Abstract
Aims
To evaluate individual variations in plasma concentrations over time in patients with naltrexone implants.
Methods
Ten opioid-dependent patients received up to four implants. Plasma samples were collected regularly for the analyses of naltrexone and the metabolite β-naltrexol.
Results
The median naltrexone Cmax was 12.3 (range 5.8–22.1) ng ml−1, the median Tmax was 1 day (range 3 h to 35 days), and the median length of time that plasma concentrations were above 1 ng ml−1 was 55 (range 30–80) days. Two patients reported heroin use without experiencing any effect. Tissue reactions were recorded in two patients after repeated implantation.
Conclusion
Marked individual and intraindividual variations in naltrexone concentrations were observed. Further studies should be performed to evaluate the need for therapeutic drug monitoring during naltrexone implant treatment.
Keywords: implant, naltrexone, opioid dependence, plasma levels
Introduction
Naltrexone is a potent competitive opioid antagonist for µ-, κ- and δ-receptors [1, 2]. Its metabolism and pharmacological effects are well characterized [2–4]. When taken orally, naltrexone has been proven to be effective in the treatment of heroin abuse [5–7]. However, only highly motivated patients continue to adhere to daily oral treatment [7]. Consequently, sustained-release forms of naltrexone such as implants or other depot formulations have been developed in an attempt to improve adherence [8–10]. Until now, few data have been published on naltrexone plasma concentrations over time in patients with implants, except for the study by Comer et al.[10, 11]. The aim of the present study was to describe the extent of individual variation in naltrexone plasma concentrations over time in patients with naltrexone implants.
Materials and methods
Ten heroin-dependent patients were recruited, five men and five women, with a median age of 30.5 (range 23–39) years, a median heroin use of 7.5 (range 4–15) years. Only those taking part in professional treatment or counselling programmes were considered eligible. Subjects received information on naltrexone, the study design and follow-up programme, including advice on antagonist protection. They were assured of their rights to withdraw, to have the implant removed, or to change to another treatment at any time. As the primary intention was to offer a more reliable therapy to already treated, fully informed patients, application to the regional ethics board was judged unnecessary. The ethics board was informed and had no objection to the study.
The patients were offered advice on pain treatment and given the contact details of a consultant physician in the event of a medical emergency. All patients gave their written informed consent. Each participant was given a medical examination focusing on hepatic function and former episodes of depression. Repeated implantation was offered, but was not a precondition for taking part.
Naltrexone implants [naltrexone (1 g), magnesium stearate and triamcinolone] were purchased from Wedgewood Pharmacy (Swedesboro, NJ, USA). A routine surgical procedure was used for implantation underneath the Scarpas fascia of the lower abdominal quadrants.
Blood samples were collected before implantation, 1, 3 and 5 h after surgery and then daily during the first week except at the weekend, and thereafter once a week until the plasma naltrexone concentration was below the limit of quantification (1 ng ml−1). The samples were centrifuged shortly after collection and plasma was stored at −20 °C until analysed.
Samples were analysed for naltrexone and the metabolite 6-β-naltrexol [3] by high-performance liquid chromatography (HPLC) using modifications of previously published methods, including a reduced plasma volume (1 ml), a different organic solvent for extraction (dichloromethane : propanol, 95 : 5) [12] and a HPLC mobile phase of 28% acetonitrile in 80 mm phosphate buffer with 2 mm sodium dodecyl sulphate [13]. The coefficients of variation for naltrexone and 6-β-naltrexol were 8 and 10% (1.7 ng ml−1) and 3 and 2% (13.6 ng ml−1), respectively. The limit of quantification was 1 ng ml−1 for both compounds.
Results
One patient received four implants, three patients three, one patient two and the remaining five patients received one implant each, a total of 20 implants. However, the results are based on only 17 implant periods. For one patient, an insufficient number of samples was collected, and two others had their implants removed after few days.
On most occasions, maximum naltrexone plasma concentrations were obtained during the day of implantation or on the next day. However, three patients showed a second concentration peak after 2–5 weeks. The median Cmax for naltrexone was 12.3 (range 5.8–22.1) ng ml−1 and the median Tmax was 1 day (range 3 h to 35 days).
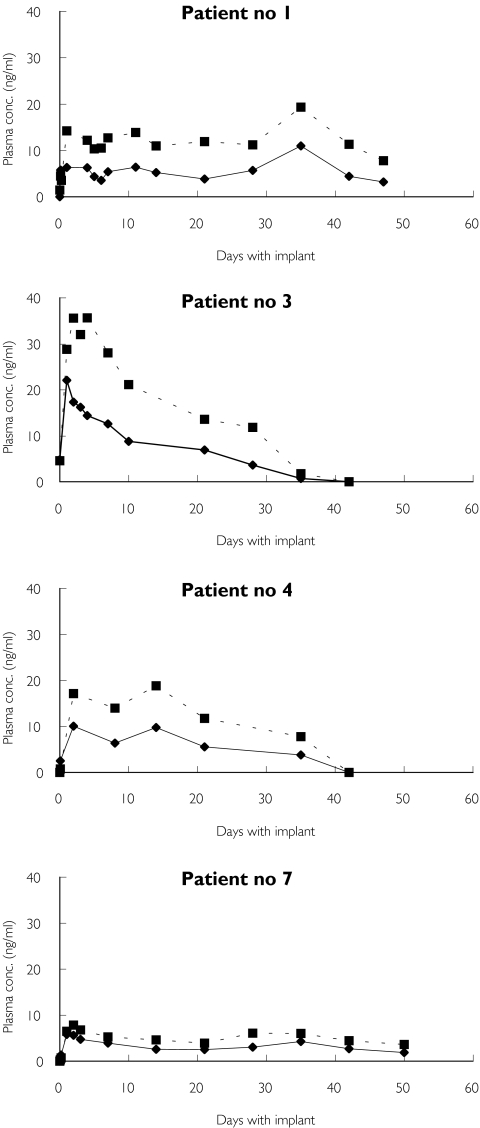
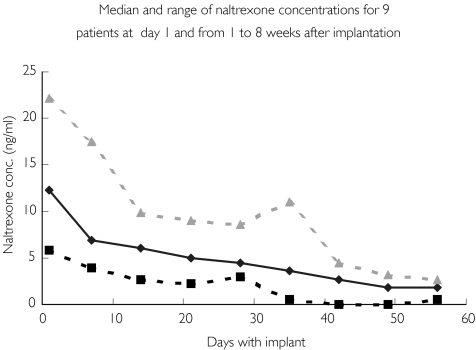
Considerable variations between individual concentration profiles were observed (Figure 1). This is further illustrated in Figure 2, which shows the median naltrexone concentrations and ranges for the first 8 weeks after implantation. The length of time that naltrexone concentrations were above 1 ng ml−1 also showed considerable variation. The median time for naltrexone to be above this concentration was 55 days (range 30–80 days). Considerable intraindividual variation for some patients receiving more than one implant was also observed. For one patient, Cmax varied from 10.8 to 22.1 ng ml−1, whereas for another patient the length of time that the concentration of naltrexone was above 1 ng ml−1 varied from approximately 52 to 80 days.
Figure 1.
Naltrexone and 6-β-naltrexol plasma concentration profiles for four patients during their first implant period, which varied from approximately 30 to >50 days. Naltrexone (♦), 6-β-naltrexol (▪)
Figure 2.
Medial naltrexone plasma concentrations and their ranges during different implant periods after day 1, and from 1 to 8 weeks after implantation. Median (♦), Min. ( ), Max. (
), Max. ( )
)
The 6-β-naltrexol concentrations were 1.5 to approximately 3 times higher than naltrexone during the implant period (30 to 80 days), with the exception of the first hours after implantation and at the end of the follow-up period until the plasma concentration was ≤1 ng ml−1.
Patients were under no particular obligation to abstain from drug use. Two patients reported intravenous heroin intake without experiencing any effects, at times when their naltrexone concentrations were above 5 ng ml−1.
All patients except one were known to be hepatitis C virus (HCV)-positive. Monitoring of hepatic function showed initial moderately elevated serum liver enzyme concentrations in some of the patients. However, in two, hepatic function deteriorated during the study period. In one case this was probably due to a recent undetected HCV infection, and antiviral therapy normalized liver function. The increased liver enzyme concentrations recorded for the other patient were probably due to heavy drinking. Liver enzyme values returned to normal after removal of the implant.
The most prevalent side-effects, probably associated with high plasma naltrexone concentrations, were irritability and dysphoria during the first week postimplant. Slight cephalagia, nausea and muscular discomfort were also reported. However, the most typical effect was a relief of craving and the ability to concentrate on nondrug-related aspects of life.
Two patients developed local tissue reactions after repeated implantation. One of these had had three earlier implantations abroad. Local necrosis developed during the second implant period in the present study, necessitating some local treatment and removal of the implant area. The other patient developed an increasing local reaction to the third implant until its removal.
One patient who was detoxified 3 days before implantation reacted with mixed abstinence phenomena and desperation, and the implant was removed.
Discussion
Our study has demonstrated that naltrexone implants may provide protective plasma drug concentrations during prolonged periods of treatment. The median plasma concentrations were comparable to those obtained after use of other depot formulations [10]. However, considerable intraindividual and individual differences were recorded, with respect to Cmax and to the time period when naltrexone concentrations remained above 1 ng ml−1. The latter varied from approximately 30 to 80 days. It has been stated earlier that adequate plasma naltrexone concentrations are critical for effective antagonist effects [14]. It has been suggested that naltrexone concentrations of at least 1 ng ml−1 are necessary for sufficient receptor blockade after challenge doses of up to 25 mg intravenous heroin [15]. However, other studies have documented sufficient protection concentrations at lower plasma naltrexone levels [1, 2].
In general, the patients seemed to tolerate the implants well. However, two of the 10 patients developed tissue reactions after repeated implantation. These may be specific to the depot formulation used, because naltrexone is not known to cause such reactions. Thus, other depot formulations should be evaluated. Further control studies of the relationship between plasma naltrexone concentrations and protection against the effect of other opioid agonists are also necessary, to evaluate the importance of therapeutic monitoring of the drug in clinical practice.
References
- 1.Dollery Therapeutic Drugs. Toronto: Churchill Livingstone; 1999. [Google Scholar]
- 2.Lee MC, Wagner HN, Jr, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 1988;29:1207–11. [PubMed] [Google Scholar]
- 3.Cone EJ, Gorodetzky CW, Yeh SY. The urinary excretion profile of naltrexone and metabolites in man. Drug Metab Dispos. 1974;2:506–12. [PubMed] [Google Scholar]
- 4.Kleber HD, Kosten TR, Gaspari J, Topazian M. Nontolerance to the opioid antagonism of naltrexone. Biol Psychiatry. 1985;20:66–72. doi: 10.1016/0006-3223(85)90136-2. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein A. Naltrexone treatment of heroin addiction: one-year follow-up. Drug Alcohol Depend. 1984;13:357–65. doi: 10.1016/0376-8716(84)90003-6. [DOI] [PubMed] [Google Scholar]
- 6.Gonzales JP, Brogden RN. Naltrexone: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- 7.Kirchmayer U, Davoli M, Verster A. Naltrexone maintenance treatment for opioid dependence (Cochrane Review) Cochrane Database Syst Rev. 2001;4:CD001333. doi: 10.1002/14651858.CD001333. [DOI] [PubMed] [Google Scholar]
- 8.Chiang CN, Hollister LE, Gillespie HK, Foltz RL. Clinical evaluation of a naltrexone sustained-release preparation. Drug Alcohol Dep. 1985;16:1–8. doi: 10.1016/0376-8716(85)90076-6. [DOI] [PubMed] [Google Scholar]
- 9.Chiang CN, Kishimoto A, Barnett G, Hollister LE. Implantable narcotic antagonist: a possible new treatment for narcotic addiction. Psychopharmacol Bull. 1985;21:672–5. [PubMed] [Google Scholar]
- 10.Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fishman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–60. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewer C. Serum naltrexone and 6-beta-naltrexol levels from naltrexone implants can block very large amounts of heroin: a report of two cases. Addict Biol. 2002;7:321–3. doi: 10.1080/13556210220139541. [DOI] [PubMed] [Google Scholar]
- 12.Davidson HF, Emm TA, Pieniaszek HJ., Jr Determination of naltrexone and its major metabolite, 6-β-naltrexol, in human plasma using liquid chromatography with electrochemical detection. J Pharmaceut Biomed Anal. 1996;14:1717–25. doi: 10.1016/0731-7085(96)01794-3. [DOI] [PubMed] [Google Scholar]
- 13.Svensson J-O. Determination of morphine, morphine-6-gluronide and normorphine in plasma and urine using high-performance liquid chromatography and electrochemical detection. J Chromatogr. 1986;14:174–8. [Google Scholar]
- 14.Ferrari A, Bertolotti M, Dell’Utri A, Avico U, Sternieri E. Serum time course of naltrexone and 6-β-naltrexol levels during long term treatment in drug addicts. Drug Alcohol Dep. 1998;52:211–20. doi: 10.1016/s0376-8716(98)00098-2. [DOI] [PubMed] [Google Scholar]
- 15.Vereby K, Volavka J, Mule SJ, Resnick RB. Naltrexone: disposition, metabolism and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;20:315–28. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]