Abstract
Aims
Chlorzoxazone is metabolized by cytochrome P450 2E1 (CYP2E1) to a single oxidized metabolite, 6-hydroxychlorzoxazone. The aim of the study was to test the robustness of chlorzoxazone as an in vivo probe of CYP2E1 activity in humans, with emphasis on investigating short-term and long-term intra-individual variabilities and effects of different doses of the drug. In addition, the influences of body build, drug metabolizing enzyme genotype, blood sampling time, and moderate recent ethanol intake were investigated.
Methods
The 6-hydroxychlorzoxazone : chlorzoxazone (metabolic) ratio in plasma was measured at 2 h in 28 male and nine female volunteers following a single oral dose of 500 mg chlorzoxazone. Similarly, the metabolic ratios at 4 h and 6 h were measured in 20 of the males. The metabolic ratio at 2 h was also determined 1.5 and 2.5 years later in 13 and seven males, respectively, and weekly for 3 weeks in seven males, after a dose of 500 mg, once at higher (750 mg) and lower (250 mg) doses, and once (500 mg) following moderate ethanol intake (0.5 g kg−1 body weight) the preceding evening. Genotypes were determined for CYP2E1 as well as for N-acetyltransferase 2 and glutathione transferase M1.
Results
Excluding an outlier (ratio = 1.6) the metabolic ratio at 2 h ranged from 0.12 to 0.61 (n = 36). A positive correlation with body weight (r = 0.61, P < 0.001) suggested dose-dependent metabolism of chlorzoxazone. The metabolic ratio decreased with increasing chlorzoxazone dose (P = 0.01), again suggesting dose-dependent metabolism. Long-term (yearly intervals) and short-term (weekly intervals) intra- and interindividual variabilities in metabolic ratio were similar (30% and 63%vs 28% and 54%, respectively). Both inter- and intra-individual variabilities tended to decrease with increasing dose of chlorzoxazone. There was no significant influence of moderate ethanol intake the preceding evening, or of CYP2E1 genotype on the metabolic ratio.
Conclusions
The relatively low intra-individual variability in the metabolism of chlorzoxazone suggests that a single-sample procedure may suffice to assess CYP2E1 activity in vivo. However, chlorzoxazone metabolism is dose-dependent at commonly used doses and it is therefore advisable to adjust the dose for body weight. Moderate intake of ethanol the preceding evening did not significantly affect the chlorzoxazone metabolic ratio.
Keywords: CYP2E1, chlorzoxazone, 6-hydroxychlorzoxazone, metabolic ratio, body build, ethanol, dose dependence, human
Introduction
Cytochrome P450 2E1 (CYP2E1) belongs to a family of enzymes that play an important role in the metabolism of endogenous substrates (steroids, bile acids, fatty acids, etc.) as well as many exogenous compounds [1]. CYP2E1 catalyzes the bioactivition of numerous industrial chemicals in the low molecular weight range (toluene, xylene, styrene, etc.) [2, 3]. It is found primarily in the liver, but it is also expressed in several extrahepatic tissues, such as kidney, lung and lymphocytes [4].
Considerable interindividual variability in human CYP2E1 activity has been observed both in vitro using liver microsomes [5, 6] and in vivo based on the 6-hydroxylation of chlorzoxazone, a probe substrate of the enzyme [7, 8]. Many factors such as fasting [9], obesity [9], liver dysfunction [10], ethanol consumption [8], and drug intake [11] have been shown to influence the CYP2E1 activity and may thus contribute to interindividual variability. Moreover, genetic factors may also be involved, as several variants of CYP2E1 have been described, some of which may be associated with altered enzyme expression or activity [12–14].
Probes for the determination of enzyme activity in population studies should ideally display low intra-individual variability. In addition, the time needed to complete the test (i.e. time from dosing to sampling) should be short and pre test restrictions (e.g. ethanol intake and diet) should be kept to a minimum. Intra-individual variability in CYP2E1 activity has not yet been assessed, at least not over longer time periods.
Chlorzoxazone is used therapeutically for the relief of painful musculoskeletal conditions. Up to 90% of the dose of chlorzoxazone is oxidized by CYP2E1 to 6-hydroxychlorzoxazone, which is subsequently glucuronidated and eliminated [15, 16], suggesting minimal influence of other elimination pathways. This substrate selectivity as well as a good safety record make chlorzoxazone a suitable in vivo probe for CYP2E1 activity in humans.
The aim of the present study was to investigate the robustness of chlorzoxazone as an in vivo measure of CYP2E1 activity. Long and short-term intra- and interindividual variabilities in activity were studied. In addition, the influence of dose, sampling time, recent moderate alcohol intake and drug metabolizing enzyme genotypes was evaluated.
Methods
Subjects
The subjects were Caucasian, all of whom were considered healthy by physical examination and had no medical history of allergy or other chronic diseases. All except subject 9 (Table 2) were nonsmokers. The subjects were instructed to refrain from taking any medication or alcohol 48 h prior to the administration of chlorzoxazone. The subjects were informed orally and in writing about the design of the study, its possible hazards, and their freedom to discontinue participation at any time. The study was approved by the regional Ethics Committee at Karolinska Institutet.
Table 2.
Metabolic ratio, urinary recovery, geno- and phenotyping of the 28 males and nine females in experiment 1. For enzyme abbreviations, see method section
| Subject | Sex | Age (years) | Metabolic ratioa | Urinary recovery (%) | CYP2E1*5 | CYP2E1*7B | CYP2E1*1C/*1D | CYP2E1*1B | GSTM1 | NAT2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 46 | 0.37 | 54.2 | c1/c1 | GG | 66 | CG | – | R |
| 2 | M | 42 | 0.41 | 54.3 | c1/c1 | GT | 66 | CC | + | R |
| 3 | M | 44 | 0.14 | 59.4 | c1/c1 | GG | 66 | CC | – | R |
| 4 | M | 32 | 0.23 | 46.5 | c1/c1 | GG | 66 | CC | + | S |
| 5 | M | 53 | 0.16 | 55.4 | c1/c1 | GG | 66 | CG | + | S |
| 6 | M | 27 | 0.12 | 42.5 | c1/c1 | GG | 68 | CG | + | S |
| 7 | M | 46 | 0.18 | 51.0 | c1/c1 | GG | 66 | CG | + | S |
| 8 | M | 29 | 0.28 | 27.0 | c1/c1 | GG | 66 | CC | + | S |
| 9 | M | 47 | 0.20 | 56.2 | c1/c1 | GG | 66 | CC | + | R |
| 10 | M | 51 | 0.33 | 54.8 | c1/c1 | GG | 66 | CC | + | R |
| 11 | M | 49 | 0.61 | 57.6 | c1/c1 | GG | 66 | CC | – | R |
| 12 | M | 49 | 0.28 | 53.1 | c1/c2 | GG | 66 | CC | + | R |
| 13 | M | 39 | 0.39 | 43.6 | c1/c1 | GG | 66 | CC | – | R |
| 14 | M | 35 | 0.23 | 35.6 | c1/c1 | GG | 66 | CG | – | S |
| 15 | M | 37 | 0.29 | 62.9 | c1/c2 | GG | 68 | CC | – | S |
| 16 | M | 27 | 0.24 | 50.9 | c1/c1 | GG | 66 | CC | – | S |
| 17 | M | 43 | 0.37 | 24.2 | c1/c1 | GG | 66 | CC | – | S |
| 18 | M | 39 | 0.17 | 55.0 | c1/c2 | GG | 66 | CC | – | S |
| 19 | M | 37 | 0.21 | 52.2 | c1/c1 | GT | 66 | CC | + | S |
| 20 | M | 32 | 0.25 | 39.8 | c1/c1 | GG | 68 | CG | – | R |
| 21 | M | 36 | 0.18 | n.a | c1/c1 | GG | 66 | CC | – | S |
| 22 | M | 46 | 0.20 | n.a | c1/c1 | GG | 66 | CC | – | S |
| 23 | M | 34 | 0.26 | n.a | c1/c1 | GG | 66 | CC | – | R |
| 24 | M | 29 | 0.29 | n.a | c1/c1 | GG | 66 | CC | + | S |
| 25 | M | 24 | 0.21 | n.a | c1/c1 | GG | 66 | CG | – | S |
| 26 | M | 46 | 0.52 | n.a | c1/c1 | GG | 66 | CC | + | R |
| 27 | M | 47 | 0.43 | n.a | c1/c1 | GG | 66 | CG | + | S |
| 28b | M | 35 | 1.60 | n.a | c1/c1 | GG | 66 | CG | + | S |
| 29 | F | 40 | 0.36 | n.a | c1/c1 | GG | 66 | CC | – | R |
| 30 | F | 35 | 0.38 | n.a | c1/c1 | GG | 66 | CG | + | S |
| 31 | F | 27 | 0.22 | n.a | c1/c1 | GG | 66 | CC | – | S |
| 32 | F | 43 | 0.25 | n.a | c1/c1 | GG | 66 | CC | – | R |
| 33 | F | 43 | 0.12 | n.a | c1/c1 | GT | 66 | CG | + | R |
| 34 | F | 40 | 0.27 | n.a | c1/c1 | GG | 66 | CC | – | S |
| 35 | F | 48 | 0.20 | n.a | c1/c1 | GG | 66 | CG | – | S |
| 36 | F | 35 | 0.39 | n.a | c1/c1 | GG | 66 | CC | – | R |
| 37 | F | 34 | 0.20 | n.a | c1/c1 | GG | 66 | CC | – | S |
n.a, not analyzed.
Measured in plasma at 2 h, ratio from first experiment is given.
Excluded from further calculations.
Design
The study designs are described in Table 1. Chlorzoxazone, in the form of 250 mg tablets, was taken together with approximately 100 ml tap water. Food was withheld from the preceding evening until at least 1 h after chlorzoxazone intake. All blood samples were collected into heparinized tubes (Venoject VT-100H). Pre-dose venous blood samples were used to confirm the absence of chlorzoxazone and to prepare analytical calibration standards. Following centrifugation, plasma was harvested and stored at −20°C until analysis. Prior to the first dose of chlorzoxazone, venous blood was also collected in a tube containing sodium citrate (Venoject VP-050SBCS), for the preparation of DNA and subsequent genotyping.
Table 1.
Study design
| Experiment | Time from previous administration | Chlorzoxazone dose | Plasma sampling time | Number of subjects per sex | Objective |
|---|---|---|---|---|---|
| 1 | – | 500 mg | 2 h | 28 M, 9 F | Population variability, sex differences, influence of body build and genotypes |
| 2 | – | 500 mg | 2, 4, 6 h | 20 M1 | |
| 3 | 1.5 years | 500 mg | 2, 4, 6 h | 13 M1 | Long-term variability (experiments 2, 3, 4) |
| 4 | 1 year | 500 mg | 2 h | 7 M1 | |
| 5 | 1 week | 500 mg | 2 h | 7 M1 | Short-term variability (experiments 4, 5, 6) |
| 6 | 1 week | 500 mg | 2 h | 7 M1 | |
| 7 | 1 day | 500 mg | 2 h | 7 M1 | Influence of ethanol (experiments 6, 7) |
| 8 | 1 week | 250 mg | 2 h | 7 M1 | Influence of dose (experiments 6, 8, 9) |
| 9 | 1 week | 750 mg | 2 h | 6 M1 |
Same individuals as in previous experiment.
Population variability, sex differences, and influence of body build and genotype were assessed in experiment 1. In this experiment, 28 males (age 20–53 years, body weight (bw) 50–110 kg, height (ht) 1.68–1.93 m, body fat 7–28%) and nine females (age 20–50 years, bw 46–83 kg, ht 1.60–1.71 m, body fat 19–40%) received a single dose of 500 mg chlorzoxazone. Body mass index was calculated from the expression bw/ht2. Lean body mass and total body fat were calculated from body weight and height according to equations described by Droz et al.[17].
The urinary recovery and the influence of sampling time on the metabolic ratio were studied in experiment 2. Blood samples were collected at 2, 4 and 6 h and urine samples at 2, 4, 6 and 8 h after the intake of chlorzoxazone in 20 males. The total volume of each urine sample was recorded and aliquots were stored at −20°C until analysis. The experiment was repeated approximately 1.5 years later in 13 of the 20 males (experiment 3). The metabolic ratio at 2 h was determined a third time after 2.5 years in seven males (experiment 4), for assessment of long-term intra-individual variability. In experiments 4, 5 and 6 dosing was repeated at weekly intervals in these seven males, for assessment of short-term intra-individual variability.
In experiment 7, the same seven males received 40% ethanol in water corresponding to 0.5 g ethanol kg−1 bw the evening before administration of chlorzoxazone. Before the drug was administered a breath test (alcometer, Palmenco AB, Sweden) was performed to confirm that there was no alcohol in the body.
In experiments 6, 8 and 9, the same seven males received 250, 500, and 750 mg (six males) of chlorzoxazone, with at least 1 week between each dose.
Chemicals
Chlorzoxazone in tablets (Paraflex®, 250 mg) was obtained from Astra AB, Södertälje, Sweden. Authentic standards of chlorzoxazone (98% pure) and 6-hydroxychlorzoxazone (100%) were purchased from Aldrich (Steinheim, Germany) and from Ultrafine Chemicals (Manchester, UK), respectively. Acetofenetidin and β-glucuronidase/sulphatase (prepared from Helix pomatia) were from Sigma (St Louis, Montana). Methanol (HPLC certi) was from Fischer (UK) and acetonitrile (HPLC ultra) from J.T. Baker (Deventer, the Netherlands). Tetrahydrofuran (extra pure), ammonium acetate (pro analysis) and sodium acetate (pro analysis) were purchased from Merck (Darmstadt, Germany).
Analysis of chlorzoxazone and 6-hydroxychlorzoxazone in plasma and urine
The analyses of chlorzoxazone and 6-hydroxychlorzoxazone were performed according to the method of Stiff & colleagues [18] with minor modifications. Acetofenitidin was used as an internal standard. Plasma and urine samples (diluted 1 : 500) were treated with β-glucuronidase/sulphatase before analysis. The HPLC instrument (Hewlett Packard model 1050) was equipped with an autosampler, a diode array detector, and an integrator (HP Chemstation v. 5.02). A reversed-phase 5 µm (4.6 mm I.D × 150 mm) Nucleosil C18 column was used for the separation. Peaks were identified by comparison of retention times and UV-VIS spectra (190–600 nm) with authentic samples. The mobile phase was a mixture of acetonitrile-tetrahydrofuran-0.1 m ammonium acetate (pH 7.0) (22.5 : 5.5 : 72), and was delivered at a flow rate of 0.95 ml min−1. The coefficients of variation for both the plasma and urine assays were less than 5%. The limit of quantification for both chlorzoxazone and 6-hydroxychlorzoxazone was about 0.5 µm.
Genotyping
DNA was prepared from white blood cells. After lysis and proteinase digestion the samples were subjected to a modified salting out procedure [19]. Thereafter DNA was isolated by precipitation with ethanol.
The RsaI polymorphism in the 5′-flanking region (CYP2E1*5) of the gene for CYP2E1 was analyzed by PCR/RFLP as described previously [20]. The insertion (96 bp) polymorphism in a repeat region of the promoter was determined by PCR, using 5′-TGG TAC ATT GTG AGA CAG TG-3′ as the forward primer and 5′-ATA CGG GAA CAC CTC GTT TG-3′ as the reverse primer [21], yielding fragments of 633 bp (6 repeats; CYP2E1*1C) and 729 bp (8 repeats; CYP2E1*1D) [22]. The G−35T (5′-flanking region, CYP2E1*7B) polymorphism was determined by PCR/RFLP as described by Fairbrother & coworkers [23]. The TaqI polymorphism (CYP2E1*1B) was determined by PCR/RFLP as described by Haufroid & collegues [24].
Glutathione transferase M1 (GSTM1) null individuals were identified by PCR essentially according to Brockmöller & coworkers [25]. Genotyping of three functional polymorphisms (341 T > C, 590 G > A, 857 G > A) in N-acetyltransferase 2 (NAT2) was performed as described previously [26]. NAT2 phenotype (R = rapid or S = slow) was deduced from the genotype.
Samples controlling for contamination (water instead of DNA), and negative and positive controls (samples from known genotypes) were included in all PCR assays.
Statistical analyses
The Shapiro-Wilk test for normality suggested that the metabolic ratios were normally distributed (Shapiro-Wilk W-test = 0.904). Metabolic dose ratios from three dose levels and their variation over time were analyzed by repeated measures analysis of variance (anova). Simple anova was used to determine associations between metabolic ratios and genotypes or phenotypes. Dose linearity was tested by fitting the chlorzoxazone dose (D) to the plasma concentration (Cp) of chlorzoxazone or 6-hydroxychorzoxazone using the power function Cp = a × Db, where a and b are constants. The two-tailed Student's paired t-test was used to compare the effect of ethanol on the metabolic ratio. Linear regression was used for correlation analyses. The Wilcoxon test was used to determine associations between recovery and genotypes or phenotypes. Shapiro-Wilk, Wilcoxon and linear regression tests were performed using the JMP® software (version 3.02, SAS Institute Inc). All other tests were carried out in StatView (v.5, SAS Institute Inc). The significance level was set at 0.05.
Results
Chlorzoxazone was generally well tolerated, but one of the males (50 kg bw) complained of dizziness when given a dose of 500 mg. Therefore he was excluded from the 750 mg study.
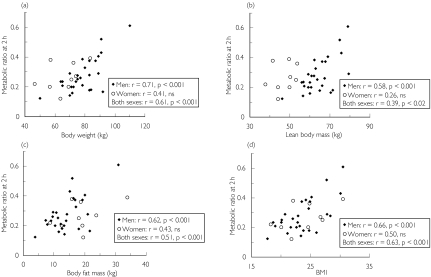
The plasma metabolic ratios in 36 subjects at 2 h ranged from 0.12 to 0.61 (Table 2) and the relative standard deviation was 40% after intake of 500 mg chlorzoxazone. One male subject with an extremely high metabolic ratio at 2 h of 1.6 (subject 28, Table 2) was excluded from all further calculations. There were statistically significant positive correlations between the metabolic ratio at 2 h and body weight, lean body mass, body fat, and body mass index in men and in both sexes combined. However, the corresponding correlations among women were weaker and not statistically significant (Figure 1). No correlation between metabolic ratio and age was detected (Table 2).
Figure 1.
Relationship between body weight (a), lean body mass (b), body fat mass (c), body mass index (BMI) (d) and the plasma 6-hydroxychlorzoxazone : chlorzoxazone metabolic ratio at 2 h after intake of 500 mg chlorzoxazone (27 males and nine females, experiment 1). The r and P values are from linear regression analysis. One subject (number 28, Table 2) was excluded
Considering CYP2E1 genotypes, the RsaI polymorphism in the 5′-flanking region (CYP2E1*5 allele) was found in three of the volunteers. Three subjects had the insertion polymorphism (CYP2E1*1D), and three other subjects had the CYP2E1*7B variant allele. The CYP2E1*1B allele was found in 12 volunteers (Table 2). There were no significant associations between these CYP2E1 genotypes and the metabolic ratio (n = 36). The GSTM1 polymorphism, 21 of the 36 subjects being of the null phenotype, also did not influence the metabolic ratio. However, a significant (P = 0.02) effect of NAT2 phenotype on the metabolic ratio was found in that the 22 slow acetylators had a lower average metabolic ratio than the 14 subjects with the rapid NAT2 phenotype.
The urinary recovery of 6-hydroxychlorzoxazone over 8 h accounted for approximately 55% (range 37–84%) of the administered dose (500 mg) (Table 2). There were no significant associations between urinary recovery and metabolic ratio, NAT2 phenotype or any of the genotypes studied (n = 20).
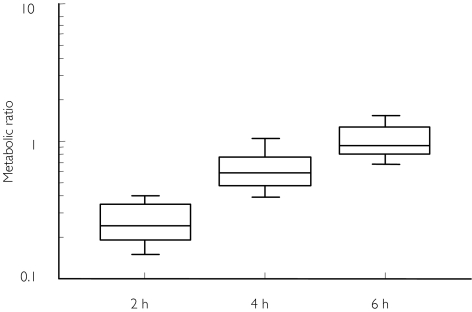
The interindividual variabilities in the metabolic ratio were similar (42%, 36% and 48%, respectively) at the three blood sampling times (n = 20, Figure 2). The correlation between the metabolic ratio at 2 h and that at 4 h was high (r = 0.90, P < 0.001) whereas the correlation between the 2 h and 6 h ratios was lower, but still significant (r = 0.56, P = 0.01) (data not shown). The metabolic ratios at 4 and 6 h were approximately two and four times higher than at 2 h (Figure 2, Table 3). When repeating the study in 13 subjects, interindividual variabilities were still similar at the three sampling times (expressed as relative standard deviations, obtained from repeated measures anova). The intra-individual variability was slightly higher at 6 h than at 2 h and 4 h (Table 3).
Figure 2.
Plasma 6-hydroxychlorzoxazone : chlorzoxazone metabolic ratios at different sampling times after intake of 500 mg chlorzoxazone. The horizontal lines represent the 10th, 30th, 50th, 70th, and 90th percentiles (20 males, experiment 2)
Table 3.
Variability in the chlorzoxazone metabolic ratio, expressed as relative standard deviation, estimated by repeated measures anova (experiments 2 and 3) (n = 13)
| Plasma sampling time | Average ratio | Interindividual variability | Intra-individual variability |
|---|---|---|---|
| 2 h | 0.27 | 63% | 30% |
| 4 h | 0.64 | 37% | 26% |
| 6 h | 1.09 | 52% | 48% |
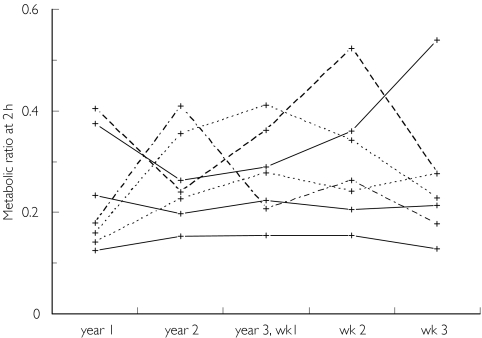
In the experiments performed with 1.5 years interval (Tables 3 and 4), the interindividual variability in metabolic ratio at 2 h was 63% and the intra-individual variability 30% (n = 13). Similar inter- and intra-individual variabilities were obtained in the seven males studied three times at yearly intervals and three times at weekly intervals (Figure 3, Table 4). Thus there was no difference between the long-term (yearly) and the short-term (weekly) intra-individual variabilities. However, the intra-individual variability in the metabolic ratio tended to be smaller in individuals with lower metabolic ratios (Figure 3). Furthermore, there was a decrease in intra-individual variability (expressed as relative standard deviation) with increasing dose (measured as mg chlorzoxazone kg−1 body weight) (r = −0.68, P = 0.07).
Table 4.
Inter- and intra-individual variability in metabolic ratio at 2 h, expressed as relative standard deviation, estimated by repeated measures anova
| Experiment | n | Interindividual variability | Intra-individual variability | |
|---|---|---|---|---|
| All subjects | 1 | 36 | 40% | – |
| Long-term variability | 2, 3 | 13 | 63% | 30% |
| Long-term variability | 2, 3, 4 | 7 | 62% | 30% |
| Short-term variability | 4, 5, 6 | 7 | 54% | 28% |
| All experiments | 2, 3, 4, 5, 6, 7 | 7 | 67% | 29% |
Figure 3.
The plasma 6-hydroxychlorzoxazone : chlorzoxazone metabolic ratio at 2 h after intake of 500 mg chlorzoxazone at yearly and weekly intervals (seven males)
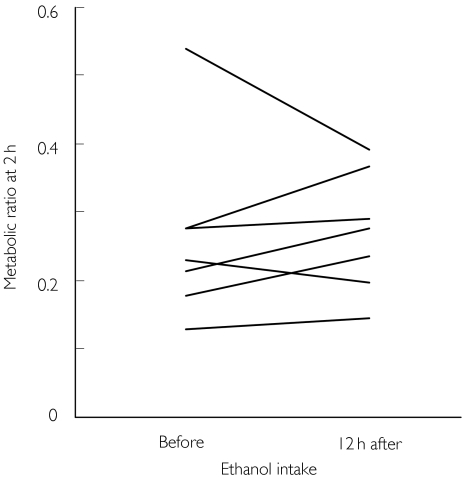
Ethanol intake (0.5 g ethanol kg−1 body weight) on the preceding evening did not influence the metabolic ratio. Thus the average increase in metabolic ratio on the day after ethanol intake was 0.7% (range −6.2% to 13.0%, n = 7), compared with the preceding day (Figure 4).
Figure 4.
The plasma 6-hydroxychlorzoxazone : chlorzoxazone metabolic ratio at 2 h after intake of 500 mg chlorzoxazone before and after intake of 0.5 g ethanol kg−1 body weight the preceding evening (seven males, experiments 6 and 7)
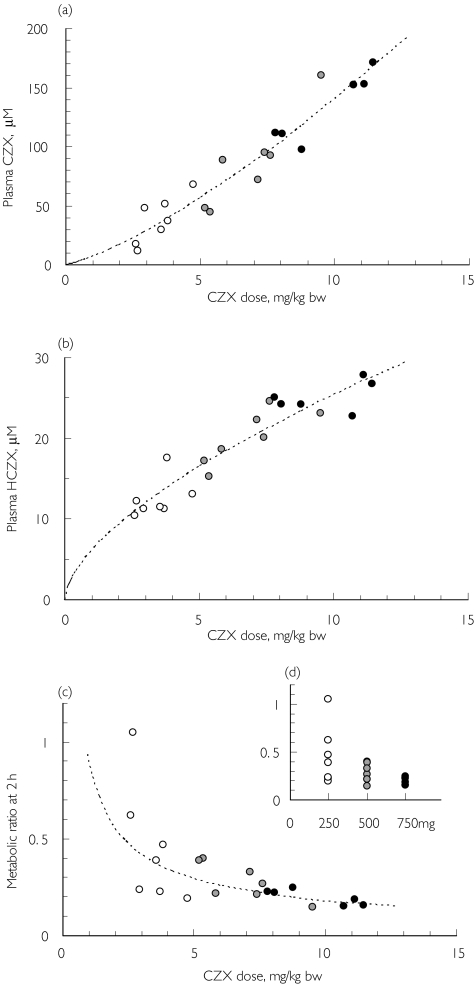
The metabolism of chlorzoxazone was found to be dose-dependent. There was a sublinear increase of the concentration of the drug in plasma (Figure 5a) and a supralinear increase in metabolite concentration (Figure 5b), suggesting some saturation of metabolism at the two higher doses. Accordingly, the metabolic ratio decreased with increasing dose (Figure 5c, d).
Figure 5.
Dose-dependence of (a) plasma chlorzoxazone (CZX), (b) plasma 6-hydroxychlorzoxazone (HCZX), and (c–d) the 6-hydroxychlorzoxazone : chlorzoxazone metabolic ratio at 2 h after intake of 250, 500, and 750 mg chlorzoxazone (seven males, experiments 6, 8 and 9, one subject (50 kg bw) was excluded from the experiment with 750 mg chlorzoxazone). The dotted curves in (a) and (b) represent the best fit of the power function (Cp = a × Db), with exponents of 1.3 and 0.61, respectively. 250 mg (○), 500 mg ( ), 750 mg (•), curve fit (
), 750 mg (•), curve fit ( )
)
Discussion
To our knowledge, this is the first study that investigates the long and short term intra-individual variability in the 6-hydroxychlorzoxazone/chlorzoxazone ratio. The influence of dose, body build and recent moderate ethanol intake was also addressed.
Intra-individual variability in the chlorzoxazone metabolic ratio was about the same following long-term (yearly) and short-term (weekly) assessments, suggesting that the CYP2E1 activity remains fairly constant with time and that the observed intra-individual variability of about 30% may to a large extent reflect the ‘method error’. There are several sources that may contribute to this ‘method error’, including variations in absorption, distribution, and excretion kinetics, as well as analytical errors. No other studies have, to our knowledge, investigated the intra-individual variation in CYP2E1 activity for such a long period of time (six times over 2.5 years). Bachmann & Sarver [27] administered chlorzoxazone to six healthy male subjects on 2 consecutive weeks and found no significant difference in clearance between the two occasions. Taken together, these results suggest that determining CYP2E1 activity in humans using chlorzoxazone only requires a single-dose, single-sample procedure.
The metabolic ratio correlated significantly with body weight, lean body mass, body fat and body mass index in men and in both sexes combined. This is in agreement with previously reported studies showing that body weight is a major contributor to the interindividual variability in the oral clearance of chlorzoxazone [14, 28]. The lack of significant correlations among women may be due to the small number of females (nine) in our study.
Experiments with different doses (250, 500, and 750 mg chlorzoxazone) given to the same individuals revealed clear signs of metabolic saturation, with sublinear (parent substance) and supralinear (hydroxy metabolite) relations with dose. As a result the metabolic ratio decreased with increasing doses. The average decreases in metabolic ratio at 2 h were 32% after 500 mg and 58% after 750 mg, compared to 250 mg. This is in close agreement with previous studies reporting that the metabolic ratio at 4 h decreased by 48% after 750 mg compared to 250 mg [29] and that the chlorzoxazone plasma clearance decreased by 50%, from approximately 0.4 l/min at 250 mg [9, 30] to approximately 0.2 l/min at 750 mg [11, 31]. It is interesting to note that also the inter-individual variability (expressed as relative standard deviation) decreased with dose. Furthermore, the repeated experiments with 500 mg indicated a tendency towards lower intra-individual variability in subjects with lower body weight, i.e. in those obtaining a higher dose of chlorzoxazone per kg body weight.
The body weight of the adult population varies by more than two-fold. This would be expected to result in at least a two-fold variation in metabolic ratio even if CYP2E1 activity were constant, provided that a fixed dose of chlorzoxazone is used. Thus, it seems more appropriate to adjust the dose by body weight. We suggest that a dose of 10 mg chlorzoxazone kg−1 body weight should be used for the determination of CYP2E1 activity. This corresponds to 750 mg in a 75 kg individual and is thus higher than the commonly used dose of 500 mg. However, 750 mg is still used therapeutically and should not result in side-effects. At this higher dose of 750 mg the biotransformation of chlorzoxazone is closer to saturation and the metabolic ratio will tend to reflect the maximum metabolic rate (Vmax). At lower doses the metabolic ratio will reflect not only the intrinsic metabolic clearance (Vmax/Km) but also the blood flow of the eliminating organ.
Frye et al.[29] have suggested the use of 250 mg chlorzoxazone to minimize saturation of metabolism. However, our data indicate that saturation occurs to some extent at the lowest dose. This finding is also supported by the study of Lucas et al.[15], in which Michaelis constants of 53 µm and 74 µm were obtained in microsomes from two human livers. These values correspond to concentrations measured 2 h after administration of the lowest chlorzoxazone dose.
Our study was not originally designed to study sex differences, therefore only a few women were included. Nevertheless, it is interesting to note that the few women in our study had a similar range of metabolic ratios as the men, in spite of lower body weights. However, when metabolic ratios were plotted against lean body mass the effect of sex became obvious. This might seem surprising since if the metabolic ratio mainly depended on absorption, distribution, or excretion phenomena or if CYP2E1 activity was only related to lean body mass or liver size, one would expect no such sex difference. In contrast, the sex difference disappeared when plotting metabolic ratio against body fat mass or body mass index (Figure 1C, 1D). This may indicate that the metabolic ratio is indeed mainly related to CYP2E1 activity which, in turn, is related to the amount of body fat. In a large population study, age-adjusted fasting insulin levels were higher in men than in women. This sex difference disappeared after adjusting for body mass index [32]. Thus, one may speculate that a higher body fat mass results in increased plasma insulin levels, higher rates of fat catabolism, formation of acetone and other ketone bodies and, ultimately, increased CYP2E1 activity and chlorzoxazone metabolic ratio. In future studies it would be of interest to measure the chlorzoxazone metabolic ratio and its relation with ketone levels in women and men with similar lean body masses.
One male subject, who was excluded from the statistical analyses, had a very high metabolic ratio of 1.6 at 2 h, for which we have no explanation. The subject was not extreme in body composition (86 kg, 1.78 m, 18% body fat), had no history of chronic diseases, was taking no medication and had normal liver enzyme values. He was a moderate social drinker and a nonsmoker. Although CYP1A has been shown to metabolize chlorzoxazone, it is of minor importance compared with CYP2E1 in vivo[15] and thus it is unlikely that induction of CYP1A2 could have explained the extremely high ratio. Furthermore, this subject was normal with respect to his metabolism of m-xylene and 2-propanol [33, 34].
In our study about 55% of a 500 mg dose was excreted in the urine over 8 h as 6-hydroxychlorzoxazone, which is similar to values reported by others [8, 29–31]. No parent drug was detected in urine. One explanation for the relatively low urinary recovery may be incomplete absorption from the gastrointestinal tract, due to slow dissolution of the tablets. In support of this De Vries & colleagues [30] found that the uptake of chlorzoxazone from tablets was 70% compared with that from a suspension. Furthermore administration of chlorzoxazone following breakfast resulted in a markedly lower urinary recovery of 5–15%[35]. Alternative explanations to the incomplete recovery are additional elimination pathways such as biliary excretion and/or additional metabolic pathways. Ring cleavage to 5-chloro-2,4-dihydroxyacetanilide via N-acetylation and dehalogenation to 6-hydroxybenzoxazolone have been identified as probably minor pathways [16]. We found no effect of NAT2 phenotype on urinary recovery further suggesting that the ring cleavage pathway may be of minor importance. However, a significant effect of NAT2 phenotype on the metabolic ratio was observed, but the reason for this was unclear.
We found no significant influence of genetic polymorphisms of CYP2E1 on the chlorzoxazone metabolic ratio or urinary metabolite recovery, although all variant genotypes tended to have slightly lower metabolic ratios. One reason for the lack of an effect may be the limited number of subjects in the study, since few individuals had the rare genotypes. The CYP2E1*5 allele has been shown to be associated with lower oral clearance of chlorzoxazone in Hawaiian subjects [14] and to be of importance in the induction of CYP2E1 by alcohol [36]. The presence of the insertion mutation (CYP2E1*1D) appears to be associated with higher CYP2E1 metabolic activity, but only among individuals who are either obese or have recently consumed ethanol [28]. The rare CYP2E1*1B allele (TaqI polymorphism) was recently shown to be associated with increased metabolism of styrene in workers from a plastics factory [37].
Isothiocyanates, to which humans are exposed via the food, can inhibit CYP2E1 [38]. These compounds are metabolized by glutathione transferases and the importance of genetic polymorphism of GSTM1 in their chemoprotective effect has recently been shown [39]. Individuals with different glutathione transferase activity will eliminate isothiocyanates at differing rates. Depending on the isothiocyanate intake, this could result in variable degrees of CYP2E1 inhibition, and thus indirectly affect chlorzoxazone metabolism. However, in the present study no influence of GSTM1 genotype on the chlorzoxazone metabolic ratio or the urinary metabolite recovery was observed.
It is unclear whether smoking affects CYP2E1 activity. No effect has been observed on the 2 h metabolic ratio by smoking (0.33 versus 0.31) in 75 smokers and 39 non-smokers [40]. However, a within-subject study performed on regular smokers showed a 24% higher (range −10 to +71%) oral clearance of chlorzoxazone when they were smoking 20 cigarettes/day compared with non-smoking conditions [41]. The single smoker in our study (subject 9, 20 cigarettes/day, Table 2) did not differ from the other males with respect to metabolic ratio or metabolite recovery in urine.
Overall, 2 h is considered to be the optimal sampling time for the assessment of CYP2E1 activity using chlorzoxazone. The high correlation between the metabolic ratios at 2 h and 4 h suggests that the latter might also be an appropriate sampling time. According to a study by Frye & colleagues [29] metabolic ratios obtained later than 4 h were not significantly related to the formation clearance of the hydroxychlorzoxazone, whereas the metabolic ratio at 2 h demonstrated the highest correlation. An additional disadvantage of late sampling times is that both parent and metabolite concentrations are lower resulting in increased analytical errors. At around 6 h, the parent drug may even approach the analytical detection limit.
We saw no influence of recent moderate intake of alcohol on CYP2E1 activity. This was somewhat unexpected, since ethanol is a well-known inducer of CYP2E1 (see e.g. review by Lieber [4]). On average, alcoholic patients metabolize chlorzoxazone five times more rapidly than do healthy subjects [40]. Plee-Gautier & colleagues [42] saw a mean increase of 77% in the metabolic ratio at 2 h following consumption of 0.8 g kg−1 ethanol, a 1.6 times higher dose than in the present study. In contrast, Oneta & colleagues [43] reported a much smaller effect of ethanol. Thus the median metabolic ratio in five males increased from 0.48 before ethanol intake to 0.63 after a week of daily intake of 40 g ethanol. The amount given in our study corresponds to a single dose of between 25 and 50 g ethanol. Overall, these results suggest that moderate intake of alcohol the preceding day does not significantly induce CYP2E1. This is an important observation since subjects are often recommended to avoid alcohol intake for several days before a study.
In conclusion, the relatively low intra-individual variability in the chlorzoxazone metabolic ratio observed in our study suggests that a single-dose, single-sample procedure is sufficient to assess CYP2E1 activity in vivo, which moderate recent intake of alcohol does not appear to affect. The metabolic ratio is clearly dose-dependent, indicating that dose should be adjusted according to body weight. We suggest a dose of 10 mg kg−1 body weight to achieve plasma concentrations of chlorzoxazone well above the reported Km for the 6-hydroxylation of chlorzoxazone. However, further studies are needed to validate this proposal.
Acknowledgments
We are grateful to Dr B. Sjögren for medical examinations. The project was financially supported by the Swedish Council for Working Life Research (Grant 98–0438) and the Swedish Council for Working Life & Social Research (Grant 2001–2242).
References
- 1.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 2.Raucy JL. Risk assessment:. toxicity from chemical exposure resulting from enhanced expression of CYP2E1. Toxicology. 1995;105:217–24. doi: 10.1016/0300-483x(95)03216-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Wang RS, Elovaara E, et al. Cytochrome P450 isozymes responsible for the metabolism of toluene and styrene in human liver microsomes. Xenobiotica. 1997;27:657–65. doi: 10.1080/004982597240253. [DOI] [PubMed] [Google Scholar]
- 4.Lieber CS. Cytochrome P-4502E1: Its physiological and pathological role. Physiol Rev. 1997;77:517–44. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 5.Yoo JS, Guengerich FP, Yang CS. Metabolism of N-nitrosodialkylamines by human liver microsomes. Cancer Res. 1988;48:1499–504. [PubMed] [Google Scholar]
- 6.Hunt CM, Strater S, Stave GM. Effect of normal aging on the activity of human hepatic cytochrome P450IIE1. Biochem Pharmacol. 1990;40:1666–9. doi: 10.1016/0006-2952(90)90470-6. [DOI] [PubMed] [Google Scholar]
- 7.Kim RB, O'Shea D. Interindividual variability of chlorzoxazone 6-hydroxylation in men and women and its relationship to CYP2E1 genetic polymorphisms. Clin Pharmacol Ther. 1995;57:645–55. doi: 10.1016/0009-9236(95)90227-9. [DOI] [PubMed] [Google Scholar]
- 8.Girre C, Lucas D, Hispard E, et al. Assessment of cytochrome P4502E1 induction in alcoholic patients by chlorzoxazone pharmacokinetics. Biochem Pharmacol. 1994;47:1503–8. doi: 10.1016/0006-2952(94)90524-x. [DOI] [PubMed] [Google Scholar]
- 9.O'Shea D, Davis SN, Kim RB, Wilkinson GR. Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone. a putative probe of CYP2E1 activity. Clin Pharmacol Ther. 1994;56:359–67. doi: 10.1038/clpt.1994.150. [DOI] [PubMed] [Google Scholar]
- 10.Dilger K, Metzler J, Bode JC, Klotz U. CYP2E1 activity in patients with alcoholic liver disease. J Hepatol. 1997;27:1009–14. doi: 10.1016/s0168-8278(97)80144-4. [DOI] [PubMed] [Google Scholar]
- 11.Kharasch ED, Thummel KE, Mhyre J, Lillibridge JH. Single-dose disulfiram inhibition of chlorzoxazone metabolism: a clinical probe for P450 2E1. Clin Pharmacol Ther. 1993;53:643–50. doi: 10.1038/clpt.1993.85. [DOI] [PubMed] [Google Scholar]
- 12.Carriere V, Berthou F, Baird S, et al. Human cytochrome P450 2E1 (CYP2E1): From genotype to phenotype. Pharmacogenetics. 1996;6:203–11. doi: 10.1097/00008571-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Haufroid V, Buchet JP, Gardinal S, Lison D. Cytochrome P4502E1 phenotyping by the measurement of the chlorzoxazone metabolic ratio: assessment of its usefulness in workers exposed to styrene. Int Arch Occup Environ Health. 2002;75:453–8. doi: 10.1007/s00420-002-0334-4. [DOI] [PubMed] [Google Scholar]
- 14.Le Marchand L, Wilkinson GR, Wilkens LR. Genetic and dietary predictors of CYP2E1 activity: a phenotyping study in Hawaii Japanese using chlorzoxazone. Cancer Epidemiol Biomarkers Prev. 1999;8:495–500. [PubMed] [Google Scholar]
- 15.Lucas D, Ferrara R, Gonzalez E, et al. Chlorzoxazone, a selective probe for phenotyping CYP2E1 in humans. Pharmacogenetics. 1999;9:377–88. doi: 10.1097/00008571-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Twele R, Spiteller G. [Identification of chlorzoxazone metabolites in human urine (author's transl) ] Arzneimittelforschung. 1982;32:759–63. [PubMed] [Google Scholar]
- 17.Droz PO, Wu MM, Cumberland WG, Berode M. Variability in biological monitoring of solvent exposure. I. Development of a population physiological model. Br J Ind Med. 1989;46:447–60. doi: 10.1136/oem.46.7.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiff DD, Frye RF, Branch RA. Sensitive high-performance liquid chromatographic determination of chlorzoxazone and 6-hydroxychlorzoxazone in plasma. J Chromatogr. 1993;613:127–31. doi: 10.1016/0378-4347(93)80205-i. [DOI] [PubMed] [Google Scholar]
- 19.Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from nucleated cells. Nucl Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson I, Johansson I, Bergling H, et al. Genetic polymorphism of cytochrome P4502E1 in a Swedish population. FEBS Lett. 1993;319:207–11. doi: 10.1016/0014-5793(93)80547-8. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Hakkola J, Oscarson M, Ingelman-Sundberg M. Structural and functional characterization of the 5′-flanking region of the rat and human cytochrome P450 2E1 genes: Identification of a polymorphic repeat in the human gene. Biochem Biophys Res Commun. 1999;263:286–93. doi: 10.1006/bbrc.1999.1362. [DOI] [PubMed] [Google Scholar]
- 22.Fritsche E, Pittman GS. Bell DA. Localization, sequence analysis, and ethnic distribution of a 96-bp insertion in the promoter of the human CYP2E1 gene. Mut Res Genom. 2000;432:1–5. doi: 10.1016/s1383-5726(99)00009-6. [DOI] [PubMed] [Google Scholar]
- 23.Fairbrother KS, Grove J, de Waziers I, et al. Detection and characterization of novel polymorphisms in the CYP2E1 gene. Pharmacogenetics. 1998;8:543–52. doi: 10.1097/00008571-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Haufroid V, Clippe A, Knoops B, Bernard A, Lison D. Genotyping in urine: an interesting tool for epidemiological studies. Clin Chem. 1998;44:2210–11. [PubMed] [Google Scholar]
- 25.Brockmöller J, Gross D, Kerb R, Drakoulis N, Roots I. Correlation between trans-stilbene oxide-glutathione conjugation activity and the deletion mutation in the glutathione S-transferase class Mu gene detected by polymerase chain reaction. Biochem Pharmacol. 1992;43:647–50. doi: 10.1016/0006-2952(92)90591-6. [DOI] [PubMed] [Google Scholar]
- 26.Tuominen R, Baranczewski P, Warholm M, et al. Susceptibility factors and DNA adducts in peripheral blood mononuclear cells of aluminium smelter workers exposed to polycyclic aromatic hydrocarbons. Arch Toxicol. 2002;76:178–86. doi: 10.1007/s00204-002-0331-0. [DOI] [PubMed] [Google Scholar]
- 27.Bachmann K, Sarver JG. Chlorzoxazone as a single sample probe of hepatic CYP2E1 activity in humans. Pharmacology. 1996;52:169–77. doi: 10.1159/000139381. [DOI] [PubMed] [Google Scholar]
- 28.McCarver DG, Byun R, Hines RN, Hichme M, Wegenek W. A Genetic polymorphism in the regulatory sequences of human CYP2E1 – Association with increased chlorzoxazone hydroxylation in the presence of obesity and ethanol intake. Toxicol Appl Pharmacol. 1998;152:276–81. doi: 10.1006/taap.1998.8532. [DOI] [PubMed] [Google Scholar]
- 29.Frye RF, Adedoyin A, Mauro K, Matzke GR, Branch RA. Use of chlorzoxazone as an in vivo probe of cytochrome P450 2E1: choice of dose and phenotypic trait measure. J Clin Pharmacol. 1998;38:82–9. doi: 10.1002/j.1552-4604.1998.tb04381.x. [DOI] [PubMed] [Google Scholar]
- 30.De Vries JD, Salphati L, Horie S, Becker CE, Hoener B-A. Variability in the disposition of chlorzoxazone. Biopharm Drug Disposit. 1994;15:587–97. doi: 10.1002/bdd.2510150706. [DOI] [PubMed] [Google Scholar]
- 31.Desiraju RK, Renzi NLJ, Nayak RK, Kung-Tat N. Pharmacokinetics of chlorzoxazone in humans. J Pharm Sci. 1983;72:991–4. doi: 10.1002/jps.2600720905. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara A, Barrett-Connor E, Wingård DL, Edelstein SL. Sex differences in insulin levels in older adults and the effect of body size, estrogen replacement therapy, and glucose tolerance status. The Rancho Bernardo Study, 1984–87. Diabetes Care. 1995;18:220–5. doi: 10.2337/diacare.18.2.220. [DOI] [PubMed] [Google Scholar]
- 33.Ernstgård L, Sjögren B, Warholm M, Johanson G. Sex differences in the toxicokinetics of inhaled solvent vapors in humans 1. m-Xylene. Toxicol Appl Pharmacol. 2003;193:147–57. doi: 10.1016/j.taap.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Ernstgård L, Sjögren B, Warholm M, Johanson G. Sex differences in the toxicokinetics of inhaled solvent vapors in humans 2. 2-Propanol. Toxicol Appl Pharmacol. 2003;193:158–67. doi: 10.1016/j.taap.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Ernstgård L, Gullstrand E, Johanson G, Löf A. Toxicokinetic interactions between orally ingested chlorzoxazone and inhaled acetone or toluene in male volunteers. Toxicol Sci. 1999;48:189–96. doi: 10.1093/toxsci/48.2.189. [DOI] [PubMed] [Google Scholar]
- 36.Lucas D, Menez C, Girre C, et al. Cytochrome P450 2E1 genotype and chlorzoxazone metabolism in healthy and alcoholic Caucasian subjects. Pharmacogenetics. 1995;5:298–304. doi: 10.1097/00008571-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Haufroid V, Buchet JP, Gardinal S, et al. Importance of genetic polymorphisms of drug-metabolizing enzymes for the interpretation of biomarkers of exposure to styrene. Biomarkers. 2001;6:236–49. doi: 10.1080/13547500010014540. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos. 2001;29:1110–13. [PubMed] [Google Scholar]
- 39.Zhao B, Seow A, Lee EJ, et al. Dietary isothiocyanates, glutathione S-transferase -M1-T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10:1063–7. [PubMed] [Google Scholar]
- 40.Lucas D, Menez C, DuPont I, et al. Clinical interest of the in vivo measurement of CYP2E1 in alcoholic liver diseases. Alcohol Clin Exp Res. 1998;22:745–6. [Google Scholar]
- 41.Benowitz NL, Peng M, Jacob P. 3rd. Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin Pharmacol Ther. 2003;74:468–74. doi: 10.1016/j.clpt.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Plee-Gautier E, Foresto F, Ferrara R, et al. Genetic repeat polymorphism in the regulating region of CYP2E1: frequency and relationship with enzymatic activity in alcoholics. Alcohol Clin Exp Res. 2001;25:800–4. [PubMed] [Google Scholar]
- 43.Oneta CM, Lieber CS, Li J, et al. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002;36:47–52. doi: 10.1016/s0168-8278(01)00223-9. [DOI] [PubMed] [Google Scholar]