Abstract
Aims
To investigate the pharmacokinetics of vancomycin in critically ill patients on continuous venovenous haemodiafiltration (CVVHDF), a continuous renal replacement therapy (CRRT) and to see if routine measures approximate vancomycin clearance.
Methods
Pharmacokinetic profiles (15) of initial and steady-state doses of 750 mg twice daily intravenous vancomycin were obtained from blood and ultrafiltrate samples from 10 critically ill patients in the intensive care unit, with acute renal failure on CVVHDF (1 l h−1 dialysate plus 2 l h−1 filtration solution; 3 l h−1 effluent; extracorporeal blood flow 200 ml min−1).
Results
CVVHDF clearance of vancomycin was 1.8 ± 0.4 l h−1 (30 ± 6.7 ml min−1). This was 1.3–7.2 times that reported previously for vancomycin using other forms of CRRT. Total vancomycin body clearance was 2.5 ± 0.7 l h−1 (41.7 ± 11.7 ml min−1). The clearance of vancomycin by CVVHDF was 76 ± 16.5% of the total body clearance. CVVHDF removed approximately half the vancomycin dose during the 12-h period (ACVVHDF = 413 mg). The fraction eliminated by all routes was 60%. The sieving coefficient for vancomycin was 0.7 ± 0.1 and for urea was 0.8 ± 0.06.
Conclusions
Vancomycin is cleared effectively by CVVHDF. Clearance was faster than other forms of CRRT, therefore doses need to be relatively high. Urea clearance slightly overestimates vancomycin clearance. The administered doses of 750 mg every 12 h were too high and accumulation occurred, as only approximately 60% of a dose was cleared over this period. The maintenance dose required to achieve a target average steady-state plasma concentration of 15 mg l−1 can be calculated as 450 mg every 12 h.
Keywords: antibiotics, continuous renal replacement therapy, pharmacokinetics, vancomycin
Introduction
Critically ill patients are at risk of Gram-positive nosocomial infections [1, 2]. Both patient and environmental factors account for an increased risk. Longer stay patients in critical care units are susceptible to infection with Enterococci and methicillin-resistant Staphylococcus aureus, which are often resistant to all the usual agents and require treatment with the glycopeptides, such as vancomycin [2].
The pharmacokinetics of many drugs are affected by critical illness and the presence of renal failure [3]. There are widely varying data, extrapolated from noncritically ill patients with renal failure and from critically ill patients without renal failure [4–12]. Various methods of renal replacement therapy are used to remove fluid and waste products from the blood of patients. Dosing and pharmacokinetic data in patients receiving intermittent haemodialysis are not applicable to those receiving continuous renal replacement therapy (CRRT) because of substantial differences in the properties of the filters used and the fact that dialysis is continuous in the latter group [13]. In addition, there are different and varying techniques of performing CRRT which will result in differing clearances [6, 7, 11].
Earlier studies collected data from people receiving continuous arteriovenous haemofiltration (CAVH) or continuous venovenous haemofiltration (CVVH) [4, 12], which achieves lower drug clearance than the currently more widely used continuous venovenous haemodialysis (CVVHD) or continuous venovenous haemodiafiltration (CVVHDF) [5]. If drug removal by CVVHDF is significantly higher then dosage supplementation may be necessary to ensure therapeutic efficacy. Therefore, knowledge of the impact of CVVHDF on a drug's pharmacokinetics and elimination is essential to optimize treatment and to provide some practical guidelines for drug dosage.
The current dosage guidelines for vancomycin vary. In the critically ill patient without renal impairment doses have ranged from 15 mg kg−1 per 24 h to 2 g every 12 h [14]. In critically ill patients with renal impairment the dose is reduced and administered from once every 24 h to once every 240 h. Because of its relatively large molecular weight, vancomycin is not significantly removed by conventional haemodialysis or peritoneal dialysis. In conventional dialysis vancomycin is given every 7–10 days [15]. However, the clearance of vancomycin is increased with the highly permeable dialysis membranes [16, 17]. A supplemental dose of vancomycin equal to one-half of a loading dose after dialysis is typical. Pollard et al. suggested that patients undergoing haemodialysis with a high-flux dialyser membrane should be given a 20 mg kg−1 loading dose and 15 mg kg−1 of drug every 7 days thereafter [18].
CVVHDF combines diffusion with convection and thus the degree of change in clearance of drug or solute may be greater than other CRRT [19]. The reported clearance of vancomycin by conventional dialysis (4 h day−1) is only 3.8 l day−1, whereas CRRT clearances range from 11.5 to 19.3 l day−1[5, 12, 20–23]. In patients receiving CRRT the volume of distribution ranges from 40.9 to 65.8 l [20, 22, 24, 25]. However, many of these studies have limited statistical power because of small sample sizes, or poorly defined CRRT conditions, such as dialysate, ultrafiltrate or blood flow rates, haemofilter type or length of therapy, or lack of documentation of adequacy of removal of a reference solute, such as urea or creatinine [19].
The progress to therapies with greater clearances and wider application of these techniques requires the re-assessment of the impact of CRRT on vancomycin concentrations. Boereboom et al. studied vancomycin clearance during continuous venovenous haemofiltration (CVVHF) in two critically ill patients and found that the cumulative amount removed was approximately 250 mg per 24 h [24]. The haemofilter clearance of vancomycin was about 1.4 l h−1. They recommended a loading dose of 15–20 mg kg−1 followed after 24 h by 250 mg to 500 mg twice daily. Santre et al. reported a mean total clearance of 2.34 l h−1 (39 ml min−1) and a mean haemofilter clearance of vancomycin of 0.25 l h−1 (4.2 ml min−1) [25]. They advised that 7.5 mg kg−1 of vancomycin should be given intravenously every 12 h to critically ill patients receiving CVVHD. Their study was limited to three patients. Joy et al. studied vancomycin clearance by CVVHF and CVVHD and found that the haemofilter clearance at an ultrafiltrate flow rate of 1.5 l h−1 was between 0.8 l h−1 and 1.4 l h−1 (13.7 ml min−1 and 22.8 ml min−1) depending on the type of haemofilter used [19]. They advised a vancomycin dosage regimen of 850–1050 mg day−1 in patients receiving CVVHF. That study was undertaken in eight stable end-stage renal failure patients undergoing controlled CVVHF and CVVHD. Therefore, the extrapolation to critically ill patients with acute renal failure must be questioned. Davies et al. determined the dose of vancomycin in patients treated with CAVHD and CVVHD and found that the filter clearances of vancomycin at dialysate flow rates of 1 l h−1 and 2 l h−1 were 0.73 l h−1 (14.6 ml min−1) and 1 l h−1 (18.6 ml min−1), respectively [20]. They suggested that in patients with acute renal failure being treated by CAVHD or CVVHD, 1000 mg of vancomycin should be administered approximately every 48 h. Macias et al. studied vancomycin pharmacokinetics in critically ill patients treated with CVVHF [22]. An empirical loading dose of 15 mg kg−1 was given followed by 750 mg to 1500 mg every 24 h. The haemofilter clearance of vancomycin ranged from 0.4 l h−1 (6.7 ml min−1) to 0.8 l h−1 (13.3 ml min−1). Bellomo et al. studied the clearance of vancomycin during therapy with CAVHD [27]. The haemofilter clearance of vancomycin was 0.64 ± 0.27 l h−1 (10.5 ± 4.46 ml min−1) and they advised that patients receiving CAVHD would require 500 mg or more of vancomycin daily. Dupuis et al. investigated vancomycin disposition in one patient undergoing CAVH [21]. The clearance of vancomycin was 9 l day−1 (0.38 l h−1 or 6.25 ml min−1).
The dose of vancomycin in critically ill patients receiving CVVHDF at the Royal Brisbane Hospital Intensive Care Unit was selected from consideration of the literature, knowing that the CVVHDF method used would produce high clearance [13]. We chose to investigate 750 mg every 12 h. The aim of therapy is to maximize the time above the minimum inhibitory concentration of 5 mg l−1 and to maintain trough concentrations between 5 and 15 mg l−1.
The objectives of this study were: to determine the pharmacokinetics of vancomycin 750 mg administered every 12 h in critically ill patients requiring continuous venovenous haemodiafiltration; to compare and contrast the calculated vancomycin clearances with those determined in other studies of patients receiving renal replacement therapies; to see if routine measurements, such as urea clearance, could predict vancomycin clearance.
Methods
The study was conducted at the Royal Brisbane Hospital (Queensland, Australia), a 785-bed general, tertiary referral, university-affiliated hospital with a number of specialities including medicine, surgery, orthopaedics, psychiatry, oncology and trauma services.
This study was conducted from March 2000 to June 2001. The study was approved by the Royal Brisbane Hospital Research Ethics Committee [no. 2000/023] and by the Medical Research Ethics Committee of the University of Queensland (H/216/PHARM/00/M). The Queensland Guardianship and Administration Tribunal approved the research project, with respect to obtaining legal consent of adults with impaired capacity (file number CR 001/2000). The study was conducted in accordance with the guidelines of the Australian NHMRC and the Declaration of Helsinki.
Ten patients were enrolled in the study. Demographic and clinical information is shown in Table 1. All patients were sedated and ventilated. This was an open-labelled study of current practice in which all consecutively admitted critically ill patients in whom vancomycin was used to treat a known or suspected infection and who required CVVHDF for acute renal failure of any cause were eligible. Informed consent was obtained from the patient or next of kin.
Table 1.
Patient demographics and clinical information
| Patient | Sex | Age (years) | Estimated weight (kg) | SOFA | Admission diagnosis | Infective diagnosis | APACHE II | Albumina (g l−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 66 | 55 | 16 | Respiratory failure | Empiric cover | 22 | 33, 16 |
| 2 | M | 68 | 79 | 20 | Ischaemic bowel, septic shock | Gram-positive cocci (blood) | 48 | 27, 25 |
| 3 | M | 70 | 80 | 14 | Abdominal aortic aneurysm, aspiration pneumonia | Staphylococcus haemolyticus and S. epidermidis (blood) | 38 | 28, 24 |
| 4 | M | 62 | 80 | 4 | Intra-abdominal surgery | Intra-abdominal sepsis | 20 | 25 |
| 5 | M | 62 | 82 | 17 | Laparatomy for chronic pelvic abscess, septic shock | Klebsiella and Enterococcus faecalis (blood) | 28 | 28 |
| 6 | F | 66 | 70 | 19 | Febrile neutropenia, acute renal failure | Multi-resistant S. aureus (blood, bronchial) | 47 | 22 |
| 7 | F | 52 | 55 | 18 | Medical | Empiric | 35 | 20 |
| 8 | F | 51 | 100 | 17 | Right hepatectomy | Empiric | 26 | 24 |
| 9 | M | 63 | 110 | 21 | Medical | Empiric | 25 | |
| 10 | M | 43 | 80 | 16 | Multi trauma | E. faecalis (blood) | 19 | 23, 16 |
SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation.
Albumin levels measured on day of study.
Patients were enrolled in the study if they were: >17 years of age; critically ill and requiring CVVHDF for renal failure of any cause; prescribed vancomycin for a known or suspected infection; and if informed consent was obtained.
Patients were excluded from the study if they were: outside age limit, ≤17 years; or if informed consent was declined or could not be obtained.
Continuous venovenous haemodiafiltration was performed with 1 l h−1 dialysate and 2 l h−1 predilution filtration solution, producing 3 l h−1 dialysis effluent. Fluid input and effluent rates were controlled using IMED PC4 volumetric pumps (Alaris Medical Systems Inc., San Diego, CA, USA) set at 999 ml h−1. The blood was pumped at a speed of 200 ml min−1 using a Gambro BMM-10 blood pump (Gambro AB, Stockholm, Sweden) through an extracorporeal circuit containing a Hospal AN69HF haemofilter (Hospal AG, Lyon, France). Unless contraindicated, anticoagulation of the circuit was titrated to the patient response.
Blood samples were collected, from an indwelling arterial cannula, in heparinized tubes. The first sample (T0, baseline) was taken immediately prior to the intravenous administration of vancomycin 750 mg, infused in 150 ml of compatible fluid over 60 min, via a central line. Specimens were again collected half way through the infusion (30 min), at the end of the infusion (1 h) and after 2, 3, 4, 6, 8 and 12 h. The 12-h sample was taken immediately prior to administration of the next dose. The blood samples were chilled on ice and centrifuged (5 min at 2000 × g at 4 °C) within 1 h to obtain plasma. The plasma samples were stored at −80 °C until analysis.
All dialysis effluent was collected in hourly batches during the 12-h profile. The volume of each hourly batch was recorded to the nearest 50 ml and a 5-ml sample of effluent was stored at −80 °C until analysis.
Initially, sampling was performed on the first day of therapy to obtain profile A. Where possible, sampling was repeated on day 3 or day 4, after steady-state concentrations had nominally been achieved, to obtain profile B. Although it was ideal to obtain both sets of data, some patients were recruited in the study with a single steady-state profile only, due to having received the vancomycin prior to research staff notification. In other patients it was possible only to obtain an initial profile.
The following data were recorded on each day in order to describe the patient population: age, sex, estimated weight, albumin, Acute Physiology and Chronic Health Evaluation II score [28], maximum Sequential Organ Failure Assessment score [29], admitting diagnosis, microbiological infection and microbiological and clinical response.
Urea concentrations were measured in plasma samples collected after 1, 2, 3, 4, 6, 8 and 12 h. The hourly batches of effluent were combined to give 4 hourly batches, i.e. 1 h to 4 h, 4 h to 8 h and 8 h to 12 h. Urea levels were measured in each 4-h batch, giving the mean urea concentration in effluent for each 4-h period.
Vancomycin and urea concentrations in plasma and effluent samples were measured by enzyme multiplied immunoassay (EMITR) according to the manufacturer's guidelines, on a COBAS MIRA Plus instrument (Basel, Switzerland). There was no sample pretreatment. A vancomycin standard curve was constructed using calibrators supplied by the manufacturer (0.0, 5.0, 10, 20, 30 and 50 mg l−1), and the limit of quantification was 5 mg l−1. In addition, vancomycin quality control samples, Moni-trol total samples (supplied by Dade Behring GmbH, Leiderbach, Germany), prepared in a synthetic matrix at specified nominal concentrations of 3–9 mg l−1 (low) and 28–48 mg l−1 (high) were used as directed by the manufacturer to ascertain immunoassay performance. The EMITR quality control samples were consistently within the manufacturer's stated criteria for all sample batches analysed and were precise at the low and high concentrations.
Similar data analyses to that used previously by Wallis et al. were employed [13]. tmax and Cmax were directly observed as the maximum measured concentration. Noncompartmental pharmacokinetic analysis was performed using the WinNonlin software (Pharsight, Mountain View, CA, USA). The terminal half life, t1/2, was calculated as ln(2)/λ2. The areas under the plasma concentration–time curves (AUCs) were calculated using the log-linear trapezoidal method. AUC for the 12-h study period (AUC0−12) was used to calculate the AUC extrapolated to infinity (AUC0–∞) after the first dose (single dose) by the equation: AUC0−12 + C12/λ2. For initial doses the percentage of the dose that was eliminated by all elimination routes during the 12-h study period (F12) was calculated as 100% × AUC0−12/AUC0–∞. The total body clearance of vancomycin (CL) was calculated as dose/AUC0–∞ for initial doses and as dose/AUC0−12 for subsequent doses. The volume of distribution at steady state (Vss) was calculated as CL × [(AUMC0–∞/AUC0–∞) − 1/2] for the initial dose and as dose × (AUMC0−12+ 12 × C12/λ2)/(AUC0−12)2 for subsequent doses. In order to calculate the clearance by CVVHDF in profiles where the dialysis was interrupted, the area under the plasma–time curve for the period while CVVHDF was in operation, AUCCVVHDFwas calculated [13]. AUCCVVHDF was equal to the AUC0−12, except when CVVHDF was interrupted.
The mean vancomycin concentration in effluent for each hour of the study period was determined by direct measurement. The volume of effluent collected for each hour (hourly flow, Q) was measured directly. The amount of vancomycin removed by the filter during the study period (ACVVHDF) was calculated as the sum of the products of concentration and volumes for each hour. The clearance of vancomycin by CVVHDF was determined as CLCVVHDF = ACVVHDF/AUCCVVHDF[9]. The percentage of vancomycin eliminated by CVVHDF was calculated as FCVVHDF = 100% × CLCVVHDF/CL.
The clearance of urea by CVVHDF (CLurea) was calculated at 4-h intervals. An extraction ratio, or sieving coefficient (Surea) was calculated from the mean concentration in effluent and mean concentration in plasma for each 4-h interval: Surea = Ceffluent/Cplasma. The clearance was calculated from Surea and the measured flow of the effluent: CLurea = Surea × Q. These calculations were repeated with the vancomycin concentrations to obtain an analogous sieving coefficient (Svanco) and associated clearance (CLvanco) for vancomycin for each 4-h period. A mean value of clearance for the 12-h study period was obtained for both vancomycin and urea from the 4-h intervals. CLvanco and CLurea were monitored at 4-h intervals over the study period in an attempt to detect any change that may have occurred in the filter performance.
The maintenance dose for a target steady-state concentration was predicted as dose/dosing interval = clearance × targeted average steady state concentration.
Results
Ten patients were enrolled. The patient demographic and clinical information are given in Table 1. There were no adverse effects attributable to the use of vancomycin in this study population.
Fifteen pharmacokinetic profiles were obtained from these 10 patients – six initial profiles (Profile 1A, 2A, 3A, 5A, 8A, 10A) and nine steady-state or subsequent profiles (Profile 1B, 2B, 3B, 4B, 6B, 7B, 8B, 9B, 10B). Five patients had two profiles measured, an initial and subsequent profile (patients 1, 2, 3, 8 and 10). Five patients had only one profile measured (patients 4, 5, 6, 7 and 9). Of these patients, one patient (patient 5) had an initial profile measured only (Profile 5A) as this patient was removed from dialysis before a subsequent profile could be measured. The other four patients had been recruited for a steady-state profile only (Profile 4B, 6B, 7B, 9B). Patients 4, 6, 7 and 9 received a 1000-mg initial dose of vancomycin and were subsequently changed to a 750-mg twice daily regimen for the steady-state profile to be obtained. Eight of the 15 profiles had CVVHDF for the entire study period, whereas CVVHDF was interrupted in Profiles 1B, 2A, 2B, 5A, 6B, 8A and 10B due to clotted circuits requiring change. The age of the filter at the start of the pharmacokinetic study, the number of filters used during the study period and the total time off the filter for each patient profile were recorded (Table 2). The full 12-h plasma profile was still obtained while changing to a second filter circuit and in the case of Profile 2B three filter circuits were used during the study period. A 8-h plasma profile was collected for Profile 9B as the patient died whilst on the study and a 6-h plasma profile was collected for Profile 10B as the patient came off the filter and it was not replaced. Plasma concentrations at 12 h were extrapolated for both these patient profiles and the data extrapolated to provide the necessary pharmacokinetic parameters based on a 12-h profile.
Table 2.
Summary of the number of filters used, the age of the filter at the start of the pharmacokinetic profile for filter 1 or at the time of replacement for subsequent filter, the total amount of time off the filter for each patient profile
| Patient profile | Number of filters | Age of filter (h) | Total time off filter (h) |
|---|---|---|---|
| 1A | 1 | 11.5 | 0 |
| 1B | 2 | 1 (filter 1), 8.3 (filter 2) | 1.67 |
| 2A | 2 | 1 (filter 1), 9.9 (filter 2) | 0.33 |
| 2B | 2 | 2 (filter 1), 4 (filter 2), 7.1 (filter 3) | 2.83 |
| 3A | 1 | 0 | 0 |
| 3B | 1 | 27 | 0 |
| 4B | 1 | 16 | 0 |
| 5A | 1 | 3 | 0.42 |
| 6B | 1 | 36 | 0.25 |
| 7B | 1 | 7 | 0 |
| 8A | 1 | 60 | 0.5 |
| 8B | 1 | 22 | 0 |
| 9B | 1 | 34 | 0 |
| 10A | 1 | 8 | 0 |
| 10B | 1 | 0 | 0.5 |
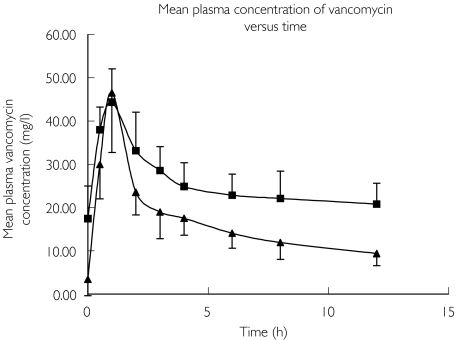
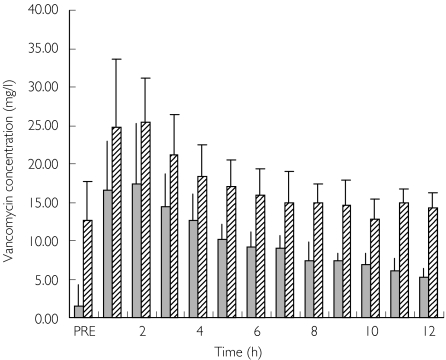
The mean (± SD) plasma vancomycin concentration–time data for the initial and subsequent doses are represented in Figure 1. Patients 1, 2, 3, 5, 8 and 10 had an initial pharmacokinetic profile (Profile A) taken on commencement of vancomycin therapy. Patients 1, 2, 3, 4, 8 and 10 had subsequent profiles (Profile B) obtained on or after day 3 of therapy. The hourly vancomycin concentration in the ultrafiltrate for both the initial and subsequent doses is depicted in Figure 2.
Figure 1.
Concentration–time data for vancomycin 750 mg administered intravenously every 12 h (mean ± SD). Initial doses (patients 1–3, 5, 8 and 10) (▴); Subsequent doses (patients 1–4, 6–10) (▪)
Figure 2.
Mean concentration of vancomycin in effluent collected hourly (mean ± SD). Initial doses (Profile A only) or patients 1–3, 5, 8 and 10 ( ); Subsequent doses (Profile B only) or patients 1–4, 6, 7, 8, 9 and 10 (
); Subsequent doses (Profile B only) or patients 1–4, 6, 7, 8, 9 and 10 ( )
)
The pharmacokinetic parameters, determined by noncompartmental analysis, are presented for each patient pharmacokinetic profile in Table 3. Clearance of vancomycin and urea through the filter are shown in Table 4.
Table 3.
Summary of pharmacokinetic data for vancomycin 750 mg i.v. every 12 h
| Patient profile | Cmax(mg l−1) | t1/2(β) (h) | AUC0–12(mg h l−1) | AUC0–∞(mg h l−1) | Cl (l h−1) | Vss(l) | F12 (%) |
|---|---|---|---|---|---|---|---|
| 1A | 67.8 | 7.8 | 243.4 | 344.0 | 2.2 | 20.3 | 71 |
| 1B | 55.0 | 25.2 | 406.7 | 1.8 | 66.5 | NA | |
| 2A | 42.9 | 9.7 | 210.6 | 362.5 | 2.1 | 27.5 | 58 |
| 2B | 43.4 | 18.3 | 318.1 | 2.4 | 57.1 | NA | |
| 3A | 47.2 | 6.2 | 196.8 | 265.6 | 2.8 | 22.8 | 74 |
| 3B | 47.2 | 22.6 | 367.6 | 2.0 | 64.7 | NA | |
| 4B | 36.3 | 11.8 | 243.9 | 3.1 | 51.6 | NA | |
| 5A | 54.0 | 13.5 | 243.6 | 509.3 | 1.5 | 27.1 | 48 |
| 6B | 49.0 | 17.1 | 300.8 | 2.5 | 56.3 | NA | |
| 7B | 53.8 | 22.1 | 350.1 | 2.1 | 62.9 | NA | |
| 8A | 39.8 | 10.9 | 190.6 | 344.9 | 2.2 | 31.8 | 55 |
| 8B | 44.9 | 38.3 | 283.8 | 2.6 | 136.7 | NA | |
| 9B | 32.6 | 6.0 | 171.9 | 4.4 | 29.9 | NA | |
| 10A | 27.1 | 11.5 | 115.6 | 205.6 | 3.6 | 53.4 | 56 |
| 10B | 39.6 | 12.8 | 293.4 | 2.6 | 36.9 | NA | |
| Mean ± SD | – | 15.6 ± 8.7 | – | – | 2.5 ± 0.7 | 49.7 ± 29.1 | 60 ± 10 |
Patient profile each separate occasion that pharmacokinetics were studied (e.g. 1B is second occasion for patient 1). Cmax, Maximum vancomycin plasma concentration; t1/2(β) elimination half life; AUC0−12, areas under the curve for study period; AUC0–∞, area under the curve extrapolated to infinity; CL, total body clearance; Vss, volume of distribution at steady state; F12, fraction of vancomycin eliminated within dosing interval of initial or first dose (Profile A only); NA, not applicable.
Table 4.
Clearance of vancomycin and urea by CVVHDF
| Patient profile | AUCCVVHDF (mg h l−1) | ACVVHDF (mg) | ClCVVHDF (l h−1) | FCVVHDF | CLurea (l h−1) |
|---|---|---|---|---|---|
| 1A | 243.4 | 366.5 | 1.5 | 69 | 2.2 |
| 1B | 256.8 | 360 | 1.4 | 78 | 1.8 |
| 2A | 210.6 | 338.3 | 1.6 | 76 | 2.2 |
| 2B | 76.3 | 79.4 | 1.0 | 43 | 1.7 |
| 3A | 196.8 | 389 | 2.0 | 71 | 2.5 |
| 3B | 367.6 | 696 | 1.9 | 95 | 2.0 |
| 4B | 243.9 | 538.4 | 2.2 | 71 | 2.3 |
| 5A | 243.6 | 376.3 | 1.5 | 100 | 1.9 |
| 6B | 300.8 | 578.6 | 1.9 | 76 | 2.2 |
| 7B | 350.1 | 723.7 | 2.1 | 100 | 2.2 |
| 8A | 190.6 | 344.4 | 1.8 | 82 | 2.3 |
| 8B | 283.8 | 655.6 | 2.3 | 88 | 2.2 |
| 9B | 171.9 | 352 | 2.1 | 48 | 2.2 |
| 10A | 115.6 | 260.6 | 2.3 | 64 | 2.4 |
| 10B | 67.8 | 135.5 | 2.0 | 77 | 2.4 |
| Mean ± SD | – | 413 ± 191 | 1.8 ± 0.4 | 76 ± 16.5 | 2.2 ± 0.2 |
AUCCVVHDF, Area under the curve while CVVHDF operating; ACVVHDF, amount of vancomycin removed by CVVHDF in dosing interval; CLCVVHDF, clearance of vancomycin through the filter; FCVVHDF, fraction of vancomycin eliminated by CVVHDF while operating; Clurea, clearance of urea through the filter.
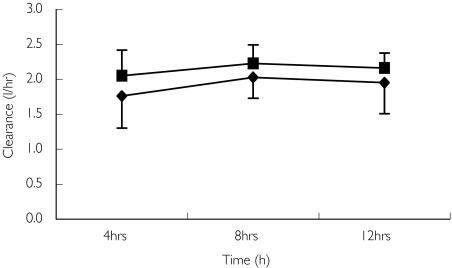
The amount of vancomycin eliminated by CVVHDF during the 12-h study period, ACVVHDF- was 413 ± 191 mg, which represents 55 ± 25% of the administered dose. On average, 60 ± 10% of the first dose was eliminated by all routes in the 12-h study period monitored (F12, Table 3). The clearance of vancomycin by CVVHDF was 1.8 ± 0.4 l h−1 (30 ± 6.7 ml min−1) which was 76 ± 16.5% of the total body clearance. In two profiles (patients 5A and 7B) all of the vancomycin was removed by CVVHDF. Estimations of the clearance of vancomycin by CVVHDF using Svanco values (CLvanco) agreed well with the directly measured values (CLCVVHDF). The estimated clearance of vancomycin using Svanco was 1.9 ± 0.3 l h−1 (31.7 ± 5 ml min−1). The estimated clearance of urea through the filter using Surea (CLurea) was 2.2 ± 0.2 l h−1 (36.7 ± 3.3 ml min−1) suggesting a slightly higher permeability of urea through the filter than vancomycin. The clearance of vancomycin and urea calculated at 4-h intervals is plotted in Figure 3.
Figure 3.
Clearance of vancomycin and urea by continuous venovenous haemodiafiltration (CVVHDF) calculated at 4-h intervals (mean ± SD). Vancomycin clearances (♦); Urea clearances (▪)
There was no significant difference between the performance of the filter in clearing vancomycin or urea over each 4-h interval of the study. The sieving coefficient for urea was 0.8 ± 0.06. The measured sieving coefficient for vancomycin was lower and more variable at 0.7 ± 0.10, possibly due to the larger fluctuations in plasma levels. Urea, which may be easier to quantify routinely, could therefore be used as an approximate indicator of vancomycin elimination.
Prediction of future average dosage requirements of vancomycin in this population indicated that to achieve an average steady-state concentration of 15 mg l−1, a dose of approximately 450 mg every 12 h would be required (using estimated average vancomycin clearance of 2.5 l h−1). This agrees well with the average amount eliminated by CVVHDF during a 12-h study period of 413 mg.
Discussion
There is a complex interaction between the patient, the type of CRRT chosen and the drug [13], which can influence the pharmacokinetics of vancomycin in critically ill patients. In general, drugs with a molecular weight of <5000 Da, low plasma protein binding and a small volume of distribution will be removed effectively by CRRT. Additional factors such as altered binding to plasma proteins, capillary leakage, hepatic and/or renal failure and changes in volume of distributions complicate dosing in critically ill patients. Vancomycin has a molecular weight of 1448, is minimally bound to plasma proteins, is renally excreted and has a relatively small volume of distribution, and therefore it might be expected to be cleared by CRRT.
The results of this study demonstrate that vancomycin is effectively cleared during CVVHDF. The results are in keeping with previous reports that CRRT, utilizing porous membranes of the polysulphone and polyacrylonitrile types, provide some degree of vancomycin clearance [15, 19–22, 24–27]. This study demonstrated that CVVHDF removed over half of the vancomycin dose during the 12-h study period (ACVVHDF = 413 mg). The overall fraction of the dose of vancomycin eliminated by all routes during the first dosing interval was 60%. CVVHDF was responsible for nearly three-quarters of all vancomycin eliminated (FCVVHDF = 76%). Extracorporeal elimination is considered clinically significant if its contribution to total body clearance exceeds 25–30%[3]. Therefore, the results of this study indicate that the extracorporeal route of elimination of vancomycin is clinically important. The difference between the total body clearance and filter clearance would suggest that there is a nonrenal and non-CVVHDF route to vancomycin elimination. The observed filter clearance is consistent with the fact that a nonrenal clearance of vancomycin of up to 30% of the total body clearance has been reported in normal subjects [26].
The clearance of vancomycin by CVVHDF (CLCVVHDF) was measured as 1.8 ± 0.4 l h−1 (30 ± 6.7 ml min−1) in this study and accounted for 76% of the total body clearance. This is 1.3–7.2 times the clearance previously reported for vancomycin by other methods of CRRT in the literature, including CVVHF, CVVHD and CAVHD [15, 19–22, 24–27]. The CVVHDF technique, used at the Royal Brisbane Hospital, combines significant convective and diffuse clearance. The results of this study reflect the superiority of utilizing both convective and diffusive forces. It is likely that the higher blood flow through the haemofilter to achieve a higher ultrafiltrate flow in this study would also allow higher vancomycin clearance in this type of patient.
The total body clearance of vancomycin observed in this study was 2.5 ± 0.7 l h−1 (41.7 ± 11.7 ml min−1, range 30–53.4 ml min−1) and was similar to that reported in previous vancomycin CRRT studies [20, 22, 24, 25].
The sieving coefficient, the ratio of drug concentration in the ultrafiltrate to the plasma, is a measure of the permeability of the haemofilter to a specific compound, in this case vancomycin [21]. Sieving coefficients near unity indicate that a drug has relatively free passage across the membrane, whereas a low value indicates retention of the drug in plasma [9, 12]. The average sieving coefficient for vancomycin in this study was 0.7 ± 0.10 and was consistent with that previously reported in the literature [19, 20, 22, 24, 26]. However, this value was lower than the unbound fraction of vancomycin, reported as 0.8–0.9 [12]. Boereboom et al. reported the average sieving coefficient for vancomycin to be 0.73, although using a different filter to the one used in this study [24]. Davies et al. used the same filter as in this study under different CRRT conditions and reported a similar average sieving coefficient of 0.66 [20]. Dupuis et al. reported a variable sieving coefficient in the range of 0.5–0.94 [21]. The increased flow of effluent in this study may be responsible for the increased clearance of vancomycin.
The sieving coefficient for urea measured in this study was 0.8 ± 0.06, which was consistent with a sieving coefficient of 0.83 ± 0.06 previously measured by Wallis et al. in a study conducted under similar conditions [13]. The sieving coefficient for urea was lower than would normally be expected given that it is a small, easily filtered molecule. The sieving coefficient of a drug can decline with time because of changes in protein binding or due to drug–protein–membrane interactions or both [21]. Other factors that may influence the sieving coefficient include changes in blood pH or in degree of uraemia, changes in serum concentration of protein, albumin or bilirubin and administration of heparin, total parenteral nutrition solutions and fat emulsion [21]. Any of these factors may have accounted for the lower than expected sieving coefficient for urea observed in this study.
Our study also demonstrated that an indication of vancomycin clearance through the filter may be obtained from knowledge of the urea clearance. However, as a marker it slightly overestimates the clearance for vancomycin (vancomycin clearance approximated 86% of the urea clearance) due to the greater permeability of urea through the filter.
The CVVHDF performance was assessed at 4-h intervals over the 12-h study period (Figure 3) and there was no significant change.
The mean vancomycin half-life of 15.6 ± 8.7 h measured during CVVHDF in this study is much longer than in patients with normal renal function, but it is considerably reduced when compared with patients with severe renal failure undergoing conventional haemodialysis and other forms of CRRT [12, 20, 25]. The half-life in this study compares well with that obtained in the study by Santre et al., who appear to have used the same filter and type of CRRT but with different blood flow and ultrafiltration rates [25]. The half-lives observed in this study reflect the data in the literature for CRRT, being between 6 and 14 h for all but five patients (Table 2). A notable exception was Profile 8B with a half-life of 38.3 h. This patient was noted to have severe hepatic and renal failure. The elimination half-life of vancomycin has previously been shown to be prolonged in patients with abnormal hepatic function [30].
One limitation of this study, and of the pharmacokinetic parameter estimates generated, is the duration of sampling relative to the apparent elimination half-life. The patients required vancomycin clinically and were receiving this on a 12-h dosage schedule. For clinical reasons doses could not be withheld to allow longer sampling times, but this did lead to potential problems with larger than ideal fractions of the areas under the concentration–time curves being extrapolated and half-lives being estimated on less than ideal data. This is always a limitation when working with such critically ill subjects, but the data generated are still preferable to the absence of data which currently exists.
The mean trough concentration for the initial dose profiles was 9.4 ± 2.8 mg l−1, which was within the acceptable range of trough concentrations at the Royal Brisbane Hospital (5–15 mg l−1). However accumulation was evident as trough concentrations (predosing and after 12 h) in seven of the nine subsequent profiles were high. The average trough concentration in the subsequent profiles was 19.2 ± 5.2 mg l−1. This is also demonstrated in Figure 2, where elimination of vancomycin in the effluent is higher for the subsequent profile due to accumulation. This is consistent with the pharmacokinetic calculations, demonstrating that only about 60% of a dose of vancomycin was eliminated during a dosing interval. Although there seems to be little evidence to support concentration-dependent toxicity, the dosage of vancomycin should be adjusted to avoid accumulation, and this can now be done readily using the calculated pharmacokinetic parameters in this specific population.
The volume of distribution measured in this study (49.7 ± 29.1 l) is in agreement with that previously reported by Santre et al. who reported a volume of distribution of 47.3 ± 6.4 l [25]. The volume of distribution is also consistent with other studies investigating the removal of vancomycin by CRRT although using different filter types and CRRT conditions [20, 22, 24]. The Vss value in this study, adjusted for estimated weights, was 0.65 ± 0.36 l kg−1, which is within the range of that reported for adult patients with normal renal function [18, 31–34]. One notable exception was Profile 8B with an estimated volume of distribution of 136.7 l (1.4 l kg−1), which may have been because of increased vascular water or could have been an artefact in this unstable patient. In critically ill patients, the volume of distribution of a drug may vary due to blood loss, massive fluid resuscitation, oedema, ascites and changes in protein and tissue binding. Clinical conditions also vary in these patients on a daily basis. Hence true steady-state concentrations may be difficult to achieve in these clinically unstable patients.
In conclusion, vancomycin is cleared effectively by this method of continuous venovenous haemofiltration. The clearance appears to be faster than that of other forms of continuous renal replacement therapy. A dose of 750 mg intravenously every 12 h provided adequate serum concentrations for the critically ill patients in this study, but accumulation occurred. The dose may need to be reduced over the longer term, as only approximately 60% of a dose was cleared over a 12-h period. To achieve an average steady-state concentration of 15 mg l−1 and using the clearance of 2.5 l h−1, a maintenance dose of 450 mg of vancomycin every 12 h would be required.
Conflict of interest statement
S.E.T., J.L. and M.E.D. have no direct conflicts of interest. J.L. has consulted for, and received an honorarium from Eli Lilly (not the manufacturers of the vancomycin used in the present study). The research grant (supported by DBL) was awarded by the Society of Hospital Pharmacists of Australia independently of DBL.
Acknowledgments
Financial support for this study was obtained from research grants given by the Society of Hospital Pharmacists of Australia (Abbott Australasia Hospital Pharmacy Research Grant, DBL Development Fund).
References
- 1.Chow AW, Azar RM. Glycopeptides and nephrotoxicity. Intensive Care Med. 1994;20(Suppl 4):S23–9. doi: 10.1007/BF01713979. [DOI] [PubMed] [Google Scholar]
- 2.Gruneberg RN, Wilson AP. Anti-infective treatment in intensive care: the role of glycopeptides. Intensive Care Med. 1994;20(Suppl 4):S17–22. doi: 10.1007/BF01713978. [DOI] [PubMed] [Google Scholar]
- 3.Schetz M, Ferdinande P, Van den Berghe G, Verwaest C, Lauwers P. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med. 1995;21:612–20. doi: 10.1007/BF01700172. [DOI] [PubMed] [Google Scholar]
- 4.Bressolle F, Kinowski JM, de la Coussaye JE, Wynn N, Eledjam JJ, Galtier M. Clinical pharmacokinetics during continuous haemofiltration. Clin Pharmacokinet. 1994;26:457–71. doi: 10.2165/00003088-199426060-00004. [DOI] [PubMed] [Google Scholar]
- 5.Cotterill S. Antimicrobial prescribing in patients on haemofiltration. J Antimicrob Chemother. 1995;36:773–80. doi: 10.1093/jac/36.5.773. [DOI] [PubMed] [Google Scholar]
- 6.Freebairn RC, Lipman J. Renal replacement therapy for the critically ill—precarious progress. Part II. Technical aspects and clinical application. S Afr J Surg. 1993;31:147–51. [PubMed] [Google Scholar]
- 7.Freebairn RC, Lipman J. Renal replacement therapy for the critically ill—precarious progress. Part I. Definitions and physiological aspects. S Afr J Surg. 1993;31:114–20. [PubMed] [Google Scholar]
- 8.Joos B, Schmidli M, Keusch G. Pharmacokinetics of antimicrobial agents in anuric patients during continuous venovenous haemofiltration. Nephrol Dial Transplant. 1996;11:1582–5. [PubMed] [Google Scholar]
- 9.Joy MS, Matzke GR, Armstrong DK, Marx MA, Zarowitz BJ. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother. 1998;32:362–75. doi: 10.1345/aph.17105. [DOI] [PubMed] [Google Scholar]
- 10.Kroh UF. Drug administration in critically ill patients with acute renal failure. New Horiz. 1995;3:748–59. [PubMed] [Google Scholar]
- 11.Meyer MM. Renal replacement therapies. Crit Care Clin. 2000;16:29–58. doi: 10.1016/s0749-0704(05)70096-8. [DOI] [PubMed] [Google Scholar]
- 12.Reetze-Bonorden P, Bohler J, Keller E. Drug dosage in patients during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clin Pharmacokinet. 1993;24:362–79. doi: 10.2165/00003088-199324050-00002. [DOI] [PubMed] [Google Scholar]
- 13.Wallis SC, Mullany DV, Lipman J, Rickard CM, Daley PJ. Pharmacokinetics of ciprofloxacin in ICU patients on continuous veno-venous haemodiafiltration. Intensive Care Med. 2001;27:665–72. doi: 10.1007/s001340100857. [DOI] [PubMed] [Google Scholar]
- 14.Chernow B, editor. Pocket Book of Critical Care Pharmacotherapy. Baltimore, MD: Williams & Wilkins; 1995. [Google Scholar]
- 15.Foote EF, Dreitlein WB, Steward CA, Kapoian T, Walker JA, Sherman RA. Pharmacokinetics of vancomycin when administered during high flux hemodialysis. Clin Nephrol. 1998;50:51–5. [PubMed] [Google Scholar]
- 16.Leader WG, Chandler MH, Castiglia M. Pharmacokinetic optimisation of vancomycin therapy. Clin Pharmacokinet. 1995;28:327–42. doi: 10.2165/00003088-199528040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm MP, Estes L. Symposium on antimicrobial agents—Part XII. Vancomycin. Mayo Clin Proc. 1999;74:928–35. doi: 10.4065/74.9.928. [DOI] [PubMed] [Google Scholar]
- 18.Pollard TA, Lampasona V, Akkerman S, et al. Vancomycin redistribution: dosing recommendations following high-flux hemodialysis. Kidney Int. 1994;45:232–7. doi: 10.1038/ki.1994.28. [DOI] [PubMed] [Google Scholar]
- 19.Joy MS, Matzke GR, Frye RF, Palevsky PM. Determinants of vancomycin clearance by continuous venovenous hemofiltration and continuous venovenous hemodialysis. Am J Kidney Dis. 1998;31:1019–27. doi: 10.1053/ajkd.1998.v31.pm9631848. [DOI] [PubMed] [Google Scholar]
- 20.Davies SP, Azadian BS, Kox WJ, Brown EA. Pharmacokinetics of ciprofloxacin and vancomycin in patients with acute renal failure treated by continuous haemodialysis. Nephrol Dial Transplant. 1992;7:848–54. [PubMed] [Google Scholar]
- 21.Dupuis RE, Matzke GR, Maddux FW, O'Neil MG. Vancomycin disposition during continuous arteriovenous hemofiltration. Clin Pharm. 1989;8:371–4. [PubMed] [Google Scholar]
- 22.Macias WL, Mueller BA, Scarim SK. Vancomycin pharmacokinetics in acute renal failure: preservation of nonrenal clearance. Clin Pharmacol Ther. 1991;50:688–94. doi: 10.1038/clpt.1991.208. [DOI] [PubMed] [Google Scholar]
- 23.Matzke GR, O'Connell MB, Collins AJ, Keshaviah PR. Disposition of vancomycin during hemofiltration. Clin Pharmacol Ther. 1986;40:425–30. doi: 10.1038/clpt.1986.201. [DOI] [PubMed] [Google Scholar]
- 24.Boereboom FT, Ververs FF, Blankestijn PJ, Savelkoul TJ, van Dijk A. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Intensive Care Med. 1999;25:1100–4. doi: 10.1007/s001340051018. [DOI] [PubMed] [Google Scholar]
- 25.Santre C, Leroy O, Simon M, et al. Pharmacokinetics of vancomycin during continuous hemodiafiltration. Intensive Care Med. 1993;19:347–50. doi: 10.1007/BF01694710. [DOI] [PubMed] [Google Scholar]
- 26.De Bock V, Verbeelen D, Maes V, Sennesael J. Pharmacokinetics of vancomycin in patients undergoing haemodialysis and haemofiltration. Nephrol Dial Transplant. 1989;4:635–9. [PubMed] [Google Scholar]
- 27.Bellomo R, Ronco C. Continuous versus intermittent renal replacement therapy in the intensive care unit. Kidney Int Suppl. 1998;66:S125–8. [PubMed] [Google Scholar]
- 28.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 29.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Cheung RP, DiPiro JT. Vancomycin: an update. Pharmacotherapy. 1986;6:153–69. doi: 10.1002/j.1875-9114.1986.tb03471.x. [DOI] [PubMed] [Google Scholar]
- 31.Cunha BA. Vancomycin. Med Clin North Am. 1995;79:817–31. doi: 10.1016/s0025-7125(16)30041-4. [DOI] [PubMed] [Google Scholar]
- 32.Begg EJ, Barclay ML, Kirkpatrick CJ. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 1999;47:23–30. doi: 10.1046/j.1365-2125.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25:433–7. doi: 10.1128/aac.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moellering RC, Jr, Krogstad DJ, Greenblatt DJ. Pharmacokinetics of vancomycin in normal subjects and in patients with reduced renal function. Rev Infect Dis. 1981;3(Suppl):S230–5. [PubMed] [Google Scholar]