Abstract
Aims
Polymorphisms of the NOSIII gene and of the CYBA gene have been associated with a number of pathological conditions such as arterial hypertension, coronary artery disease, and myocardial infarction. Because endothelium-dependent vasodilation is impaired in these disorders, we hypothesized that polymorphisms of NOSIII or CYBA might modulate endothelial function of venous capacitance vessels already before cardiovascular disease becomes overt.
Methods
Endothelium-dependent and -independent venodilation was assessed by measuring local vascular responses to bradykinin and sodium nitroprusside in the dorsal hand vein after preconstriction with phenylephrine in 72 healthy male Caucasians after careful exclusion of cardiovascular risk factors. Genotyping was performed for polymorphisms of the NOSIII gene (T-786C, G894T, (CA)n) and the CYBA gene (C242T).
Results
Genotype distribution for each polymorphism followed the Hardy–Weinberg equilibrium. In all studied single nucleotide polymorphisms no significant difference between the respective genotypes and the venodilator response to either sodium nitroprusside or bradykinin was observed, and the number of CA repeat copies was not related to the venodilator response to bradykinin. Mean venodilation induced by bradykinin 50 ng min−1 (±SEM) for homozygote carriers of the single nucleotide polymorphisms was 48.9 ± 8.5% venodilation (G894T; wild type: 49.8 ± 6.9), 50.3 ± 11.0% venodilation (T-786C; wild type: 42.6 ± 5.2), and 30.4 ± 9.1% venodilation (C242T; wild type: 49.2 ± 6.0), respectively.
Conclusions
This study suggests that the studied polymorphisms of NOSIII and CYBA do not significantly modulate endothelium-dependent venodilation in individuals without vascular risk factors.
Keywords: nitric oxide; endothelial function; endothelial nitric-oxide synthase; NADPH oxidase polymorphism (C242T); NOSIII polymorphisms (G894T, T-786C and (CA)n)
Introduction
Nitric oxide (NO) released from vascular endothelial cells is a multifunctional antiproliferative molecule that modulates vessel tone, inhibits platelet aggregation, and prevents the adhesion of platelets and leucocytes to the vessel wall [1]. Genetic polymorphisms of enzymes involved in NO metabolism are supposed to modulate oxidative stress and have been associated with the individual risk of cardiovascular diseases [2].
The responsible enzyme for the generation of NO in the vasculature is constitutive endothelial NO synthase (NOSIII), which is encoded by a gene containing several polymorphic sites [3, 4]. For the point mutation G894T, resulting in the replacement of glutamate by aspartate at codon 298 in exon 7, associations with pathological conditions such as coronary vasospasm, essential hypertension, coronary artery disease, and myocardial infarction have been reported [5–8]. For the point mutation T-786C in the 5′ flanking region and for the (CA)n polymorphism, consisting of variations in the CA repeat copy number in intron 13 of NOSIII[4], the clinical relevance is less well investigated and the results of the few published studies are conflicting [9–12].
The metabolic fate of NO and thus its duration of action in the tissue is modulated by various metabolic pathways with reactive oxygen species (e.g. superoxide anion) being a major cause for accelerated degradation [13]. A significant source of superoxide anion in the vascular wall is the NAD(P)H oxidase system [14], of which p22phox is an important component [15]. A single nucleotide polymorphism (C242T) in the CYBA gene, encoding p22phox, reduces vascular NAD(P)H oxidase activity in vitro and might thus restrict local superoxide anion formation [15]. Hence, the C242T polymorphism might prevent generation of reactive oxygen species and diseases associated with oxidative stress such as atherosclerosis [2]. To date studies about the clinical importance of this polymorphism are sparse and contradictory [16–19] and no information about its effects on NO-mediated endothelial function in vivo is available.
Studies analyzing the consequences of NOSIII (T-786C and (CA)n) and CYBA (C242T) polymorphisms on vascular function are lacking. Vascular function has been directly assessed only for one polymorphism (G894T polymorphism). In a study in patients with expected endothelial dysfunction due to arterial hypertension or hypercholesterolaemia a relevant effect on endothelium-dependent vasodilation was not found [20]. In another study on the G894T polymorphism in healthy volunteers [21] acetylcholine-induced relaxation in hand veins and in forearm resistance arteries was preserved, whereas endogenous NO production as measured by urinary NOx excretion was impaired. Neither of these studies attempted to exclude the potential contribution of other endothelial vasodilators released in response to acetylcholine, e.g. by administering effective doses of acetylsalicylic acid. At present, the pathogenetic links between the NOSIII and CYBA polymorphisms and cardiovascular diseases are still unclear.
We hypothesized that different polymorphisms of NOSIII and CYBA might act in concert and modulate endothelial function of venous capacitance vessels before cardiovascular disease becomes overt. We thus assessed endothelial and endothelium-independent responses in well characterized healthy male individuals without clinical signs of cardiovascular disease or vascular risk factors.
Methods
Participants
This was a prospective study in 72 healthy male nonsmoking Caucasians, mainly students from the Heidelberg area. After approval of the study by the responsible Ethics Committee of the Medical Faculty of the University of Heidelberg the study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The individuals were enrolled after giving written informed consent if they met all of the following inclusion criteria: normal physical examination, normal ECG, normal urinalysis and routine laboratory investigations including cardiovascular risk factors (serum lipids, glucose, homocysteine). Exclusion criteria were: a history of allergies, known conditions causing endothelial dysfunction such as diabetes, hyperlipidaemia, arterial hypertension, and hyperhomocysteinaemia, regular medication and/or treatment with drugs within the last 6 weeks, acute or chronic illness, smoking in the 12 months preceding the study, and drug and/or alcohol abuse. Investigators and participants were blinded for the genotypes of the volunteers.
Genotyping
All participants were genotyped for polymorphisms in the NOSIII (G894T, T-786C and CA-repeat) and the CYBA (C242T) gene. Genomic DNA was isolated from white blood cells using either QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany) or NucleoSpin® Blood Quick Pure Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions.
The NOSIII (G894T) and the CYBA (C242T) polymorphism were determined using the hybridization probes format on the LightCycler™ (Roche Applied Science, Mannheim, Germany) as recently developed in our group [22].
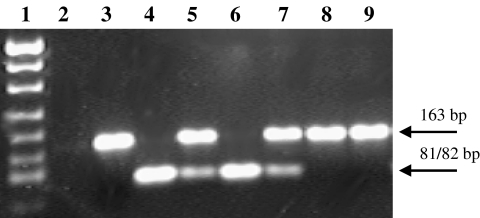
The NOSIII (T-786C) polymorphism was determined using a newly developed PCR-RFLP analysis [23]. PCR was performed in the LightCyclerTM (Roche Applied Science, Mannheim, Germany) in a total volume of 20 µl using the LightCycler-FastStart DNA Master SYBR Green I Kit (Roche Applied Science, Mannheim, Germany) and the primers 5′-GGTGTACCCCACC TGCATTCT-3′ (sense) and 5′-CACCCCCACCCTGT CATTC-3′ (antisense), which were designed and synthesized by TIB MOLBIOL, Berlin, Germany. The reaction mixture contained 0.4 mm of each primer, 3 mm MgCl2, and about 50 ng of genomic DNA. For removing the heat-labile blocking groups of the FastStart Taq DNA polymerase, the samples were preincubated at 95 °C for 10 min, thus activating the enzyme. Amplification was performed using 40 cycles of denaturation (95 °C for 4 s), annealing (65 °C for 8 s), and extension (72 °C for 10 s) with temperature transition rates of 20 °C s−1. To monitor amplification, fluorescence was measured at the end of each elongation period. The163 bp PCR product was purified with the QIAquick® PCR Purification Kit (Qiagen, Hilden, Germany) and digested with the restriction enzyme PdiI (MBI Fermentas, St Leon-Rot, Germany). PdiI recognizes the mutant allele (C at position −786) and cleaves the 163 bp product in two fragments of 81 and 82 bp. The products of the digestion were size-separated by agarose gel electrophoresis (2%, stained with GelStarTM). The PCR product of an individual carrying two wild type alleles (TT) digested with PdiI revealed no fragments, whereas digestion of an individual homozygote for the C allele resulted in two fragments (which appear as one band on the agarose gel, due to their similar size). Digestion of the PCR product of a heterozygote person (TC) resulted in three fragments (163, 81 and 82 bp, visible as two bands on the gel) (Figure 1). The genotyping of each individual was repeated twice to verify the result.
Figure 1.
T-786C polymorphism of the NOSIII gene detected by PdiI restriction digestion of the 163 bp PCR product: Lane 1: molecular size marker pUC19/MspI; lane 2: no template control; lanes 3 +8 + 9: restriction pattern corresponding to homozygosity for the T-allele; lanes 4 +6: restriction pattern corresponding to homozygosity for the C-allele; lanes 5 +7: restriction pattern corresponding to heterozygosity
The NOSIII intron 13 CA dinucleotide repeat was determined by PCR following the protocol of Nadaud et al.[4] with the following modifications. The following oligonucleotides were synthesized by GeneScan (Freiburg, Germany), and used for the amplification: forward 5′-Cy*5-TGAGGAGAGACTCAGAATTGGA-3′ and reverse 5′-GCTTGTGTGGGGTTTCAGGCT-3′. The 25 µl reaction mixture contained 50 ng genomic DNA, 10 pmol of each primer, 0.24 mm dNTP, 1.5 mm MgCl2, 0.5 U Taq polymerase (Invitek, Berlin, Germany), 16 mm ammoniumsulphate, 50 mm Tris-HCl pH 8.8, and 0.01% Tween 20. Amplification consisted of an initial denaturation step of 5 min at 95 °C, followed by 35 cycles of 1 min at 95 °C, 1 min at 58 °C, 1 min at 72 °C, and finished with a final extension step at 72 °C for 10 min. The amplification products were analyzed on an ALFexpress sequencer (Amersham Pharmacia, Freiburg, Germany) by a 6% denaturating polyacrylamide gel.
Assessment of endothelium-dependent and -independent venodilator responses
The participants were allowed to have a light breakfast until 2 h prior to the investigation. They abstained from alcohol for 24 h and from methylxanthine-containing beverages for at least 12 h before the measurements were made. Endothelium-dependent and -independent venodilator responses were investigated in a quiet room maintained at a constant temperature between 23° and 25 °C using the dorsal hand vein compliance technique according to Aellig [24] with modifications as described previously [25–27]. Hand vein compliance measurements always started in the morning and the participants were asked to remain in a semirecumbent position throughout the study.
In brief, the hand under investigation was placed on a vacuum pillow (Germa®, Sweden) sloping upwards at an angle of 30° from the horizontal. All vasoactive compounds were administered through a butterfly needle at a constant flow rate (0.25 ml min−1) into the investigated vein with the help of a syringe infusion pump (Pilote C, Fresenius®, Brezins, France). Changes of the diameter of the vein were recorded using a linear variable differential transformer (Schaevitz®, Type 100 MHR, Pennsauken, NJ, USA) with a freely movable core (weight 0.5 g) resting over the centre of the vein under investigation. Transformer signals were amplified by a Schaevitz® CAS series signal conditioner and the output was recorded on a strip-chart recorder (LKB 2210 recorder, LKB Produkter AB®, Bromma, Sweden) at a paper speed of 0.5 cm min−1. The difference between the position of the core before and during inflation of a sphygmomanometer cuff on the same arm to 40 mmHg gave a measure of the diameter changes under a given congestion pressure. Peak heights on the strip-chart recorder were linearly proportional to the movement of the core and were measured manually.
Design and materials
Half an hour before an investigation a single intravenous dose of 500 mg acetylsalicylic acid (Aspisol®, Bayer, Leverkusen, Germany) was administered into a cubital vein of the opposite arm to minimize modulatory vascular effects of prostaglandins released from the endothelium. After establishing a stable first baseline with normal saline (NaCl 0.9%, Braun, Melsungen, Germany), defined as 100% relaxation, increasing doses of the α1-adrenoceptor agonist phenylephrine (Neo-Synephrine®, Abbott Laboratories, North Chicago, USA; dosages: 1.25–8000 ng min−1) were infused to constrict the vein by about 80% (defined as 0% relaxation). Then while continuing the administration of the venoconstrictor increasing dose-rates of sodium nitroprusside (Nipruss®, Schwarz Pharma, Monheim, Germany; dosages: 0.02–2000 ng min−1) were co-infused to construct a complete cumulative dose–response curve to the endothelium-independent vasodilator. After a wash-out phase of sufficient length to allow the vein to return to a stable preconstriction (at least 40 min) the endothelium-dependent venodilator bradykinin (Clinalfa, Läufelfingen, Switzerland) was co-administered with phenylephrine. To prevent the occurrence of tolerance only three cumulative dose-rates of bradykinin (1, 50, 250 ng min−1) were used [28]. Bradykinin was dissolved in gelatine-polysuccinate (Gelafundin 4%, Braun, Melsungen, Germany) to avoid it adhering to infusion lines and adapters. Gelatine-polysuccinate is not known to have any vasoactive effect. Each dose-rate was administered for at least 5 min (phenylephrine: 6 min, sodium nitroprusside: 5 min, bradykinin: 6 min) to allow sufficient time to equilibrate. Dose-rates administered were intended not to result in any systemic effects which were monitored by repeated measurements of heart rate and blood pressure. Venoconstriction was expressed as the percentage reduction in vein diameter from baseline maximum dilation in the absence of vasoactive compounds (set as 100%). Venodilation was expressed as percentage reversal of phenylephrine-induced constriction which was set to 0%[24].
Data analysis and statistical evaluation
Data are expressed as mean values ± SEM, unless otherwise stated. The distribution of genotypes for each polymorphism was assessed for deviation from the Hardy–Weinberg equilibrium by chi square testing. Individual dose–response curves to sodium nitroprusside were fitted to a four-parameter logistic equation [29] by means of a computerized nonlinear least-squares regression program (Allfit, version 2.7, Laboratory of Theoretical and Physical Biology, National Institutes of Health, Bethesda, USA). This iterative curve-fitting program provides estimates of the maximum effect (Emax) and of the dose-rate producing a half-maximal response (ED50). ED50 values were log transformed and the geometric mean was calculated as the antilog of the mean of log values.
Analysis of variance (anova) was used to compare the results for log ED50 and Emax of sodium nitroprusside for the different genotypes of G894T, T 786C, and C242T. Repeated measures anova was performed to assess differences between these genotypes regarding endothelium-dependent venodilation with bradykinin. Similar analyses were performed for assessing the influence of the CA repeat polymorphism of NOSIII on endothelium-independent (sodium nitroprusside) and endothelium-dependent (bradykinin) venodilation, using a cut-off value of 34 CA repeats for creation of two groups of participants. This cut-off level was previously shown to be associated with coronary artery disease [12] and was therefore selected for dichotomization. The influence of the number of CA repeats on venodilator responses was analysed using univariate regression analysis. Multivariate regression analyses were performed to analyse the influence of selected factors on venodilator responses. A P value of less than 0.05 was considered significant.
Power calculations were performed (nQuery Advisor 4.0: three groups, one-way anova, unequal group size) based on sample sizes as for G894T, i.e. 29-28-15 per group and for C242T, i.e. 32-31-9 per group, assuming a common standard deviation of 20% venodilation. Potential absolute differences to be detected at the 5% level with a power of 80% were 10% venodilation for the G894T genotypes and 11% venodilation for the C242T genotypes.
Results
Demographic characteristics
Table 1 shows the frequencies of genotypes in the participants. All genotype distributions followed the Hardy–Weinberg equilibrium and allele frequencies were similar to those reported in previous European and Australian studies [12, 17, 30].
Table 1.
Genotype frequencies in 72 healthy male individuals without cardiovascular risk factors
| Genotype | n | Frequency | |
|---|---|---|---|
| NOSIII G894T | GG | 29 | 0.40 |
| GT | 28 | 0.39 | |
| TT | 15 | 0.21 | |
| NOSIII T-786C | TT | 27 | 0.38 |
| TC | 32 | 0.44 | |
| CC | 13 | 0.18 | |
| CYBA C242T | CC | 32 | 0.44 |
| CT | 31 | 0.43 | |
| TT | 9 | 0.13 |
n = number of individuals with respective genotype.
The clinical characteristics of the total study population were as follows: age 27 ± 1 years; body weight 75 ± 1 kg; height 181 ± 1 cm; body mass index 22.8 ± 0.2 kg m−2, cholesterol 168 ± 3 mg dl−1, HDL cholesterol 50 ± 1 mg dl−1, LDL cholesterol 105 ± 4 mg dl−1, homocysteine 9.5 ± 0.3 µmol l−1, resting systolic blood pressure 124 ± 1 mmHg, resting diastolic blood pressure 75 ± 1 mmHg. Clinical characteristics were similar across the genotypes.
Local infusion of phenylephrine, sodium nitroprusside or bradykinin into dorsal hand veins had no local or systemic adverse effects. There was no significant difference between groups in absolute hand-vein diameter or in the dose of phenylephrine necessary to produce 80% constriction of the hand vein. Mean venoconstriction with phenylephrine before sodium nitroprusside infusions and before bradykinin infusions was not significantly different (data not shown).
Impact of G894T and T-786C polymorphism in the NOSIII gene on venous response
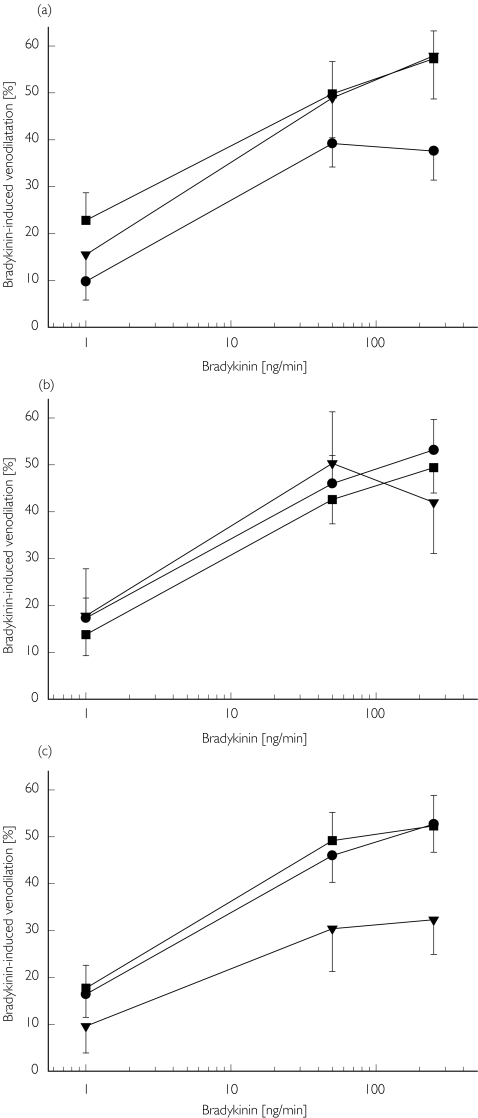
There was no significant difference between the three genotypes of G894T (GG, GT, TT) or T-786C (TT, TC, CC) with respect to the effect of sodium nitroprusside (Table 2) or bradykinin (P = 0.44 (G894T); P = 0.51 (T-786C); Figure 2).
Table 2.
Sodium nitroprusside-induced relaxation of dorsal hand veins during preconstriction with phenylephrine shown for individual genotypes
| Genotype | Emax (%) | ED50 (ng min−1) | |
|---|---|---|---|
| NOSIII G894T | GG | 102 ± 4 | 20 ± 6 |
| GT | 113 ± 8 | 40 ± 11 | |
| TT | 99 ± 4 | 18 ± 5 | |
| P value* | 0.21 | 0.65 | |
| NOSIII T-786C | TT | 108 ± 8 | 25 ± 7 |
| TC | 103 ± 4 | 26 ± 9 | |
| CC | 107 ± 7 | 36 ± 13 | |
| P value* | 0.77 | 0.51 | |
| NOSIII (CA)n | n < 34 | 110 ± 6 | 22 ± 6 |
| n ≥ 34 | 100 ± 3 | 34 ± 10 | |
| P value* | 0.14 | 0.97 | |
| CYBA C242T | CC | 108 ± 6 | 25 ± 7 |
| CT | 106 ± 5 | 29 ± 8 | |
| TT | 97 ± 8 | 31 ± 20 | |
| P value* | 0.65 | 0.87 |
Overall comparison (anova).
Figure 2.
Response of dorsal hand veins of 72 healthy males to bradykinin (1, 50, and 250 ng min-1) during preconstriction with phenylephrine with regard to the NOSIII G894T (a) GG (G894T wild type) (▪), GT (•), TT (▾); and T-786C (b) TT (T-786C wild type) (▪), TC (•), CC (▾); genotypes and the CYBA C242T genotype (c) CC (242T wild type) (▪), CT (•), TT (▾)
Impact of (CA)n polymorphism in the NOSIII gene on venous response
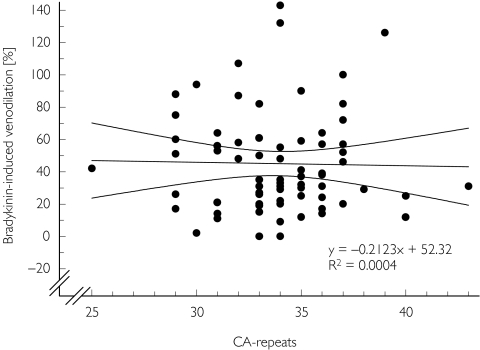
Eighteen different alleles were identified containing 21–43 CA-repeats (mean (CAn = 32). The venodilator response to bradykinin and the CA repeat copy number were not correlated (Figure 3; similar results were found for bradykinin 1 and 250 ng min−1). The presence of at least one allele with ≥34 CA repeats was not associated with an altered sodium nitroprusside or bradykinin response (P = 0.07).
Figure 3.
Relationship between response of preconstricted dorsal hand veins to bradykinin (50 ng min−1) and CA repeat copy number of NOSIII (univariate correlation analysis; P = NS). The straight line is the linear regression line, curved lines indicate the 95% confidence interval
Impact of C242T polymorphism in the CYBA gene on venous response
There was no significant difference between the three genotypes (CC, CT, TT) with respect to the sodium nitroprusside (Table 2) or bradykinin venodilator responses (P = 0.78; Figure 2), although there was a tendency towards reduced bradykinin-induced venodilation in the individuals being homozygote carriers of the mutant T-allele.
Relationship between venous responses to bradykinin and serum concentrations of cholesterol
Selected clinical parameters (total cholesterol, HDL, LDL, homocysteine, glucose) were tested by univariate regression analysis. None of the parameters was significantly correlated with the venous response to any of the bradykinin doses (P = NS).
Discussion
We hypothesized that the observed associations between polymorphisms of the NOSIII gene or CYBA gene and various cardiovascular disorders might be explained on the basis of altered endothelium-dependent vasodilation even before cardiovascular disease becomes overt. However, our data do not support a relevant effect of the C242T polymorphism of the CYBA gene nor of the studied NOSIII polymorphisms on endothelium-dependent venodilation in healthy individuals.
NOSIII is the key enzyme for production of nitric oxide and consequently plays an essential role in endothelium-dependent vasodilation. Local intravenous administration of bradykinin and assessment of dorsal hand vein compliance is a suitable method to determine nitric oxide-mediated responses [26, 31]: bradykinin promotes venodilation mainly via activation of NO synthase through endothelial receptors [32], and in contrast to other NO-releasing compounds like acetylcholine [33] its response in veins is not biphasic. However, bradykinin also acts to a lesser extent through prostaglandins [26, 28]. In order to minimize the well-known contribution of cyclooxygenase products to venous bradykinin effects all measurements in this study have been made during inhibition of prostaglandin synthetase with a systemically effective dose of acetylsalicylic acid. Therefore potential differences between venodilator responses to bradykinin will preferentially reflect deficits at the level of NO production in the endothelium. It may, however, not have blocked all NO-independent venodilator pathways potentially activated by bradykinin in the human vasculature. However, while bradykinin-induced endothelium-derived hyperpolarization is well documented in human resistance arteries [34] the available evidence in human hand veins suggests that additional pathways do not significantly contribute to bradykinin-induced venodilation [26, 35]. Our study (n = 72) had a power of 80% to detect a difference of 11% venodilation between the genotypes at a 5% level and thus our study population appeared to be large enough to detect a clinically relevant effect of the studied polymorphisms on endothelium-dependent vasodilation, if biologically present.
The absence of an effect of the G894T polymorphism on nitric oxide-mediated vasodilation in vivo is in accordance with findings of two recent smaller studies, where no association of NOSIII allele status with the variability in the acetylcholine response of the hand vein was observed [21, 36]. Epidemiological studies examining the association of the G894T polymorphism with clinical outcome have been conflicting. Whereas some studies did not find an association of the G894T polymorphism with coronary artery disease [30] or hypertension [37], several studies observed a significant association with the risk of coronary artery disease [7] or hypertension [6]. Despite these clinical associations there was no effect on nitric oxide-mediated vascular responses in our study. This inconsistency may be explained by the fact that differences in nitric oxide production between genotypes do not result in relevant differences in vascular response in healthy individuals, but may do so under conditions of endothelial dysfunction. This concept of a gene–environmental interaction was recently confirmed in a study investigating flow-mediated brachial artery dilation [38]. No influence of the NOSIII G894T polymorphism on endothelial function was observed in the whole group of young adults. However, carriage of the T-allele increased the likelihood of a smoking-associated impairment of endothelial function and also the positive vascular effect of increased circulating n-3 fatty acid concentrations.
Studies on the possible implication of the T-786C polymorphism in the predisposition to cardiovascular disease suggested that ethnic differences may be present. In Caucasians, the T-786C polymorphism is not associated with coronary artery disease [30] or myocardial infarction [11], whereas in Japanese populations the mutant C-allele is associated with coronary spasm [9] and myocardial infarction [10]. Interestingly, the T-786C polymorphism had no effect on the cerebrovascular circulation in healthy Japanese individuals, but in smokers, a group known to have endothelial dysfunction, CC homozygotes showed a significant decrease of cerebral blood flow and a significant increase in cerebrovascular resistance [39]. This finding again suggests that NOSIII polymorphisms may only become an important contributor to vascular function in the presence of cardiovascular risk factors.
In a recent matched case-control study investigating the (CA)n polymorphism of the NOSIII gene the presence of at least one allele containing ≥34 CA-repeats was associated with an excess risk of coronary artery disease [12]. In contrast to these data no correlation between the CA repeat copy number and the venodilator response to bradykinin was detectable in our population. Besides the smaller number of participants our results might be related to the fact that we included only healthy individuals without any cardiovascular risk factors, whereas in the patients included into the case-control study a broad spectrum of diseases, cardiovascular risk factors, and medications was present.
The association of the CYBA C242T polymorphism with atherosclerosis has been studied previously, and the results have been conflicting as well. It had been anticipated that a substitution in the heme-binding site of the p22phox protein leads to a loss of oxidase function and, thus, reduced production of reactive oxygen species and oxidative stress in the vasculature [2]. In line with this hypothesis, Inoue et al.[16] found that the mutant T-allele conferred protection against atherosclerosis in a Japanese population. However, all other studies either did not find any association of the C242T polymorphism with coronary artery disease [17], or even showed the opposite effect, that the T-allele is significantly associated with progression of atherosclerosis [19] and cerebrovascular disease [18]. Our data, which are the first directly assessing the impact of the C242T polymorphism on vascular function in vivo, do not support the hypothesis that the mutant T-allele is protective, because the vascular response to bradykinin was, if altered at all, rather impaired in participants homozygote for the T-allele.
One important limitation of this study is the fact that an abbreviated dose–response curve was constructed to avoid the occurrence of tolerance [28]. The doses were selected on the basis of the dose–response-curves assessed by Dachman et al.[28]. The fact that no dose between 1 and 50 ng min−1 was studied may have meant that small shifts in the bradykinin dose–response relationship may have been missed.
In conclusion, the studied polymorphisms of the NOSIII and CYBA gene did not have a clinically re-levant effect on endothelium-dependent venodilation in healthy individuals. These polymorphisms may be associated with subclinically altered endothelial function, which only becomes manifest in the presence of cardiovascular risk factors.
Acknowledgments
C. Hesse's work was supported in part by a Hans-Dengler-Forschungsstipendium.
References
- 1.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead A, FitzGerald GA. Twenty-first century phox – not yet ready for widespread screening. Circulation. 2001;103:7–9. doi: 10.1161/01.cir.103.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Marsden P, Heng H, Scherer S, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268:17478–88. [PubMed] [Google Scholar]
- 4.Nadaud S, Bonnardeaux A, Lathrop M, Soubrier F. Gene structure, polymorphism and mapping of the human endothelial nitric oxide synthase gene. Biochem Biophys Res Commun. 1994;198:1027–33. doi: 10.1006/bbrc.1994.1146. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura M, Yasue H, Nakayama M, et al. A missense Glu298Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum Genet. 1998;103:65–9. doi: 10.1007/s004390050785. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Srinivasan SR, Elkasabany A, Ellsworth DL, Boerwinkle E, Berenson GS. Combined effects of endothelial nitric oxide synthase gene polymorphism (G894T) and insulin resistance status on blood pressure and familial risk of hypertension in young adults: the Bogalusa Heart Study. Am J Hypertens. 2001;14:1046–52. doi: 10.1016/s0895-7061(01)02192-6. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani A, Liang CF, Fatibene J, et al. A common variant of the endothelial nitric oxide synthase (Glu298Asp) is a major risk factor for coronary artery disease in the UK. Circulation. 1999;100:1515–20. doi: 10.1161/01.cir.100.14.1515. [DOI] [PubMed] [Google Scholar]
- 8.Shimasaki Y, Yasue H, Yoshimura M, et al. Association of the missense Glu298Asp variant of the endothelial nitric oxide synthase gene with myocardial infarction. J Am Coll Cardiol. 1998;31:1506–10. doi: 10.1016/s0735-1097(98)00167-3. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama M, Yasue H, Yoshimura M, et al. T-786C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999;99:2864–70. doi: 10.1161/01.cir.99.22.2864. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama M, Yasue H, Yoshimura M, et al. T-786C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with myocardial infarction, especially without coronary organic stenosis. Am J Cardiol. 2000;86:628–34. doi: 10.1016/s0002-9149(00)01041-9. [DOI] [PubMed] [Google Scholar]
- 11.Poirier O, Mao C, Mallet C, et al. Polymorphisms of the endothelial nitric oxide synthase gene – no consistent association with myocardial infarction in the ECTIM study. Eur J Clin Invest. 1999;29:284–90. doi: 10.1046/j.1365-2362.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 12.Stangl K, Cascorbi I, Laule M, et al. High CA repeat numbers in intron 13 of the endothelial nitric oxide synthase gene and increased risk of coronary artery disease. Pharmacogenetics. 2000;10:133–40. doi: 10.1097/00008571-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Miles AM, Bohle DS, Glassbrenner PA, Hansert B. Wink DA, Grisham MB. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996;271:40–7. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK, Sorescu D, Ushio-Fukai M NAD. (P)H oxidase. role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 15.Guzik T, West N, Black E, et al. Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation. 2000;102:1744–7. doi: 10.1161/01.cir.102.15.1744. [DOI] [PubMed] [Google Scholar]
- 16.Inoue N, Kawashima S, Kanazawa K, Yamada S, Akita H, Yokoyama M. Polymorphism of the NADH/NADPH oxidase p22 phox gene in patients with coronary artery disease. Circulation. 1998;97:135–7. doi: 10.1161/01.cir.97.2.135. [DOI] [PubMed] [Google Scholar]
- 17.Gardemann A, Mages P, Katz N, Tillmanns H, Haberbosch W. The p22 phox A640G gene polymorphism but not the C242T gene variation is associated with coronary heart disease in younger individuals. Atherosclerosis. 1999;145:315–23. doi: 10.1016/s0021-9150(99)00083-0. [DOI] [PubMed] [Google Scholar]
- 18.Ito D, Murata M, Watanabe K, et al. C242T polymorphism of NADPH oxidase p22 PHOX gene and ischemic cerebrovascular disease in the Japanese population. Stroke. 2000;31:936–9. doi: 10.1161/01.str.31.4.936. [DOI] [PubMed] [Google Scholar]
- 19.Cahilly C, Ballantyne CM, Lim DS, Gotto A, Marian AJ. A variant of p22 (phox), involved in generation of reactive oxygen species in the vessel wall, is associated with progression of coronary atherosclerosis. Circ Res. 2000;86:391–5. doi: 10.1161/01.res.86.4.391. [DOI] [PubMed] [Google Scholar]
- 20.Schneider MP, Erdmann J, Delles C, Fleck E, Regitz-Zagrosek V, Schmieder RE. Functional gene testing of the Glu298Asp polymorphism of the endothelial NO synthase. J Hypertens. 2000;18:1767–73. doi: 10.1097/00004872-200018120-00010. [DOI] [PubMed] [Google Scholar]
- 21.Sofowora G, Dishy V, Xie HG, et al. In vivo effects of Glu298Asp endothelial nitric oxide synthase polymorphism. Pharmacogenetics. 2001;11:809–14. doi: 10.1097/00008571-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Weiss J, Fricker R, Haefeli WE. Rapid detection of polymorphisms of the nitric oxide cascade. Clin Chem Lab Med. 2002;40:341–4. doi: 10.1515/CCLM.2002.054. [DOI] [PubMed] [Google Scholar]
- 23.Weiss J, Haefeli WE, Gasse C, Hoffmann MM, Weyman J, Gibbs S, Mansmann U, Bärtsch P. Lack of evidence for association of high altitude pulmonary edema and polymorphisms of the NO pathway. High Alt Med Biol. 2003;4:355–66. doi: 10.1089/152702903769192313. [DOI] [PubMed] [Google Scholar]
- 24.Aellig WH. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981;11:237–43. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haefeli WE, Srivastava N, Kongpatanakul S, Blaschke TF, Hoffman BB. Lack of role of endothelium-derived relaxing factor in effects of α-adrenergic agonists in cutaneous veins in humans. Am J Physiol. 1993;264:H364–H369. doi: 10.1152/ajpheart.1993.264.2.H364. [DOI] [PubMed] [Google Scholar]
- 26.Bedarida GV, Bushell E, Haefeli WE, Blaschke TF, Hoffman BB. Responsiveness to bradykinin in veins of hypercholesterolemic humans. Circulation. 1993;88:2754–61. doi: 10.1161/01.cir.88.6.2754. [DOI] [PubMed] [Google Scholar]
- 27.Strobel WM, Lüscher TF, Simper D, Linder L, Haefeli WE. Substance P in human hand veins in vivo: tolerance, efficacy, potency, and mechanisms of venodilator action. Clin Pharmacol Ther. 1996;60:435–43. doi: 10.1016/S0009-9236(96)90200-3. [DOI] [PubMed] [Google Scholar]
- 28.Dachman WD, Ford GA, Blaschke TF, Hoffman BB. Mechanism of bradykinin-induced venodilation in humans. J Cardiovasc Pharmacol. 1993;21:241–8. doi: 10.1097/00005344-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 29.De Lean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay and physiological dose–response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 30.Granath B, Taylor RR, van Bockxmeer FM, Mamotte CD. Lack of evidence for association between endothelial nitric oxide synthase gene polymorphisms and coronary artery disease in the Australian Caucasian population. J Cardiovasc Risk. 2001;8:235–41. doi: 10.1177/174182670100800408. [DOI] [PubMed] [Google Scholar]
- 31.Webb DJ. The pharmacology of human blood vessels in vivo. J Vasc Res. 1995;32:2–15. doi: 10.1159/000159072. [DOI] [PubMed] [Google Scholar]
- 32.Mombouli JV, Vanhoutte PM. Kinins and endothelial control of vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1995;35:679–705. doi: 10.1146/annurev.pa.35.040195.003335. [DOI] [PubMed] [Google Scholar]
- 33.Collier J, Vallance P. Biphasic response to acetylcholine in human veins in vivo: the role of the endothelium. Clin Sci (Lond) 1990;78:101–4. doi: 10.1042/cs0780101. [DOI] [PubMed] [Google Scholar]
- 34.Honing ML, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35:1314–8. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- 35.Bedarida GV, Kim D, Blaschke TF, Hoffman BB. Characterization of an inhibitor of nitric oxide synthase in human-hand veins. Horm Metab Res. 1994;26:109–12. doi: 10.1055/s-2007-1000784. [DOI] [PubMed] [Google Scholar]
- 36.Grossmann M, Dobrev D, Siffert W, Kirch W. Heterogeneity in hand veins responses to acetylcholine is not associated with polymorphisms in the G-protein β3-subunit (C825T) and endothelial nitric oxide synthase (G894T) genes but with serum low density lipoprotein cholesterol. Pharmacogenetics. 2001;11:307–16. doi: 10.1097/00008571-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Tsujita Y, Baba S, Yamauchi R, et al. Association analyses between genetic polymorphism of endothelial nitric oxide synthase gene and hypertension in Japanese: The Suita Study. J Hypertens. 2001;19:1941–8. doi: 10.1097/00004872-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Leeson CPM, Hingorani AD, Mullen MJ, et al. Glu298Asp endothelial nitric oxide synthase gene polymorphism interacts with environmental and dietary factors to influence endothelial function. Circ Res. 2002;90:1153–8. doi: 10.1161/01.res.0000020562.07492.d4. [DOI] [PubMed] [Google Scholar]
- 39.Nasreen S, Nabika T, Shibata H, et al. T-786C polymorphism in endothelial NO synthase gene affects cerebral circulation in smokers: Possible gene–environmental interaction. Arterioscler Thromb Vasc Biol. 2002;22:605–10. doi: 10.1161/01.atv.0000013286.60021.fe. [DOI] [PubMed] [Google Scholar]