Abstract
The prevalence of obesity in the western world is dramatically rising, with many of these individuals requiring therapeutic intervention for a variety of disease states. Despite the growing prevalence of obesity there is a paucity of information describing how doses should be adjusted, or indeed whether they need to be adjusted, in the clinical setting. This review is aimed at identifying which descriptors of body size provide the most information about the relationship between dose and concentration in the obese. The size descriptors, weight, lean body weight, ideal body weight, body surface area, body mass index, fat-free mass, percent ideal body weight, adjusted body weight and predicted normal body weight were considered as potential size descriptors. We conducted an extensive review of the literature to identify studies that have assessed the quantitative relationship between the parameters clearance (CL) and volume of distribution (V) and these descriptors of body size. Surprisingly few studies have addressed the relationship between obesity and CL or V in a quantitative manner. Despite the lack of studies there were consistent findings: (i) most studies found total body weight to be the best descriptor of V. A further analysis of the studies that have addressed V found that total body weight or another descriptor that incorporated fat mass was the preferred descriptor for drugs that have high lipophilicity; (ii) in contrast, CL was best described by lean body mass and no apparent relationship between lipophilicity or clearance mechanism and preference for body size descriptor was found. In conclusion, no single descriptor described the influence of body size on both CL and V equally well. For drugs that are dosed chronically, and therefore CL is of primary concern, dosing for obese patients should not be based on their total weight. If a weight-based dose individualization is required then we would suggest that chronic drug dosing in the obese subject should be based on lean body weight, at least until a more robust size descriptor becomes available.
Keywords: body mass index, body surface area, body weight, obesity, pharmacokinetics
Background
It has been suggested that the level of obesity in western countries is reaching epidemic proportions, with the USA, UK and Australia recording a prevalence in adults of around 20%[1]. Accompanying the increasing prevalence of obesity is a corresponding increase in the occurrence of chronic disease states such as depression, hypertension, dyslipidaemia, diabetes, cardiovascular disease, osteoarthritis and some cancers [2–4]. The current definition of obesity according to the World Health Organization (WHO) [5] is a body mass index (BMI) = 30 kg m−2, discussed further under Body mass index.
In a pharmacological sense, obesity presents a challenging role for clinicians, as the effects of altered body composition on the time course of drug response are poorly understood. There are some data describing general physiological or pathophysiological changes associated with obesity [6]. Although not the focus of this review, limited evidence has shown that glomerular filtration rate (GFR) and renal perfusion appear similar in obese and normal weight individuals [7, 8]. In contrast to renal function, changes in metabolic processes have been poorly characterized, which may be due to large interindividual variability in enzyme activity irrespective of body composition. At the least it could be predicted that for those drugs that are hydrophilic and extensively renally cleared, clearance would depend upon creatinine clearance. The question of how to best calculate creatinine clearance (CLCR) in the obese, using for instance the Cockcroft and Gault equation [9], remains to be determined; however, empirical evidence for gentamicin [10] demonstrated that CLCR is best calculated using ideal body weight. Overall, there is a deficit of information on the influence of obesity on pharmacokinetics and pharmacodynamics, primarily as most drug-dosing data have resulted from clinical studies performed in groups that rarely included patients with obesity. With the growing number of obese patients requiring drug dosing, this knowledge deficit may have significant ramifications when interpreting the relationship between dose and outcomes or risk of toxicity. Current methods to dose individualize drugs for the obese patient are based on some measure of the patient's size, such as total body weight or body surface area, and assume that the structural and functional aspects of the body are similar in the obese and non-obese population. Although this assumption is likely to be beneficial for ‘normal weight’ patients of varying size, it is unlikely that this assumption is true for scaling to the obese population since excess weight does not arise from similar proportions of adipose tissue and lean body mass [11].
Several pharmacokinetic studies have been conducted in the obese population to overcome this information deficit, which have been reviewed by Cheymol in 1993 [12] and 2000 [13]. This manuscript has included those studies reviewed by Cheymol (and more recent studies of a similar nature), to identify any commonality in the preference for a size descriptor that helps explain variability in pharmacokinetic parameters in the obese population. We initially consider why and how the various size descriptors evolved (see Description of size), which is followed by a summary of the methodology employed in pharmacokinetic studies that have addressed size descriptors for obesity (Methods used to assess the impact of obesity). We then provide a summary of the quantitative pharmacokinetic studies performed in obesity (Influential size descriptors), followed by overall conclusions.
Description of size
Drug dosing, based on mass, e.g. total body weight (TBW), is a common method of dose individualization. A dose recommendation per kilogram on the drug label is often assumed to be TBW, and rarely is an alternative weight descriptor explicitly defined. There are, however, many other weight and size descriptors presented in the pharmacokinetic literature, such as: BMI, body surface area (BSA), ideal body weight (IBW), fat-free mass (FFM), lean body weight (LBW), adjusted body weight (ABW), percent of ideal body weight (%IBW) and predicted normal weight (PNWT). Allometric scaling of the normalized size descriptor with the exponent equal to 0.75 has also been used for cross species scaling. Since this metric requires the selection of an appropriate size descriptor (from those listed above), its potential merits will not be the focus of this review. The equations used to compute these size descriptors and the demographics of the patients used in their derivation are shown in Table 1, and are discussed in the chronological order that they appeared in the literature. However, before reviewing these descriptors it seems useful to make some comment on the a priori expectation of a suitable size descriptor. Based on biological grounds and transportability, we would expect that a body size descriptor would incorporate: age, height, weight, sex and race either directly or indirectly as a covariate. In addition, these covariates should be combined in a manner that does not introduce mathematical inconsistencies at extremes of the covariate, thereby allowing the descriptor to be internally robust and suitable for a wide range of covariate combinations.
Table 1.
Size descriptors used in pharmacokinetic studies
| Subject demographics from where size descriptor derived | ||||||
|---|---|---|---|---|---|---|
| Size descriptor | Formula | Weight − kg mean ± SD (range) | Height – m mean ± SD (range) | |||
| Body mass index (BMI), kg m−2 | Males = 7426 [15] | |||||
| TBW/HT (m)2 | 69.5 ± 10.5* | 1.69 ± 0.062* | ||||
| Body surface area (BSA), m2 | Male / females = 43 [17] | |||||
| TBW0.425 × HT (cm)0.725 × 0.007184 | 52.4 ± 18.5(6.27 – 93.0) | 1.61 ± 0.204(0.782 – 1.87) | ||||
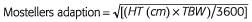 [19] [19] |
||||||
| Ideal body weight (IBW), kg | Males [26] | |||||
| Tables dependent on frame size and height | (29.5 – 156) | (1.47 – 2.01) | ||||
| Females [26] | ||||||
| Devines estimation = 45.4 + 0.89 × (HT (cm) − 152.4) + 4.5 (if male) [28] | (29.5 – 156) | (1.37 – 1.91) | ||||
| Fat-free mass (FFM), kg | Males = 24 [36] | |||||
| 0.285 × TBW + 12.1 × HT (m)2 for males | 80.3 ± 22.0(43.5 – 126) | 1.72 ± 0.07(1.57 – 1.86) | ||||
| Females = 104 [36] | ||||||
| 0.287 × TBW + 9.74 × HT (m)2 for females | 91.7 ± 19.5(42.3 – 133.5) | 1.63 ± 0.07(1.44 – 1.79) | ||||
| Lean body weight (LBW), kg | Males = 89 | |||||
| 1.1 × TBW − 0.0128 × BMI × TBW for males | 68.0 ± 9.86(47.2 – 92.3) [49] | NR | ||||
| 71.8 ± 17.1(36.4 – 122) [30] | ||||||
| 68.0(57.0 – 81.2) [50] | ||||||
| Females = 44 | ||||||
| 1.07 × TBW − 0.0148 × BMI × TBW for females | 54.7 ± 7.12(45.4 – 62.4) [49] | |||||
| 64.7 ± 23.5(32.3 – 108) [30] | NR | |||||
| 57.0(44.4 – 68.3) [50] | ||||||
| Adjusted body weight(ABW), kg | Males = 24, females = 24 | |||||
| IBW + CF × (TBW − IBW)CF = correction factor of 0.4 | Gentamicin | |||||
| Obese = 138.3 ± 15.2 | ||||||
| Normal = 73.2 ± 7.5 | ||||||
| Amikacin | ||||||
| Obese = 151.1 ± 17.2 | ||||||
| Normal = 71.0 ± 8.5 | ||||||
| Tobramycin | ||||||
| Obese = 147.3 ± 16.3 | ||||||
| Normal = 72.4 ± 4.2 | ||||||
| Percent IBM (%IBW), % | N/A [42] | |||||
| Subject demographics from where size descriptor derived | Weight − kg | Height − m | ||||
| Size discriptor | Formula | mean ± SD (range) | mean ± (range) | |||
| Predicted normal weight(PNWT), kg | Males = 1226 [10] | |||||
| 1.57 × TBW − 0.0183 × BMI × TBW − 10.5 for males | 72.7 ± 11.9(36.0 – 112) | 1.72 ± 7.88(1.47 – 1.96) | ||||
| Females = 1121 [10] | ||||||
| 1.75 × TBW – 0.0242 × BMI × TBW – 12.6 for females | 61.8 ± 9.77(25.2 – 95.0) | 1.62 ± 6.83(137 – 182) | ||||
TBW = total body weight (kg), HT = height.NA = not applicable, NR = not reported.
Mean of pooled mean weights / heights / SD from the 12 recruitment centres.
Body mass index
BMI or ‘Quetelet's Index’ was initially reported by Quetelet in 1869 [14]. It was intuitively recognized that body size should be related to weight and height, and Quetelet initially thought that body volume would ideally be related to height cubed (HT3). This concept was explored further in 1972, when various relationships of TBW to HT, HT2 and HT3 were assessed to identify that which seemed preferable to describe the incidence of coronary heart disease in men. The result was the ratio of TBW to HT2 (Quetelet's Index), which was subsequently renamed ‘body mass index’ (BMI) [15]. BMI is now the international metric recommended to classify obesity [5], with overweight defined as a BMI = 25–29.9 kg m−2, and obesity as a BMI ≥ 30 kg m−2. Obesity is further classified as moderate (BMI = 30.0–34.9 kg m−2), severe (BMI = 35–39.9 kg m−2) or morbid (BMI ≥ 40 kg m−2). BMI increases with TBW, but cannot differentiate adipose tissue from muscle mass. It is not therefore useful for assessment of those with a larger than average muscle mass compared with fat, and its role as a dosing scalar is limited as patients with a large muscle mass would receive the same dose as patients with a large fat mass. Due to the different compositions of these tissues [16], it is likely that obese patients need dose individualization based on some alternative metric that accounts for varying proportions of muscle to fat. It should also be noted that BMI is not gender specific, was not derived from women and its predictive value for morbidity has not been evaluated in women.
Body surface area
BSA was initially developed by Du Bois et al. in 1916 [17]. It was a more refined and precise estimate of BSA from that previously estimated by Meeh [18], and was used within respiratory and metabolism experiments in obese patients. The formula was derived based on the assumption that HT, TBW and some constant (C) were related to BSA. Combinations of these known variables were then regressed against the ‘true’ BSA, which was identified from a series of anatomical measurements. Additional constants in the exponent were then determined by graphical interpolation. The result was the equation: BSA = TBW0.425 × HT0.725 × 0.007184. Table 1 shows the demographics and unit values of the covariates used in the development of BSA, with few subjects enrolled in the study considered obese based on today's standards. Table 1 also shows the refined version of BSA presented by Mosteller [19]. Whilst this simplification is slightly less accurate [19], it has been widely used in clinical practice to dose chemotherapeutic agents, and is probably considered the ‘gold standard’ metric for dosing such drugs [20]. Other methods for computing BSA presented in the literature have not been considered as accurate [21], and have seldom been used in pharmacokinetic (PK) studies. BSA does seem a biologically plausible size descriptor as it considers height and weight in its derivation. Unfortunately, it does not consider sex, and is therefore less likely to be a useful dosing scalar for the obese individual (in clinical practice many drug doses are capped to a value of BSA = 2 m2) [20].
Ideal body weight
IBW was a size descriptor derived from insurance data collected by the Metropolitan Life Insurance Company of New York. It represents a large quantity of evidence that relates size to mortality, and was first presented in the literature in 1942 [22] and 1943 [23] for women and men, respectively. This was updated in 1959 [24] and 1960 [25] from data obtained during the Build and Blood Pressure Study [26]. This study included over 4.5 million people; however, ideal weights for height were derived on a subset of 360 000 life insurance policy holders. The value of IBW from these tables is unrelated to TBW, and is an estimate of weight corrected for sex, height and frame size. Equations to approximate these tables (excluding frame size) have been presented by Blackburn in 1977 [27], although the previous empirical estimate of IBW by Devine in 1974 [28] is the most common reference cited in the PK literature. It appears that the formula presented by Devine was unrelated to the Metropolitan Life Insurance Company data, although it does share some similarities. As IBW was developed for purposes unrelated to pharmacokinetics, extrapolation of its use as a dosing scalar is of questionable merit, especially considering all patients of the same height would receive the same dose. This does not seem biologically plausible, as TBW would seem to add further knowledge about the size of an individual over height alone.
Fat-free mass
FFM was a size descriptor derived by Rathbun and Pace in 1945, as an experimental validation of previous postulated relationships between weight and fat mass [29]. The metric was derived in guinea pigs, where the live weight and eviscerated wet and dry weights were used to determine the total fat mass of the animal. Human measures have subsequently been suggested, and have been derived from HT and TBW [30], skinfold thickness [31], density testing (underwater weighing) [31, 32] and total body potassium [32]. Many other estimates of FFM have used bioelectrical impedance analysis (BIA) in combination with height, TBW and sex [33–35]. The regressed FFM equations presented by Garrow and Webster [36] have been used to ascertain the impact of obesity on the pharmacokinetics of glibenclamide [37]. These equations were developed to ascertain how well BMI correlated with fat mass, which was individually estimated as the mean fat mass identified from skinfold thickness, density and total body potassium testing. It should be noted that this descriptor has desirable properties as a dosing scalar, as it depends upon sex, TBW and HT.
Lean body weight
Fractional fat mass (FMfrac) was initially computed by TP Eddy to describe the increasing prevalence of obesity in the UK. This metric was incorporated into a 1976 report by James for the Department of Health and Social Security Medical Research Council [38]. It has subsequently been re-arranged to LBW, where lean body weight is equal to TBW minus the product of FMfrac and weight [13, 39]. The original purpose of FMfrac was to relate patient size to epidemiological trends in morbidity and mortality, although it has since been adopted as a metric to help describe variability between subjects in their pharmacokinetic parameter values. It should be noted that subjects used in the derivation of FMfrac weighed considerably less than those considered obese today [40], which has lead to some inconsistencies in the calculation of LBW at extremes of WT and HT [40]. Nevertheless, LBW is a potentially useful predictor of the PK behaviour of drugs that are highly water soluble. It is of interest that the original coefficient value for males of 1.28 × 10x reported by James [38] has been commonly misquoted as 1.20 × 10x in other publications [13, 39], where x is dependent on units.
Adjusted body weight
ABW was the first size descriptor specifically developed for use in pharmacokinetic experiments, and was presented in 1983 as part of a noncompartmental analysis of aminoglycoside dosing [41]. The descriptor was derived as a tool to normalize V, where some proportion (termed a correction factor) of excess weight above IBW was added to IBW. The mean correction factor (CF) was estimated at 0.45 for gentamicin, 0.37 for tobramycin and 0.42 for amikacin [41]. Because of the variability in the constant CF, it would seem prudent to estimate a population value on a case by case basis. ABW does however, seem a plausible size descriptor as it considers sex, TBW and HT.
Percent ideal body weight
Percent ideal body weight is a metric designed to quantitatively describe TBW as a percentage of IBW [42]. It was developed to present data on obesity trends, and has been modified, by some, to the ratio of the difference between total and ideal body weight to ideal body weight [43]. For this review %IBW has been considered as the ratio of TBW to IBW (shown in Table 1).
Predicted normal weight
Predicted normal weight (PNWT) is a new size descriptor derived in 2003 [10], specifically to overcome some of the limitations associated with the alternative size descriptors. It was derived to better describe the pharmacokinetics of drugs (rather than for prediction of patient morbidity) and represents the expected normal weight of an obese individual as the sum of their lean body mass and their predicted ‘normal’ fat mass (excluding excess fat mass). Since this descriptor includes LBW in its derivation it shares the potential for similar mathematical inconsistencies as with LBW. Nevertheless, it has appropriate properties to be useful in PK studies as it considers sex, TBW and HT.
Methods used to assess the impact of obesity on PK parameters
There are essentially three methods that have been used in the PK literature to assess the influence of various size descriptors in the obese patient. These are:
-
1
Comparison of parameter estimates (e.g. CL) between an obese and a non-obese control population.
-
2
Regression of individual parameter values against a size descriptor.
-
3
Incorporation of the size descriptor into a population PK analysis.
Method 1 has traditionally been favoured by kineticists, presumably due to the numerical ease of analysing the data. The choice of the descriptor used to determine obesity will typically be the size descriptor found to be predictive of a change in the parameter value – which is circular. Either the investigators have to try all descriptors to find the metric that provides a statistically significant relationship (which would seem unjustifiable due to multiplicity in a statistical sense and inconsistent with biological principles) or to choose a descriptor a priori. In either case, this method will not allow identification of the best size descriptor, and at best will provide information on the quantal difference in the typical value of a parameter in normal vs. the obese population. It is not possible to determine a quantitative and therefore predictive relationship between the size descriptor and the PK parameter value, and this method is therefore suitable for hypothesis testing only. For these reasons, it is not considered further in this review unless as an accompaniment with methods 2 or 3.
Method 2 is more informative than method 1, as it attempts to quantify the relationship between the parameter value and a given size descriptor, the idea being to assess the relationship between pairs of parameter-weight descriptor values taken from many individuals simultaneously. The assumption inherent in this process is that all parameter values for all individuals are known to the same level of accuracy, which for an unbalanced PK study design is an unlikely and possibly inappropriate assumption. A further limitation of this method lies in the marginal analysis of the parameter–covariate relationship, which excludes interactions between the covariate–parameter relationship and other parameters in the PK model. Nevertheless, this method is widely used and is a valuable tool both in its own right and when used in conjunction with a fully population method (e.g. perhaps within a generalized additive modelling framework or using the Wald's approximation method) [44, 45]. However, it is unfortunately common that results of such studies are published without reporting the coefficient estimates of the regression relationship, in these cases typically describing only the degree of association between covariate and parameter values as R2. This makes it impossible to understand the quantitative nature of the relationship, and indeed even the direction of the association, which means that the reporting of the analysis holds no predictive potential. In these circumstances it is not possible to identify the likely clinical impact of the association, since it is not possible to determine the likely change in parameter value over a reasonable range of the covariate. Despite the potential limitation of the reporting of this method we have included those studies that have reported regression relationships in the absence of publishing the coefficient parameter values, since it does add to the understanding of what was found to be the ‘best’ size descriptor of those size descriptors that were studied.
Method 3 is the most formal assessment of the relationship between various size descriptors and the parameter values. It is currently the least commonly used technique in this regard, which is at least in part due to the complexity of the analysis techniques and the need for specialized software. In this method, the covariates are explicitly combined within the structural pharmacokinetic model, by way of a user-defined regression relationship and the data analysed within the framework of a nonlinear mixed effects model. This allows the mean population parameter values as well as the random between-subject variability and residual variability to be quantified. Following the addition of an influential covariate there should be evidence of both a statistically significant fall in the objective function and also a reduction in the unexplained random variability in the dependent parameter. For example, addition of weight as a covariate on clearance should reduce the unexplained between-subject variability in clearance in the study population, since some of that variability will be explained by variability in the weight of the participants. The influence of the covariate should also be based on both the clinical significance and the biological plausibility of the relationship. The benefits of this approach lie in the predictive performance of the model for both interpolating PK responses that were not explicitly studied as well as predicting PK responses that arise from situations that exceed the scope of the original study (although care should be taken in this latter scenario). The interested reader is referred to Mandema [45], Wade [46] and Whalby [47, 48] for reviews and considerations when building covariate models in population analysis.
Influential size descriptors on PK parameters
This review has sought to identify which size descriptors seem the most important when describing variability in the pharmacokinetic parameters CL and V. Only studies that used methods 2 and 3 have been considered, unless further analysis was possible based on original data presented in the manuscript. Studies that included obese subjects alone without ‘normal’ weighted subjects were also excluded.
Studies were identified in the medical and pharmaceutical literature from the databases MEDLINE, EMBASE and IPA. These databases were searched using the terms obesity, pharmacokinetics, weight, NONMEM, modelling, population, size, LBW, ABW, IBW, FFM, BMI and BSA (both as acronyms and in full text). The search was limited to adults and humans, with the term ‘pharmacokinetics’ incorporated in all searches. Statistical significance was assumed if a covariate was included in a population pharmacokinetic model but the change in objective function was not reported. It should be noted that many additional studies used method 1 and found statistically significant differences in parameter estimates. However, as regression analysis was not performed, the information from these studies does not contribute towards knowledge synthesized in this manuscript. Finally, for consistency it should be noted that the size descriptors presented in these tables relate to definitions presented in the section of this manuscript entitled Description of size. Where the author of a manuscript used an alternative terminology, i.e. the authors quoted LBW but the descriptor was computed as IBW, the ‘true’ descriptor IBW is reported.
Investigation of the best size descriptors
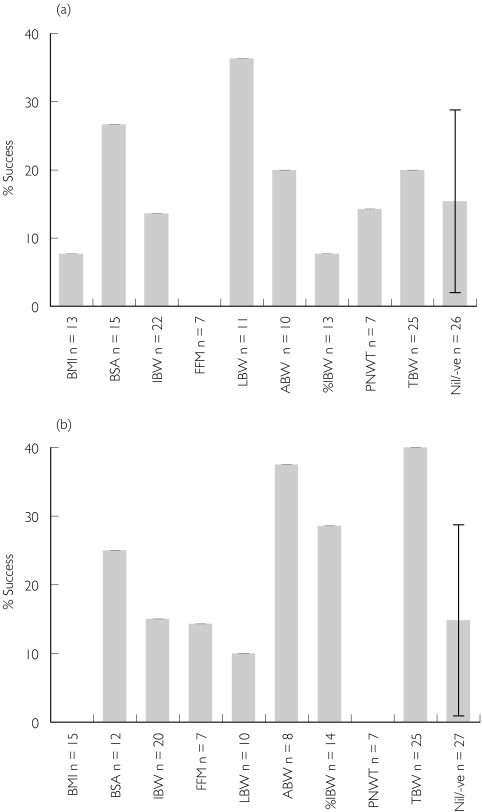
The data presented in Appendix I and II are indeed formidable. At face value it requires that the studies are considered to be well conducted, and that variability in their conclusions is more a function of the characteristics of the drug rather than the patient group or the study design and analysis techniques used. Clearly, these assumptions are likely to be erroneous on occasion, nevertheless some holistic interpretation of the results would be of general interest. We have summarized the results of the best size descriptors for CL and V of distribution in Figure 1a,b, respectively. For both parameters the figures show the proportion of studies that found a particular size descriptor to be best, where the proportion is given by the ratio of the number of studies that found the weight descriptor to be best to the number of studies that considered the weight descriptor as a potential covariate. For example, for CL only 13 studies considered BMI as a potential descriptor and hence the results are expressed as the proportion of times BMI was found to be the best descriptor of clearance out of the 13 times it was considered. To help interpretation of the likely significance of the influence of various size descriptors, an additional ‘no-descriptor’ bar has been added to each chart. This describes the proportion of times that no descriptor was correlated with the parameter of interest (it should be noted that all studies potentially had the option of finding no relationship). Interpretation of the results fromAppendix I and II also revealed a number of studies that found a negative correlation between the size descriptor and weight. Since our a priori belief was that both CL and V increase with patient size, then a negative relationship was considered biologically implausible and was assumed to represent a type I statistical error. These results were subsequently incorporated into the ‘no-descriptor’ category termed the null model. Finally, the binomial approximation to the asymptotic normal 95% confidence interval (CI) is shown for the null model proportion in order to allow informal inference to be gained about the statistical significance of other descriptors.
Figure 1.
The percent success of the size descriptor, dependent upon the number of times it was considered in the regression or population pharmacokinetic analysis; n denotes the number of studies in which the size descriptor was evaluated. The error bar on the null model represents the 95% confidence interval. (a) The pharmacokinetic parameter clearance (CL). (b) Volume of distribution (V)
Clearance
CL is a parameter that is related to the functional capacity of the body and is characterized by the intrinsic elimination capacity of the various organs and their perfusion. It is clear from Appendix I that the relationship between CL and various size descriptors is highly variable, and no single size descriptor is always the best descriptor of CL. However, the pathophysiological changes that accompany obesity in the otherwise healthy individual are principally related to changes in body composition, with increased excess fat mass and an accompanying but significantly smaller increase in lean body mass [16]. Unless the fat tissue has intrinsic extraction properties, then a proportionate increase in CL with total body weight is a priori unlikely. The results from Figure 1a support this notion, and indicate that LBW was the best single size descriptor of CL, being considered best in 35% of the studies in which it was considered. Most other size descriptors were encompassed within the 95% CI of the null model. Although this cannot be interpreted to provide an associated P-value, it does suggest that other size descriptors seem no better than ‘no-descriptor’, and their representation is characteristic of ‘noise’ within the meta-analysis. Of interest is the finding that fat-free mass [36] did not appear to be the best descriptor in any of the seven studies where it was considered. Since this metric is similar in principle to LBW, it is unclear how to interpret this finding, except that the number of studies that considered this metric was small.
Volume of distribution
V is a parameter that is related to structural aspects of the body, and is defined by the apparent volume in which a drug would theoretically distribute. This parameter does not make the distinction between whether a drug is evenly distributed throughout the body (the assumption commonly considered in its interpretation) or concentrated in a particular region. Similar to CL, it is clear fromAppendix II that the relationship between V and various size descriptors is highly variable. In contrast to CL, however, the pathophysiological changes that accompany obesity in the otherwise healthy individual would be expected to have a significant effect on the distribution of some drugs in the body, particularly those that are lipophilic and therefore more likely to distribute into adipose tissue. The results from Figure 1b support this notion and indicate that TBW was the best single descriptor of V, being considered best in 40% of the studies in which it was considered. A somewhat surprising outcome was the finding that adjusted body weight was also a relatively good descriptor of V. However, since the value of the constant CF was not given and could have been > 0.9, interpretation of these findings is not possible. Most other size descriptors are encompassed within the 95% CI of the null model. Of interest is the finding that %IBW did not fare particularly well, which is surprising in that it is a relative measure of excess fat weight, and should therefore be well correlated with distribution of drugs into fat mass.
Interpretation of the influence of size descriptors
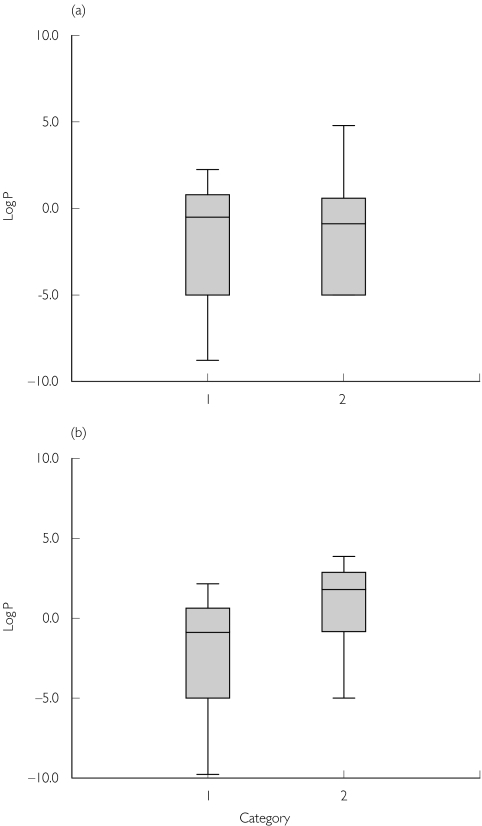
Although this review was not designed to interpret the particular influence of size descriptors on CL and V, we conducted a post hoc analysis comparing the best descriptor vs. a measure of the lipophilicity of the drug. The lipophilicity was estimated based on non-ionized log octanol-water partition coefficients (Log P) of the various drugs, which were obtained for most drugs from the logkow website (http://esc.syrres.com/interkow/kowdemo.htm). We were unable to locate Log P values for c-peptide, dalteparin, enoxaparin, lithium, tinzaparin, remifentanil, vinorelbine and doxacurium. However since the former drugs (c-peptide, dalteparin, enoxaparin, lithium, tinzaparin) are highly water soluble they were assumed to have similar Log P values to the aminoglycosides and were therefore set to a value of −5. The remainder (remifentanil, vinorelbine and doxacurium) were excluded from the analysis. For the purposes of this summary we considered that the actual value of LogP for drugs that are highly hydrophilic and in all likelihood would have values that are much less than −2 is probably unimportant. The other assumption we made was to divide the weight descriptors into two categories in order to get sufficient numbers of studies in each group. Category 1 were those descriptors that appeared to compensate for excess fat mass, which were ABW, BSA, PNWT, IBW and LBW. ABW compensates for excess fat mass by reducing the slope of the linear relationship between WT and ABW, and BSA compensates by raising TBW to the power of 0.425 (approximating the square root of TBW). Category 2 descriptors were those that did not compensate for increased excess fat, i.e. fat mass is considered physiologically comparable to lean mass. These were: TBW, %IBW and BMI. Percent IBW reflects, in theory, the increase in fat mass since it is the relative proportion that fat mass contributes over and above lean mass, and for BMI for any given height its value increases linearly with TBW. Based on these assumptions, the LogP value was plotted against the best size descriptors (based on their categories) for both CL and V(Figure 2a, b). Although the numbers are small, it is clear that category 2 descriptors were preferred to describe V for drugs that have higher log P-values (P = 0.036 using a Mann–Whitney U-test). This supports the finding that V was better described by TBW rather than by LBW. In contrast, for CL neither category was associated with higher log P-values (P = 0.831), suggesting that the best size descriptor for CL is independent of the lipophilicity of the drug, and hence a size descriptor that accounts for increased adipose tissue such as TBW is likely to be erroneous. In addition, there was no apparent link between the route of elimination (e.g. hepatic or renal) and the category of size descriptor that was favoured (P = 0.20, Fisher's exact test).
Figure 2.
A box plot of log P-values for each drug vs. category of the best descriptor for that drug. Category 1 size descriptors were adjusted body weight (ABW), body surface area (BSA), predicted normal weight (PNWT), ideal body weight (IBW) and lean body weight (LBW) and category 2 were total body weight (TBW), %IBW and body mass index (BMI). (a) The pharmacokinetic parameter clearance (CL). (b) Volume of distribution (V)
Conclusions
The one clear conclusion that can be drawn from the available studies is that there is no single size descriptor that is undeniably better than the others for describing the pharmacokinetics of drugs in the obese patient. Despite this overwhelming lack of conclusive evidence, there is strong empirical and mechanistic evidence that support some interim conclusions: (i) that in general CL does not increase in proportion with total body weight in obese individuals, and (ii) that V is consistently increased in patients with excess adipose tissue and that this increase at least in part seems to be related to the physicochemical properties of the drug. This implies that drugs that are dosed acutely, e.g. anaesthetic agents, will require different dosing considerations from those that are dosed chronically.
Variability in the findings between studies is likely to be multifactorial: (i) the variety of choice and implementation of size descriptors, (ii) the presence of co-morbidities in the obese patient, (iii) the interaction of obesity with other covariate relationships (e.g. estimation of creatinine clearance), (iv) the highly variable physicochemical characteristics of the drugs studied, and (v) poor study design (e.g. the use of hypothesis testing studies to learn about drug actions). Currently, few size descriptors have been developed specifically for scaling doses to obese patients, indeed most have been derived for risk assessment in actuarial summaries of population morbidity and mortality. Adaptation of the original descriptor for pharmacokinetic purposes has often been performed ad hoc, or in some circumstances no adaptation was performed and the size descriptor used in its original form.
Despite these potential flaws it appears that a descriptor that accounts for increased adipose mass (e.g. total body weight) would be expected to be an appropriate descriptor of V for drugs that are moderate to highly lipophilic. It is equally clear that chronic dosing regimens should not be based on TBW in the obese patient. The exact nature of the required adjustment to total body weight is not clear cut, although LBW seems to be preferred. In addition, there was no evidence of drug-related factors, such as lipophilicity, or patient-related factors, such as the effects of obesity on the route of elimination, that were linked to a preference for a particular size descriptor. Needless to say, the complexity of clearance processes, coupled with small numbers of studies and potentially inadequate size descriptors, makes it difficult to draw specific conclusions from the available evidence.
Appendix I
Table Appendix I.
Studies evaluating the impact of obesity on clearance (CL)
| Drug | Subjects’ TBW, kg mean ± SD (range) | Clearance (CL), L h−1 mean ± SD | Size descriptors considered | Best descriptor | Correlation and direction or change in OBJ |
|---|---|---|---|---|---|
| Lithium†[51] | Normal = 69.7 ± 9.3 | Normal 1.38 ± 0.372** | TBW/IBW/%IBW/LBW/ABW/ | BMI | R2= 0.39 |
| Obese = 106.4 ± 22.1 | Obese = 1.97 ± 0.400 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| CL = 0.0275 x BMI + 0.846 | |||||
| Dalteparin [52] | Normal = 69.7 ± 9.3 | Normal 1.11 (25.2%CV)NS | TBW/IBW/ABW | TBW | R2= 0.39 |
| Obese = 106.4 ± 22.1 | Obese 1.3 (45.4%CV) | + ve | |||
| Vancomycin [53] | Not reported | CL ml min-1= 2.939 – 0.012 x AGE (years) | TBW/IBW/%IBW | TBW | R2 = NR |
| − 0.009 × TBW (kg) − 0.68 x SCr (mg dl−1) | – ve | ||||
| Busulphan [54] | Not reported | (BMI <18 = 9.96 ± 2.04 | TBW/BSA/ABW/IBW/HT/ | TBW | R2 = 0.30 |
| BMI 18–26.9 = 8.55 ± 2.7)*** | BSA/BMI | + ve | |||
| (BMI 27–35 = 13.4 ± 3.18 | |||||
| BMI >35 = 15.0 ± 2.82) | |||||
| Vancomycin [55] | Normal = 68 ± 6 | Normal = 4.62 ± 1.32*** | TBW/IBW | TBW | R2 = 0.90 |
| Obese = 165 ± 46 | Obese = 11.8 ± 4.62 | + ve | |||
| Ifosfamide [56] | Normal = 64.2 (47.7–77.0) | Normal = 4.33 (3.19–11.3)NS | TBW/IBW/%IBW | IBW | R2 = 0.49 |
| Obese = 76.8 (70.0–86.0) | Obese = 4.56 (3.9–5.50) | + ve | |||
| Doxorubicin [42] | Normal = 66 ± 14 | Normal = 94.1 ± 15.4 | TBW/IBW/%IBW/BSA | % IBW | R2 = 0.56 |
| Overweight = 78 ± 15 | Overweight = 73.6 ± 16.8 | – ve | |||
| Obese = 81 ± 14 | Obese = 53.5 ± 17.1* | ||||
| Glyburide [37] | Normal = 135 ± 14NS | Normal = 3.10 ± 1.98 | TBW/IBW/BMI/FFM | TBW | R2 = 0.34 |
| Obese = 182 ± 26 | Obese = 3.26 ± 2.19 | + ve | |||
| Caffeine†[57] | Normal = 66.9 ± 13.3NS | Normal = 4.96 ± 2.04 | TBW/IBW/%IBW/LBW/ABW/ | ABW | R2 = 0.31 |
| Obese = 110.4 ± 19.2 | Obese = 6.04 ± 2.97 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| CL = 0.1071 x ABW − 2.3647 | |||||
| Ranitidine [58] | Normal = 54.8 ± 4.5NS | Normal = 32.3 ± 6.1 | TBW/IBW/LBW | LBW | R2 = 0.16 |
| Obese = 103.5 ± 10.4 | Obese = 34.5 ± 6.9 | + ve | |||
| Procainamide†[43] | Normal = 68.4 ± 11.5NS | Normal = 41.9 ± 13.6 | TBW/IBW/%IBW/LBW/ABW/ | IBW | R2 = 0.24> |
| Obese = 100.2 ± 17.3 | Obese = 51.7 ± 9.17 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| CL = 0.6205 x IBW + 6.6157 | |||||
| Carbamazepine†[59] | Normal = 62.2 ± 8.3NS | Normal = 1.38 ± 0.276 | TBW/IBW/%IBW/LBW/ABW/ | IBW | R2 = 0.18 |
| Obese = 111.4 ± 19.9 | Obese = 1.19 ± 0.312 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| CL = 0.0159 x IBW + 0.2992 | |||||
| Amikacin[60] | 166.5 ± 36.9 | 8.57 ± 1.79 | TBW/IBW/%IBW/LBW/ABW/ | LBW | R2 = 0.45 |
| CL = 0.0429 x LBW + 6.2527 | BMI/BSA/PNWT/HT/FFM | + ve | |||
| Vancomycin[61] | Normal = 74.6 ± 10.1* | Normal = 4.85 ± 0.68 | TBW/IBW/%IBW/LBW/ABW/ | BSA | R2 = 0.98 |
| Obese = 166 ± 44.0 | Obese = 11.3 ± 3.88 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| CL = 7.594 x BSA − 10.011 | |||||
| Enoxaparin [62] | Normal = 65.9 ± 9.1 | Normal = 0.74*** | BSA/BMI/TBW/LBW/IBW/ | BSA | R2 = 0.43 |
| Obese = 99.6 ± 15.5 | Obese = 0.99 | %IBW | + ve | ||
| Enoxaparin [63] | 85.0 ± 20.5 | 1.03 l h−1.70 kg−1 (6.80 %CV) | TBW/IBW/LBW/ABW/BMI/ | LBW | = 28.9¶ |
| BSA/PNWT | |||||
| Tobramycin¶¶[64] | 124.5 ± 19.5 | 0.136 l h−1 kg−1 (10.2%CV) | TBW/IBW/%IBW/LBW/ABW/ | PNWT | = 1336 |
| BMI/BSA/PNWT/HT/FFM | |||||
| Carboplatin [65] | (65–112) | 6.72 l h−1 kg−1 | IBW/TBW/ABW | ABW (CF estimated at 0.512) | > 3.84¶ |
| Tinzaparin [66] | Treatment group | CL = (− 2.13 SCr−1) – (0.006 %IBW−1) | IBW/%IBW/BMI | %IBW | = 5¶ |
| = 76.6 ± 18.4 | |||||
| Prophylaxis group | |||||
| = 70.5 ± 11.4 | |||||
| Remifentanil [67] | 88.7 ± 28.6 | CL (l min−1 kg−1) = (0.0185 x LBW) + 1.88 | TBW/LBW | LBW | = 45¶¶ |
| p-Aminohippurate [68] | 99 ± 27 | CL = (11.5 x BSA) − (0.107 x SCr) + 17.2 | TBW/BSA/HT | BSA | >3.84¶ |
| Lithium [69] | (44.5–111) | CL = (0.0093 x LBW) + (0.0885 x CLcr) | TBW/IBW/LBW/BSA | LBW | = 46¶ |
| For LBW alone | |||||
| Doxacurium [70] | NR | CL (ml min−1) = 2.11 x (1 − [0.001 x | TBW/IBW/%IBW/HT | %IBW | = 10.16¶ |
| %IBW]) x (1 + 0.00606 x [CLcr − 80]) | For %IBW alone | ||||
| Vinorelbine [71] | 66 | CL = 29.2 x BSA x (1 − 0.0009 x plat count) | TBW/BSA/HT | BSA | = 35¶ |
| (39–114) | + 6.7 x Wt/SCr) | For BSA alone | |||
| Etoposide [72] | Median = 65 | CL (ml min−1) = θ1 + 1.1 x (TBW/SCr) | TBW/BSA | TBW | = 46¶ |
| (35–106) | θ1 not reported | ||||
| Sufentanil [73] | 125.4 ± 23.3 | 76.2 (23%CV) | TBW/BMI/IBW/HT | None stat. sig. | N/A |
| (82–155) |
NR, not reported; SCr, serum creatinine; OBJ, objective function;
, not significantly different between obese and normal group.
Change in OBJ from baseline model.
Combined change in OBJ with CL, Vc and V p scaled to LBW.Δ, Change in the objective function.Difference between obese and normal group where
P < 0.05;
P < 0.01;
P < 0.001.
Linear regression performed for this review (by B.G.) using raw data presented in the original paper.
Data from original paper analysed by author (B.G.) using NONMEM v5 and G77 compiler.
Appendix II
Table Appendix II.
Studies evaluating the impact of obesity on volume of distribution (V)
| Drug | Subjects’ TBW, kg mean ± SD (range) | Central volume (Vc), l, mean ± SD | Size descriptors considered | Best descriptor | Correlation and direction or change in OBJ |
|---|---|---|---|---|---|
| Alprazolam [74] | Normal = 63.3 ± 2.9 | Normal = 73.1 ± 3.6 (SE)** | TBW/IBW/TBW | TBW | R2 = 0.67 |
| Obese = 111.6 ± 11.8 | Obese = 113.5 ± 11.4 (SE) | + ve | |||
| Lithium††[51] | Normal = 69.7 ± 9.3 | Normal 0.662 ± 0.157 | TBW/IBW/%IBW/LBW/ABW/ | BMI | R2 = 0.63 |
| Obese = 106.4 ± 22.1 | Obese = 0.418 ± 0.0858 | BMI/BSA/PNWT/HT/FFM | – ve | ||
| V = − 0.0125 x BMI + 0.9181 | |||||
| Dalteparin [52] | Normal = 69.7 ± 9.3 | Normal 8.36 (51.1%CV)NS | TBW/IBW/ABW | ABW | R2 = 0.55 |
| Obese = 106.4 ± 22.1 | Obese 12.39 (54.1%CV) | + ve | |||
| V = 0.33 x ABW − 14.1 | |||||
| Vancomycin [53] | NR | V = 0.219 x AGE (years) + 0.814 x TBW | TBW/IBW/%IBW | TBW + %IBW | R2 = 0.58 |
| + 0.536 x %IBW | + ve | ||||
| Propranolol [75] | Normal = 66.8 ± 11.3 | Normal = 198 ± 8* | TBW/IBW/BMI | TBW | R2 = 0.95+ ve |
| Obese = 136.5 ± 35.8 | Obese = 339 ± 22 | ||||
| Dexfenfluramine [76] | NR | Normal = 668.7 ± 139.6** | IBW/%IBW/BMI | %IBW | R2 = 0.30 |
| Obese = 969.7 ± 393.3 | + ve | ||||
| V = 6.113 x %IBW (± 2.2) + 88.5 (±273) | |||||
| Vancomycin [55] | Normal = 68 ± 6 | Normal = 46 ± 16NS | TBW/IBW | TBW | R2= 0.24 |
| Obese = 165 ± 46 | Obese = 52 ± 13 | + ve | |||
| Ifosfamide [56] | Normal = 64.2 (47.7–77.0) | Normal = 33.7 (17.8–50.6)* | TBW/IBW/%IBW | TBW & %IBW | R2= 0.37 |
| Obese = 76.8 (70.0–86.0) | Obese = 42.8 (35.5–51.9) | + ve | |||
| Bisoprolol [77] | Normal = 51 ± 4 | Normal = 135 ± 14*** | TBW/%IBW/BMI | %IBW | R2= 0.76+ ve |
| Obese = 91 ± 17 | Obese = 182 ± 26 | ||||
| Glyburide [37] | Normal = 135 ± 14 | Normal = 56.8 ± 60.3NS | TBW/IBW/BMI/FFM | BMI | R2= 0.31 |
| Obese = 182 ± 26 | Obese = 47.0 ± 47.0 | – ve | |||
| Caffeine[57] | Normal =66.9 ± 13.3 | Normal = 40.1 ± 13 | TBW/IBW/%IBW/LBW/ABW/ | ABW | R2 = 0.70 |
| Obese = 110.4 ± 19.2 | Obese = 49.9 ± 9.3 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| V = 0.7667 x ABW − 12.071 | |||||
| C-peptide [78] | Normal = 69.4 ± 11.4 | Normal = 4.18 ± 0.83*** | TBW/BMI/BSA | BSA | R2 = 0.23 |
| Obese = 107.9 ± 24.9 | Obese = 4.77 ± 1.27 | + ve | |||
| Males V = 1.92 x BSA + 0.64 | |||||
| Females V = 1.11 x BSA + 2.04 | |||||
| Ranitidine [58] | Normal = 54.8 ± 4.5 | Normal = 79.5 ± 14.0NS | TBW/IBW/LBW | IBW | R2 = 0.26 |
| Obese = 103.5 ± 10.4 | Obese = 83.3 ± 21.9 | + ve | |||
| Procainamide††[43] | Normal = 68.4 ± 11.5 | Normal = 150 ± 26.0NS | TBW/IBW/%IBW/LBW/ABW/ | IBW | R2 = 0.14 |
| Obese = 100.2 ± 17.3 | Obese = 158 ± 33.0 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| V = 1.0982 x IBW + 82.778 | |||||
| Carbamazepine†[59] | Normal = 62.2 ± 8.3*** | Normal = 60.7 ± 8.5 | TBW/IBW/%IBW/LBW/ABW/ | TBW | R2 = 0.86 |
| Obese = 111.4 ± 19.9 | Obese = 98.4 ± 26.9 | BMI/BSA/PNWT/HT/FFM | + ve | ||
| V = 0.9031 x TBW + 0.2001 | |||||
| Desmethyldiazepam [79] | Normal = 67 (51–91) | Normal = 63 (38–119)*** | TBW/%IBW | TBW | R2 = 0.74 |
| Obese = 105 (77–197) | Obese = 159 (91–340) | + ve | |||
| Amikacin†[60] | 166.5 ± 36.9 | 27.4 ± 7.75 | TBW/IBW/%IBW/LBW/ABW/ | FFM | R2 = 0.35 |
| V = 0.3107 x FFM + 3.5167 | BMI/BSA/PNWT/HT/FFM | + ve | |||
| Theophylline [80] | Lean = 53 ± 5.5 | Lean = 22.5 ± 3.7** | TBW/IBW/%IBW/BMI | TBW | R2 = 0.56 |
| Control = 63 ± 10.5 | Control = 27.7 ± 8.1 | + ve | |||
| Obese = 81 ± 11.5 | Obese = 35.8 ± 5.1 | ||||
| Very obese = 115 ± 26.6 | Very obese = 42.4 ± 9.2 | ||||
| V = 11.9 + 0.262 x TBW | |||||
| Vancomycin†[61] | Normal = 74.6 ± 10.1 | Normal = 33.3 ± 3.4* | TBW/IBW/%IBW/LBW/ABW/ | ABW | R2 = 0.94 |
| Obese = 166 ± 44.0 | Obese = 50.2 ± 11.5 | BMI/BSA/PNWT/HT/FFM | |||
| V = 0.4927 x ABW − 1.9227 | + ve | ||||
| Phenytoin [81] | Normal = 67 ± 3 | Normal = 40.2 ± 1.9** | IBW/%IBW | IBW | R2 = 0.77 |
| Obese = 124 ± 10 | Obese = 82.2 ± 7.9 | + ve | |||
| Enoxaparin [62] | Normal = 65.9 ± 9.1 | Normal = 4.37*** | BSA/BMI/TBW/LBW/IBW/ | BSA | R2 = 0.60 |
| Obese = 99.6 ± 15.5 | Obese = 5.77 | %IBW | + ve | ||
| Enoxaparin [63] | 85.0 ± 20.5 | 3.67 l kg−1 (24.5 %CV) | TBW/IBW/LBW/ABW/BMI/ | TBW | = 47.6¶ |
| BSA/PNWT | |||||
| Remifentanil [67] | 88.7 ± 28.6 | Vc (l kg−1) = (0.121 x LBW) − 0.0713 | TBW/LBW | Vc = LBW | = 45¶¶ |
| Vp (l kg−1) = (0.165 x LBW) − 0.0713 | Vp = LBW | ||||
| p-Aminohippurate [68] | 99 ± 27 | V = (0.05 x TBW) + 5.58 | TBW/BSA/HT | TBW | >3.84¶ |
| Vinorelbine [71] | 66 | V = 2340 x (1 − 0.000849 x Plt count) | TBW/BSA/HT | None stat. sig. | >3.84¶ for BSA alone, |
| (39–114) | x (1 + 0.26 x SEX) | NOT included in final model | |||
| Etoposide [72] | Median = 65 | 5.6 l m−2 | TBW/BSA | BSA | = 26¶ |
| (35–106) | |||||
| Sufentanil [73] | 125.4 ± 23.3 | 37.1 (20%CV) | TBW/BMI/IBW/HT | None stat. sig. | N/A |
| (82–155) |
NR, not reported; OBJ, Objective function;
, not significantly different between obese and normal group.
Change in OBJ from baseline model.
Combined change in OBJ with CL, Vc and V p scaled to LBW.
, Change in the objective function. Difference between obese and normal group where
P < 0.05;
P < 0.01;
P < 0.001.
Linear regression performed for this review (by B.G.) using raw data presented in the original paper.
Data from original paper analysed by author (B.G.) using NONMEM v5 and G77 compiler.
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: the Evidence Report. Bethesda, MD: US Department of Health and Human Services; 1998. [Google Scholar]
- 3.Lurbe E, Alvarez V, Redon J. Obesity, body fat distribution, and ambulatory blood pressure in children and adolescents. J Clin Hypertens. 2001;3:362–7. doi: 10.1111/j.1524-6175.2001.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JW, Konz EC. Obesity and disease management: effects of weight loss on comorbid conditions. Obes Res. 2001;9:326S–334S. doi: 10.1038/oby.2001.138. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Report of a WHO Consultation on Obesity: Preventing and Managing the Global Epidemic. Geneva: WHO; 1998. [PubMed] [Google Scholar]
- 6.Blouin RA, Warren GW. Pharmacokinetic considerations in obesity. J Pharm Sci. 1999;88:1–7. doi: 10.1021/js980173a. [DOI] [PubMed] [Google Scholar]
- 7.Anastasio P, Spitali L, Frangiosa A, et al. Glomerular filtration rate in severely overweight normotensive humans. Am J Kidney Dis. 2000;35:1144–8. doi: 10.1016/s0272-6386(00)70052-7. [DOI] [PubMed] [Google Scholar]
- 8.Nowack R, Raum E, Blum W, Ritz E. Renal hemodynamics in recent-onset type II diabetes. Am J Kidney Dis. 1992;20:342–7. doi: 10.1016/s0272-6386(12)70296-2. [DOI] [PubMed] [Google Scholar]
- 9.Cockroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 10.Duffull S, Dooley M, Green B, Poole S, Kirkpatrick C. A standard weight descriptor for dose adjustment in the obese patient. Clin Pharmacokinet. 2004 doi: 10.2165/00003088-200443150-00007. in press. [DOI] [PubMed] [Google Scholar]
- 11.Kjellberg J, Reizenstein P. Body composition in obesity. Acta Med Scandanavia. 1970;188:161–9. [PubMed] [Google Scholar]
- 12.Cheymol G. Clinical pharmacokietics of drugs in obesity. Clin Pharmacokinet. 1993;25:103–14. doi: 10.2165/00003088-199325020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Cheymol G. Effects of obesity on pharmacokinetics. Clin Pharmacokinet. 2000;39:215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 14.Quetelet LAJ. 1869. Physique Sociale vol 2; p. 92. Brussels, C. Muquardt. [Google Scholar]
- 15.Keys A, Fidanza F, Karvonen M, Kimura N, Taylor H. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 16.Morse W, Soeldner JS. The composition of adipose tissue and the nonadipose body of obese and nonobese man. Metabolism. 1963;12:99–107. [Google Scholar]
- 17.Du Bois D, Du Bois EF. Clinical calorimetry. Tenth paper. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863. [PubMed] [Google Scholar]
- 18.Meeh K. Oberflächenmessungen des menschlichen körpers. Ztschr f Biol, München. 1879;15:425–58. [Google Scholar]
- 19.Mosteller RD. Simplified calculation of body surfaced area. N Eng J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 20.Dooley M, Singh S, Poole S, Toner G, Michael M. Distribution and discordance of body surface area (BSA) and body mass index (BMI) in 4514 patients with malignancy. The utility of BMI to identify obese patients and its implications in BSA adjusted dosing of cytotoxics. Proc Am Soc Clin Oncol. 2002;21:356. [Google Scholar]
- 21.Turcotte G. Erroneous nomograms for body-surface area. N Eng J Med. 1979;300:1339. doi: 10.1056/nejm197906073002320. [DOI] [PubMed] [Google Scholar]
- 22.Stat Bull Metropolitan Life Insurance Co; 1942. Ideal Weights for Women. October. [Google Scholar]
- 23.Stat Bull Metropolitan Life Insurance Co; 1943. Ideal Weights for Men. June. [Google Scholar]
- 24.New Weight Standards for Men and Women. Stat Bull Metropolitan Life Insurance Co. 959;40:1–4. Nov–Dec. [Google Scholar]
- 25.Mortality among overweight men and women. Stat Bull Metropolitan Life Insurance Co. 1960;41:6. [Google Scholar]
- 26.Society of Actuaries. Build and Blood Pressure Study. New York: Peter Mallon; 1959. [Google Scholar]
- 27.Blackburn GL, Bistrian BR, Maini BS, Schlamm HT, Smith MF. Nutritional and metabolic assessment of the hospitalized patient. J Parenter Enteral Nutr. 1977;1:11–22. doi: 10.1177/014860717700100101. [DOI] [PubMed] [Google Scholar]
- 28.Devine D. Case study number 25 gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650–5. [Google Scholar]
- 29.Rathbun EN, Pace N. The determination of total body fat by means of the body specific gravity. J Biol Chem. 1945;158:667–76. [Google Scholar]
- 30.Hume R, Weyers E. Relationship between total body water and surface area in normal and obese subjects. J Clin Pathol. 1971;24:234–8. doi: 10.1136/jcp.24.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durnin JVGA, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21:681–9. doi: 10.1079/bjn19670070. [DOI] [PubMed] [Google Scholar]
- 32.Boddy K, King PC, Hume R, Weyers E. Estimation of the fat-free mass of twenty subjects from measurements of total body potassium, body density, skinfold thickness, and height and weight. Proc Nutr Soc. 1972;31:35A. [PubMed] [Google Scholar]
- 33.Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol. 1986;60:1327–32. doi: 10.1152/jappl.1986.60.4.1327. [DOI] [PubMed] [Google Scholar]
- 34.Segal KR, Van Loan M, Fitzgerald PI, Hodgdon JA, Van Itallie TB. Lean body mass estimation by bioelectrical impedance analysis: a four-site cross-validation study. Am J Clin Nutr. 1988;47:7–14. doi: 10.1093/ajcn/47.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Gray DS, Bray GA, Gemayel N, Kaplan K. Effect of obesity on bioelectrical impedance. Am J Clin Nutr. 1989;50:255–60. doi: 10.1093/ajcn/50.2.255. [DOI] [PubMed] [Google Scholar]
- 36.Garrow J, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–53. [PubMed] [Google Scholar]
- 37.Jaber LA, Antal EJ, Slaughter RL, Welshman IR. The pharmacokinetics and pharmacodynamics of 12 weeks of glyburide therapy in obese diabetics. Eur J Clin Pharmacol. 1993;45:459–63. doi: 10.1007/BF00315518. [DOI] [PubMed] [Google Scholar]
- 38.James W. Research on Obesity. London: Her Majesty's Stationery Office; 1976. [Google Scholar]
- 39.Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Clin Pharmacokinet. 1994;26:292–307. doi: 10.2165/00003088-199426040-00005. [DOI] [PubMed] [Google Scholar]
- 40.Green B, Duffull S. Caution when lean body weight is used as a size descriptor for obese subjects. Clin Pharmacol Ther. 2002;72:743–4. doi: 10.1067/mcp.2002.129306. [DOI] [PubMed] [Google Scholar]
- 41.Bauer LA, Edwards WA, Dellinger EP, Simonowitz DA. Influence of weight on aminoglycoside pharmacokinetics in normal weight and morbidly obese patients. Eur J Clin Pharmacol. 1983;24:643–7. doi: 10.1007/BF00542215. [DOI] [PubMed] [Google Scholar]
- 42.Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol. 1988;6:1321–7. doi: 10.1200/JCO.1988.6.8.1321. [DOI] [PubMed] [Google Scholar]
- 43.Christoff PB, Conti DR, Naylor C, Jusko WJ. Procainamide disposition in obesity. Drug Intell Clin Pharm. 1983;17:516–22. doi: 10.1177/106002808301700704. [DOI] [PubMed] [Google Scholar]
- 44.Kowalski KG, Hutmacher MM. Efficient screening of covariates in population models using Wald's approximation to the liklihood ratio test. J Pharmacokinet Pharmacodynam. 2001;28:253–75. doi: 10.1023/a:1011579109640. [DOI] [PubMed] [Google Scholar]
- 45.Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic–pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–28. doi: 10.1007/BF01061469. [DOI] [PubMed] [Google Scholar]
- 46.Wade JR, Beal SL, Sambol NC. Interaction between structural, statistical, and covariate models in population pharmacokinetic analysis. J Pharmacokinet Biopharm. 1994;22:165–77. doi: 10.1007/BF02353542. [DOI] [PubMed] [Google Scholar]
- 47.Wahlby U, Jonsson EN, Karlsson MO. Comparison of stepwise covariate model building strategies in population pharmacokinetic–pharmacodynamic analysis. AAPS Pharm Sci. 2002;4:27. doi: 10.1208/ps040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahlby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28:231–52. doi: 10.1023/a:1011527125570. [DOI] [PubMed] [Google Scholar]
- 49.Boddy K, King PC, Hume R, Weyers E. The relationship of total body potassium to height, weight and age in normal subjects. J Clin Pathol. 1972;25:512–7. doi: 10.1136/jcp.25.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Womersley J, Boddy K, King PC, Durnin JVGA. A comparison of the fat free mass of young adults estimated by anthropometry, body density and total potassium content. Clin Sci. 1972;43:469–75. doi: 10.1042/cs0430469. [DOI] [PubMed] [Google Scholar]
- 51.Reiss RA, Haas CE, Karki SD, Gumbiner B, Welle SL, Carson SW. Lithium pharmacokinetics in the obese. Clin Pharmacol Ther. 1994;55:392–8. doi: 10.1038/clpt.1994.47. [DOI] [PubMed] [Google Scholar]
- 52.Yee JY, Duffull SB. The effect of body weight on dalteparin pharmacokinetics. A preliminary study. Eur J Clin Pharmacol. 2000;56:293–7. doi: 10.1007/s002280000141. [DOI] [PubMed] [Google Scholar]
- 53.Vance-Bryan K, Guay DR, Gilliland SS, Rodvold KA, Rotschafer JC. Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother. 1993;37:436–40. doi: 10.1128/aac.37.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibbs JP, Gooley T, Corneau B, et al. The impact of obesity and disease on busulfan oral clearance in adults. Blood. 1999;93:4436–40. [PubMed] [Google Scholar]
- 55.Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54:621–5. doi: 10.1007/s002280050524. [DOI] [PubMed] [Google Scholar]
- 56.Lind MJ, Margison JM, Cerny T, Thatcher N, Wilkinson PM. Prolongation of ifosfamide elimination half-life in obese patients due to altered drug distribution. Cancer Chemother Pharmacol. 1989;25:139–42. doi: 10.1007/BF00692355. [DOI] [PubMed] [Google Scholar]
- 57.Caraco Y, Zylber-Katz E, Berry EM, Levy M. Caffeine pharmacokinetics in obesity and following significant weight reduction. Int J Obes Relat Metab Disord. 1995;19:234–9. [PubMed] [Google Scholar]
- 58.Davis RL, Quenzer RW, Bozigian HP, Warner CW. Pharmacokinetics of ranitidine in morbidly obese women. Drug Intell Clin Pharm. 1990;24:1040–3. doi: 10.1177/106002809002401101. [DOI] [PubMed] [Google Scholar]
- 59.Caraco Y, Zylber-Katz E, Berry EM, Levy M. Carbamazepine pharmacokinetics in obese and lean subjects. Ann Pharmacother. 1995;29:843–7. doi: 10.1177/106002809502900902. [DOI] [PubMed] [Google Scholar]
- 60.Bauer LA, Blouin RA, Griffen WO, Jr, Record KE, Bell RM. Amikacin pharmacokinetics in morbidly obese patients. Am J Hosp Pharm. 1980;37:519–22. [PubMed] [Google Scholar]
- 61.Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO., Jr Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21:575–80. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanderink GJ, Le Liboux A, Jariwala N, et al. The pharmacokinetics and pharmacodynamics of enoxaparin in obese volunteers. Clin Pharmacol Ther. 2002;72:308–18. doi: 10.1067/mcp.2002.127114. [DOI] [PubMed] [Google Scholar]
- 63.Green B, Duffull S. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol. 2002;56:96–103. doi: 10.1046/j.1365-2125.2003.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blouin RA, Mann HJ, Griffen WO, Jr, Bauer LA, Record KE. Tobramycin pharmacokinetics in morbidly obese patients. Clin Pharmacol Ther. 1979;26:508–612. doi: 10.1002/cpt1979264508. [DOI] [PubMed] [Google Scholar]
- 65.Benezet S, Guimbaud R, Chatelut E, Chevreau C, Bugat R, Canal P. How to predict carboplatin clearance from standard morphological and biological characteristics in obese patients. Ann Oncol. 1997;8:607–9. doi: 10.1023/a:1008259009500. [DOI] [PubMed] [Google Scholar]
- 66.Barrett JS, Gibiansky E, Hull RD, et al. Population pharmacodynamics in patients receiving tinzaparin for the prevention and treatment of deep vein thrombosis. Int J Clin Pharmacol Ther. 2001;39:431–46. [PubMed] [Google Scholar]
- 67.Egan TD, Huizinga B, Gupta SK, et al. Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology. 1998;89:562–73. doi: 10.1097/00000542-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Kinowski JM, Rodier M, Bressolle F, et al. Bayesian estimation of p-aminohippurate clearance by a limited sampling strategy. J Pharm Sci. 1995;84:307–11. doi: 10.1002/jps.2600840309. [DOI] [PubMed] [Google Scholar]
- 69.Jermain DM, Crismon ML, Martin ES3rd. Population pharmacokinetics of lithium. Clin Pharm. 1991;10:376–81. [PubMed] [Google Scholar]
- 70.Fisher D, Schoolar Reynolds K, Schmith VD, et al. The influence of renal function on the pharmacokinetics and pharmacodynamics and simulated time course of doxacurium. Anesth Analg. 1999;89:789–95. doi: 10.1097/00000539-199909000-00049. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen L, Tranchand B, Puozzo C, Variol P. Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br J Clin Pharmacol. 2002;53:459–68. doi: 10.1046/j.1365-2125.2002.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen L, Chatelut E, Chevreau C, et al. Population pharmacokinetics of total and unbound etoposide. Cancer Chemother Pharmacol. 1998;41:125–32. doi: 10.1007/s002800050718. [DOI] [PubMed] [Google Scholar]
- 73.Slepchenko G, Simon N, Goubaux B, Levron JC, Le Moing JP, Raucoules-Aime M. Performance of target-controlled sufentanil infusion in obese patients. Anesthesiology. 2003;98:65–73. doi: 10.1097/00000542-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Abernethy DR, Greenblatt DJ, Divoll M, Smith RB, Shader RI. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9:177–83. doi: 10.2165/00003088-198409020-00005. [DOI] [PubMed] [Google Scholar]
- 75.Bowman SL, Hudson SA, Simpson G, Munro JF, Clements JA. A comparison of the pharmacokinetics of propranolol in obese and normal volunteers. Br J Clin Pharmacol. 1986;21:529–32. doi: 10.1111/j.1365-2125.1986.tb02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheymol G, Weissenburger J, Poirier JM, Gellee C. The pharmacokinetics of dexfenfluramine in obese and non-obese subjects. Br J Clin Pharmacol. 1995;39:684–7. [PMC free article] [PubMed] [Google Scholar]
- 77.Le Jeunne C, Poirier JM, Cheymol G, Ertzbischoff O, Engel F, Hugues FC. Pharmacokinetics of intravenous bisoprolol in obese and non-obese volunteers. Eur J Clin Pharmacol. 1991;41:171–4. doi: 10.1007/BF00265912. [DOI] [PubMed] [Google Scholar]
- 78.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–77. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 79.Abernethy DR, Greenblatt DJ, Divoll M, Shader RI. Prolongation of drug half-life due to obesity: studies of desmethyldiazepam (clorazepate) J Pharm Sci. 1982;71:942–4. doi: 10.1002/jps.2600710827. [DOI] [PubMed] [Google Scholar]
- 80.Rizzo A, Mirabella A, Bonanno A. Effect of body weight on the volume of distribution of theophylline. Lung. 1988;166:269–76. doi: 10.1007/BF02714057. [DOI] [PubMed] [Google Scholar]
- 81.Abernethy DR, Greenblatt DJ. Phenytoin disposition in obesity. Determination of loading dose. Arch Neurol. 1985;42:468–71. doi: 10.1001/archneur.1985.04060050066010. [DOI] [PubMed] [Google Scholar]