | 3 (1) | 7 (2) | 27 (8) | 33 (10) |
| Moderate | 3 (1) | 13 (4) | 7 (2) | |
| Severe | 3 (1) | |||
| Other | ||||
| Mild | 3 (1) | |||
| Moderate | ||||
| Severe | 7 (2) | |||
Discussion
In the present study an attempt was made, for the first time, to test the morphine–oxycodone synergistic hypothesis in humans. The results failed to demonstrate a synergistic morphine–oxycodone effect in any of the three tested parameters. The 0.5 mg kg−1 dose of oxycodone produced a significantly greater effect on latency and tolerance compared with an equal dose of morphine, while the combination of 0.25 mg of each drug fell in between. Therefore, in agreement with previous findings [11], these results demonstrate the relative higher potency of oxycodone than that of morphine, rather than a synergistic effect. The results also show that adverse effects following the administration of the study medications were reported by 80–100% of subjects. This observation indicates that, at the doses tested, there is no side-effect attenuating synergy between the two drugs.
These findings contradict those reported by Ross et al.[3] in animals, by not showing evidence that the coadministration of morphine and oxycodone results in enhanced analgesia and reduced opioid-related CNS adverse effects. There appear to be some similarities between the two studies. In both, opioids were administered prior to administration of the nociceptive stimulus, and latency for the response to the nociceptive stimulus was tested. Yet there are significant differences between the two, which may explain the inconsistency in results. First, the animals were exposed to the radiant heat-induced Tail Flick Latency Test, whereas the humans immersed their hand in ice-cold water. It is noteworthy that even within one species conflicting responses to heat and cold noxious stimuli have been reported [12]. Second, either subcutaneous, intraperitoneal, or intraventricular routes of opioid administration were used in the animals, while the humans received the medications orally. Previous reports indicate that administration of one opioid in two routes simultaneously, e.g. intraventricular and intrathecal [13] or intrathecal and intraperitoneal [14], can result in analgesic synergy in animals. In humans, concomitant administration of intrathecal morphine and intravenous buprenorphine enhanced postoperative pain reduction and lowered untoward effects [15]. Thus, the route of administration per se may have an effect on the degree of synergy. Third, the dosages of the opioids in the two studies differed considerably. The opioid dosages injected into the animals were subnociceptive. In the humans, the morphine (and possibly the oxycodone) dosage administered was subantinociceptive specifically for the CPT. However, in clinical reality a dose of 0.25 mg kg−1 of both drugs is well within the antinociceptive range. It is possible that the use of clinically subantinociceptive dosages in humans would have yielded different results.
The present study was designed to test the synergistic hypothesis in a well-defined population and under strict conditions. The study population consisted of healthy female volunteers only, and was further narrowed by the exclusion of ‘stoic’ subjects as well as of those who were defined as placebo responders.
Only female volunteers were chosen to participate because of the well-known gender difference in responsiveness to opioids. While most animal studies reported male rodents displaying significantly higher levels of opioid-induced analgesia than females [16–20], in humans, women demonstrated a more pronounced analgesic effect in response to the administration of opioids than men [21–26] (see [27] for review). The enrolment of female subjects was therefore meant to avoid possible between-sex differences in the response to the administered opioids.
The enriched enrolment strategy has been used in an attempt to improve the ability of the study to detect treatment effects [5, 6]. The removal of ‘stoic’ subjects from the study was meant to avoid testing those whose baseline measures indicated low pain perception, because the likelihood of detecting differences in the magnitude of analgesia induced by different drugs in such subjects is not very high. The ‘placebo responders’, on the other hand, were excluded due to their tendency to respond to any intervention regardless of the type of drug administered. Before excluding ‘placebo responders’ from the study it was important to make sure that the effect of the intervention was indeed a placebo response rather than an adaptation to repeated CPT immersions at successive time points. In a pilot study conducted at our laboratory (unpublished data) a group of 25 healthy subjects were exposed to repeated CPT immersions every 30 min for 3 h. No significant changes in latency, magnitude or tolerance occurred over time in any of these subjects and there were no signs of adaptation. The exclusion of placebo responders, as part of the enriched enrolment design, was therefore justified. Yet the main limitation of the enriched enrolment approach is that the results apply only to a selected population and cannot be generalized.
The experimental model of cold-induced pain (CPT) was tested in the present study for the following reasons: (i) it is regarded as a highly repeatable and reliable test; (ii) it allows the testing of several dimensions of pain such as latency to onset (latency), intensity, and tolerance, and therefore increases the likelihood of detecting a previously unstudied effect; (iii) in contrast to the equivocal results with heat pain [28–31], the effect of morphine (although not of oxycodone) on the different CPT-induced pain parameters is well established [8]. The CPT is also considered to bear resemblance to tonic clinical pain. Nonetheless, in contrast to ongoing clinical pain, which usually exists prior to the initiation of treatment, in the present study drugs were administered first and pain was inflicted only subsequently. For all the above reasons, we emphasize that these results are applicable only to this specific population and under these strict experimental conditions and dose levels tested, and are in no way meant to indicate that no synergistic analgesic effects of morphine and oxycodone exist in humans. This is especially true in light of the fact that in clinical settings opioids are mainly used for the reduction of pain magnitude, an effect that could not be demonstrated in the present experiment. Controlled clinical trials are required to evaluate the possible synergistic interaction between morphine and oxycodone.
Lastly, while morphine is clearly a µ-opioid receptor agonist, it is unclear through which opioid receptor oxycodone produces its analgesic effect. Traditionally, it has been assumed that morphine and oxycodone produce their antinociceptive effect by interacting with the same opioid receptor [32]. Recent binding study in rats has shown that oxycodone is a µ-receptor agonist [33]. In contrast, Ross and Smith [4] have shown that the antinociceptive effect of oxycodone is antagonized by the selective κ-opioid receptor antagonist nor-BNI, but not by the selective µ-opioid receptor antagonist nalxonazine. Intracerebroventricular administration of oxycodone and other κ-agonists has a rapid onset of a single antinociceptive phase (5–7 min), whereas morphine produces a two-phase delayed-onset antinociceptive effect [4]. Moreover, subcutaneous administration of oxycodone to Dark Agouti rats, which lack the enzyme (CYP2D1) required for O-demethylation of oxycodone to its potent µ-agonist metabolite oxymorphone, still produced large antinociceptive effects [34]. Humans also metabolize oxycodone to oxymorphone to a very limited extent [35, 36]. Thus, oxymorphone concentrations in plasma samples taken from subjects who received intravenous oxycodone were very low, suggesting that the analgesic effect of oxycodone in humans is unlikely to result from µ-opioid receptor activation by oxymorphone [35, 36]. Based on these understandings, Ross et al.[3] assumed that the coadministration of subantinociceptive doses of putative κ- with µ-opioid receptor agonists can result in a synergistic antinociceptive effect. The results of our study do not support their hypothesis.
Acknowledgments
This study was supported by grant number 20012010-C from the Israeli Cancer Association, by grant number 001/1062/03 from the Israeli Ministry of Defense, and an independent research grant by Rafa Laboratories Ltd. (Jerusalem, Israel).
References
- 1.Kalso E, Vainio A, Mattila MJ, Rosenburg PH, Seppala T. Morphine and oxycodone in the management of cancer pain: plasma levels determined by chemical and radioreceptor assays. Pharmacol Toxicol. 1990;67:322–8. doi: 10.1111/j.1600-0773.1990.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 2.Cherny N, Ripamonti C, Pereira J, et al. Expert Working Group of the European Association of Palliative Care Network. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–54. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 3.Ross FB, Wallis SC, Smith MT. Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain. 2000;84:421–8. doi: 10.1016/s0304-3959(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 4.Ross FB, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be kappa opioid receptor mediated. Pain. 1997;73:151–7. doi: 10.1016/S0304-3959(97)00093-6. [DOI] [PubMed] [Google Scholar]
- 5.Byas-Smith MG, Max MB, Muir J, Kingman A. Transdermal clonidine compared to placebo in painful diabetic neuropathy using a two-stage ‘enriched enrollment’ design. Pain. 1995;60:267–74. doi: 10.1016/0304-3959(94)00121-t. [DOI] [PubMed] [Google Scholar]
- 6.Galer BS, Rowbotham MC, Perander J, Friedman E. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain. 1999;80:533–8. doi: 10.1016/S0304-3959(98)00244-9. [DOI] [PubMed] [Google Scholar]
- 7.Wolff BB. Methods of testing pain mechanisms in normal man. In: Wall PD, Melzack R, editors. Textbook of Pain. 2. Edinburgh: Churchill Livingstone; 1984. pp. 186–94. [Google Scholar]
- 8.Cleeland CS, Nakamura Y, Howland EW, Morgan NR, Edwards KR, Backonja M. Effects of morphine on cold pressor tolerance time and neuropsychological performance. Neuropsychopharmacology. 1996;15:252–62. doi: 10.1016/0893-133X(95)00205-R. [DOI] [PubMed] [Google Scholar]
- 9.Hoskin PJ, Hanks GW, Aherne GW, Chapman D, Littleton P, Filshie J. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. Br J Clin Pharmacol. 1989;27:499–505. doi: 10.1111/j.1365-2125.1989.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyhia R, Seppala T, Olkkola KT, Kalso E. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol. 1992;33:617–21. doi: 10.1111/j.1365-2125.1992.tb04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patt RB, Szalados JE, Wu CL. Pharmacotherapeutic guidelines. In: Patt RB, editor. Cancer Pain. Philadelphia: J.B. Lippincott Co.; 1993. pp. 565–75. [Google Scholar]
- 12.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci USA. 1999;96:7744–51. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pick CC, Roques B, Gacel G, Pasternak GW. Supraspinal mu 2-opiod receptors mediate spinal/supraspinal morphine synergy. Eur J Pharmacol. 1992;220:275–7. doi: 10.1016/0014-2999(92)90761-r. [DOI] [PubMed] [Google Scholar]
- 14.Niv D, Nemirovsky A, Rudick V, Geller E, Urca G. Antinociception induced by simultaneous intrathecal and intraperitoneal administration of low doses of morphine. Anesth Analg. 1995;80:886–9. doi: 10.1097/00000539-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Beltrutti D, Niv D, Ben-Abraham R, Di Santo S, Weinbroum AA. Late antinociception and lower untoward effects of concomitant intrathecal morphine and intravenous buprenorphine in humans. J Clin Anesth. 2002;14:441–6. doi: 10.1016/s0952-8180(02)00397-5. [DOI] [PubMed] [Google Scholar]
- 16.Islam AK, Cooper ML, Bodnar RJ. Interactions among aging, gender and gonadectomy effects upon morphine antinociception in rats. Physiol Behav. 1993;54:45–54. doi: 10.1016/0031-9384(93)90042-e. [DOI] [PubMed] [Google Scholar]
- 17.Cicero TJ, Nock B, Meyer ER. Gender related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–73. [PubMed] [Google Scholar]
- 18.Cicero TJ, Nock B, Meyer ER. Sex related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–44. [PubMed] [Google Scholar]
- 19.Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav. 2000;65:91–6. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 20.Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–47. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 21.DeKock M, Scholtes J. Postoperative PCA in abdominal surgery. Analysis of 200 consecutive patients. Acta Anaesth Belgica. 1991;42:85–91. [PubMed] [Google Scholar]
- 22.Gear RW, Miaskowski C, Gorgon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–50. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 23.Sidebotham D, Dijkhuizen MRJ, Schug SA. The safety and utilization of patient controlled analgesia. J Pain Symptom Manage. 1997;14:202–9. doi: 10.1016/s0885-3924(97)00182-6. [DOI] [PubMed] [Google Scholar]
- 24.Miaskowski C, Levine JD. Does opioid analgesia show a gender preference for females? Pain Forum. 1999;8:34–44. [Google Scholar]
- 25.Miaskowski C, Gear RW, Levine JD. Sex-related differences in analgesic response. In: Fillingim RB, editor. Sex, Gender and Pain. Progress in Pain Research Management. Vol. 17. Seattle: IASP Press; 2000. pp. 209–30. [Google Scholar]
- 26.Sarton E, Olofsen E, Romberg R, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiol. 2000;93:1245–54. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Craft R. Sex differences in opioid analgesia: ‘From mouse to man’. Clin J Pain. 2003;19:175–86. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Angst MS, Ramaswamy B, Riley ET, Stansky DR. Lumbar epidural morphine in humans and supraspinal analgesia to experimental heat pain. Anesthesiology. 2000;92:312–24. doi: 10.1097/00000542-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Brennum J, Dahl JB, Moiniche S, Arendt-Nielsen L. Quantitative sensory examination of epidural anasthesia and analgesia in man: effects of pre and post traumatic morphine on hyperalgesia. Pain. 1994;59:261–71. doi: 10.1016/0304-3959(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 30.Ekblom A, Segerdahl M, Sollevi A. Adenosin increases the cutaneous heat pain threshold in healthy volunteers. Acta Anaesth Scand. 1995;39:717–22. doi: 10.1111/j.1399-6576.1995.tb04158.x. [DOI] [PubMed] [Google Scholar]
- 31.Warncke T, Stubhaug A, Jorum E. Ketamine, an NMDA receptor antagonist, suppresses spatial and temporal properties of burn induced secondary hyperalgesia in man: a double blind, cross over comparison with morphine and placebo. Pain. 1997;72:99–106. doi: 10.1016/s0304-3959(97)00006-7. [DOI] [PubMed] [Google Scholar]
- 32.Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. II. Comparisons of intramuscular oxycodone with intramuscular morphine and codeine. J Pharmacol Exp Ther. 1978;207:101–8. [PubMed] [Google Scholar]
- 33.Monory K, Greiner E, Sartania N, et al. Opioid binding profiles of new hydrazone, oxime, carbazone and semicarbazone derivatives of 14-alkoxymorphinans. Life Sci. 1999;64:2011–20. doi: 10.1016/s0024-3205(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 34.Cleary J, Mikus G, Somogyi A, Bochner F. The influence of pharmacogenetics on opioid analgesia: studies with codeine and oxycodone in the Sprague-Dawley/Dark Agouti rat model. J Pharmacol Exp Ther. 1994;271:1528–34. [PubMed] [Google Scholar]
- 35.Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD. Pharmacokinetic–pharmacodynamic relationships of controlled release oxycodone. Clin Pharmacol Ther. 1996;59:52–61. doi: 10.1016/S0009-9236(96)90024-7. [DOI] [PubMed] [Google Scholar]
- 36.Lacouture PG, Iwan T, Benziger D, et al. Proceedings of the 8th World Congress on Pain. Seattle: IASP Press; 1996. Plasma disposition of oxycodone (OC), oxymorphone (OM), and noroxycodone (NO) with long-term controlled release (CR) oxycodone (Oxycontin™ Tablets) dosing; p. (Abstract) 286. [Google Scholar]