Abstract
Aim
To assess determinants of treatment failure after antimicrobial therapy of urinary tract infections in women.
Methods
In primary care 16 703 Dutch women who received a first course (3, 5 or 7 days) of trimethoprim, nitrofurantoin or norfloxacin between 1 January 1992 through 31 December 1997 and who were between 15 and 65 years old at the day of first use were selected. Failure of the initial treatment was defined as a further prescription for one of these three antibiotics or for cotrimoxazole, amoxicillin, ciprofloxacin or ofloxacin, within 31 days after the end of the initial therapy.
Results
Treatment failure rate was 14.4% in patients treated with trimethoprim and nitrofurantoin and 9.6% in patients treated with norfloxacin. A multivariate analysis showed that 5 days’[RRNIT 0.67, 95% confidence interval (CI) 0.57, 0.82, RRTRI 0.82, 95% CI 0.73, 0.91] and 7 days’ (RRNIT 0.64, 95% CI 0.53, 0.77, RRTRI 0.85, 95% CI 0.71, 1.02) trimethoprim and nitrofurantoin treatment appeared to be more effective than a 3-day treatment (reference category). Other factors increasing treatment failure rates were the age of the patient, the year of therapy and previous hospitalization.
Conclusions
It may be concluded that 3-day courses of nitrofurantoin and trimethoprim are less effective than 5- and 7-day courses in the treatment of uncomplicated urinary tract infections in women.
Keywords: antibiotic treatment, nitrofurantoin, treatment failure, trimethoprim, urinary tract infection
Introduction
Recently, the comparable efficacy of 3, 5 and 7 days’ treatment for uncomplicated urinary tract infections was reviewed [1]. One of the conclusions was that the evidence on the efficacy of short 3-day courses was mostly derived from small studies in which different dosages, durations and combinations of antibiotics were compared. Moreover, the results of randomized clinical trials on the efficacy of 3-day courses of cotrimoxazole or fluoroquinolones were extrapolated to trimethoprim or nitrofurantoin [1, 2].
The Dutch College of General Practitioners (NHG) recommends a short course (3 days) of trimethoprim or nitrofurantoin in the case of an uncomplicated urinary tract infection in females [3]. This recommendation was partly based on the fact that the antimicrobial resistance to trimethoprim and nitrofurantoin in Escherichia coli, the main causative pathogen, remained low in the Netherlands in the last 10 years (1% resistance for nitrofurantoin and 10% resistance for trimethoprim in 1999) [4, 5].
However, the percentage of patients who return to the general practitioner (GP) within 1 or 2 months after the initial treatment for cystitis with a treatment failure is unknown. It is recognized that in some pathogens, such as E. coli, in vitro microbiological resistance is not always predictive of the success or failure of antibiotic treatment [6]. As a result, the percentage of treatment failures may change over the years, while antibiotic resistance remains constant. The aim of this study was to study treatment failure after prescription of antibiotics for uncomplicated cystitis in females in relation to changes in the duration of prescribing of antibiotics for urinary tract infections, rates of therapy failure for different treatment regimes, and factors that are associated with treatment failure.
Methods
Data sources
Data for this study were obtained from the PHARMO medical record linkage system in the Netherlands. The PHARMO medical record linkage scheme includes the drug-dispensing records from community pharmacies and hospital discharge records of all 320 000 community-dwelling inhabitants of eight medium-sized cities in the Netherlands [7, 8]. PHARMO is a noncommercial database. The prescription data are locally entered at the participating pharmacies and sent by tape to PHARMO every 3 months [7–10] For all residents, the drug-dispensing histories are linked to the hospital discharge records of the same patient, using a probabilistic algorithm, based on characteristics such as date of birth, gender, and a code for the GP. Validation of 9822 linked records showed that these registries were linked with a sensitivity and specificity exceeding 95%, which is comparable to record linkage systems based on unique personal identifiers [7, 8]. The computerized drug-dispensing histories contain data concerning the dispensed drug, type of prescriber, dispensing date, dispensed amount, prescribed dose regimens, and the prescription length. The hospital records include detailed information concerning the primary and secondary diagnoses, procedures, and dates of hospital admission and discharge. All diagnoses are coded according to the ICD-9-CM (International Classification of Diseases, 9th Revision, Clinical Modification).
Ethical approval was not relevant because data were anonymized before entering the PHARMO database. Researchers only have information on the gender and age of the patient. All other identifying information is deleted after the linkage with the hospital records from the National Medical Registry. This approach was approved by the Dutch Data Protection Authority.
Patient selection
All women aged 15–64 years between 1991 and 2000 with at least 1 year of data in the PHARMO database were included. Because there was no information on the prescribing diagnosis, we only included patients with a first prescription of trimethoprim, nitrofurantoin and norfloxacin for 3, 5 or 7 days, because common use of these short courses is restricted as advised by the NHG [3] to therapy of uncomplicated urinary tract infections. A ‘first’ prescription with one of these three antibiotics was defined when the patients did not use any antibiotic in the previous 6 months. In the case that a patient had more than one ‘first’ antibiotic prescription, for instance in 1993 and 1996, random selection, instead of selection of the earliest, was used to select just one ‘first’ prescription per patient. Because there was only information on a patient for a restricted period (1992–1998) there was no possibility of assessing whether an earliest ‘first’ prescription could be selected and random selection was used instead. Patients were followed from the first day of treatment (index date) and monitored until 31 days after the end of the initial treatment.
Outcomes
A further prescription for one of three initial antibiotics or for cotrimoxazole, amoxicillin, ciprofloxacin or ofloxacin, within 31 days after the end of the initial therapy, was defined as an indicator for failure of the initial treatment. Cotrimoxazole, pipemidic acid (not very commonly used; before the introduction of norfloxacin sometimes used after an initial treatment failure), ofloxacin and ciprofloxacin were included because they were also regularly prescribed for the treatment of urinary tract infections. In case of concomitant prescription of one of these antibiotics with other medications from the ATC (Anatomical Therapeutic Chemical classification system) main groups A (gastrointestinal), D (skin), R (respiratory) and S (ear, eye), this further treatment was not assigned as failure to initial treatment.
Additional information on other possible determinants of treatment failure such as the use of oral contraceptives (ATC code G03A), hormone replacement therapy (ATC code G03 except G03A), oral corticosteroids (ATC code H02), antidiabetics (ATC code A10) and hospital admission was assessed in the previous year [11–13]. Crude and multivariate analyses to identify independent determinants of treatment failure of antibiotic therapy were conducted using Cox's proportional hazard analyses (SAS ver. 8.2 for Windows, Cary, NC, USA). Variables significantly associated with treatment failure (P < 0.05) in the crude analyses were included in the multivariate analysis.
Results
The treatment failure analysis included a group of 16 703 women between 15 and 64 years who started 3-, 5- or 7-day courses of trimethoprim, nitrofurantoin or norfloxacin (5 or 7 days’ treatment); 10 195 patients started with trimethoprim, 5760 with nitrofurantoin and 748 with norfloxacin (Table 1). For trimethoprim, most prescriptions were for 3 or 5 days, while for nitrofurantoin 3-, 5- and 7-day treatments were common (Table 1). For nitrofurantoin, 3454 prescriptions were furabid (capsule, sustained release, 100 mg, twice daily), 1424 were 50 mg nitrofurantoin, and 882 100 mg nitrofurantoin (both four times daily).
Table 1.
Duration of the initial treatment for nitrofurantoin, trimethoprim and norfloxacin in the cohort of females between 15 and 64 years
| Antibiotic | Duration of initial treatment | ||
|---|---|---|---|
| 3 days | 5 days | 7 days | |
| Nitrofurantoin (n = 5760) | 1463 (25.4) | 2687 (46.6) | 1610 (28.0) |
| Trimethoprim (n = 10 195) | 4737 (46.5) | 4509 (44.2) | 949 (9.3) |
| Norfloxacin (n = 748) | – | 220 (29.4) | 528 (70.6) |
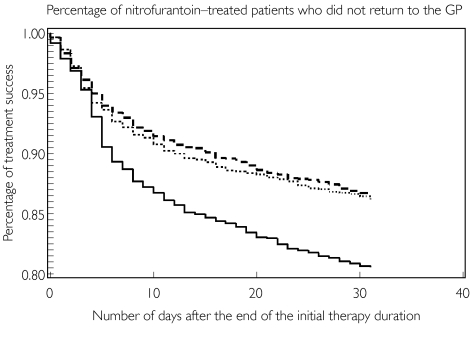
Of the 16 703 patients, 2369 (14.2%) required a new prescription within 31 days after the end of the initial treatment. Treatment failure was comparable after initial therapy with trimethoprim or nitrofurantoin (14.4%) and relatively low after initial therapy with norfloxacin (9.6%). Univariate analysis indicated that treatment failure was higher in older patients and in more recent years (Table 2). Previous hospitalization increased treatment failure rates. Treatment failure in nonhospitalized patients was 13.9%, 16.2% in patients hospitalized 3–12 months before the index date, and 19.9% in patients hospitalized within 3 months before the index date. Treatment failure was highest in patients who had a 3-day course of nitrofurantoin (18.9%) (see also Figure 1) and, to a lesser extent, in patients who had a 3-day course of trimethoprim (15.6%). Previous use of oral contraceptives, hormone replacement therapy, oral corticosteroids and antidiabetics did not significantly affect the treatment failure rate (Table 2). In a multivariate model with a 3-day treatment with nitrofurantoin as a reference, longer treatments with nitrofurantoin and trimethoprim demonstrated lower therapy failure rates (Table 2). Results from an analysis with 3 days’ treatment with trimethoprim as a reference showed similar results (data not shown).
Table 2.
Crude and adjusted rate ratios from Cox regression analyses of determinants of therapy failure for antibiotic treatment of urinary tract infections
| Crude RR | 95% CI | Adjusted* RR 95% | 95% CI | Failure (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics at index date Age group (years) | ||||||||||
| 15–24 | 1.0 | 1.0 | 13.5 | |||||||
| 25–34 | 0.99 | 0.88, 1.12 | 0.99 | 0.87, 1.11 | 13.3 | |||||
| 35–44 | 0.99 | 0.85, 1.12 | 0.99 | 0.88, 1.13 | 13.3 | |||||
| 45–54 | 1.16 | 1.03, 1.31 | 1.19† | 1.05, 1.34 | 15.5 | |||||
| 55–64 | 1.22 | 1.07, 1.39 | 1.26† | 1.10, 1.43 | 16.2 | |||||
| Year start | ||||||||||
| 1992–1993 | 1.0 | 1.0 | 12.4 | |||||||
| 1994–1996 | 1.19 | 1.07, 1.32 | 1.17† | 1.05, 1.31 | 14.6 | |||||
| 1997–1998 | 1.21 | 1.08, 1.36 | 1.22† | 1.08, 1.37 | 14.9 | |||||
| Type of antibiotic and duration of therapy | ||||||||||
| Nitrofurantoin | ||||||||||
| 3 days | 1.0 | 1.0 | 18.9 | |||||||
| 5 days | 0.67 | 0.57, 0.79 | 0.67† | 0.57, 0.79 | 13.1 | |||||
| 7 days | 0.64 | 0.53, 0.77 | 0.63† | 0.52, 0.75 | 12.5 | |||||
| Trimethoprim | ||||||||||
| 3 days | 0.82 | 0.71, 0.94 | 0.82† | 0.72, 0.95 | 15.6 | |||||
| 5 days | 0.68 | 0.59, 0.79 | 0.69† | 0.60, 0.80 | 13.2 | |||||
| 7 days | 0.71 | 0.58, 0.88 | 0.71† | 0.58, 0.88 | 13.7 | |||||
| Norfloxacin | ||||||||||
| 5 days | 0.62 | 0.42, 0.92 | 0.60† | 0.40, 0.89 | 12.3 | |||||
| 7 days | 0.43 | 0.31, 0.59 | 0.42† | 0.30, 0.57 | 8.5 | |||||
| One year prior to start of antibiotic therapy Oral corticosteroids | ||||||||||
| No | 1.0 | 14.2 | ||||||||
| Yes | 1.08 | 0.86, 1.36 | 15.2 | |||||||
| Hormone replacement therapy | ||||||||||
| No | 1.0 | 14.1 | ||||||||
| Yes | 1.04 | 0.92, 1.17 | 14.6 | |||||||
| Antidiabetic therapy | ||||||||||
| No | 1.0 | 14.2 | ||||||||
| Yes | 0.86 | 0.66, 1.12 | 12.3 | |||||||
| Oral contraceptives | ||||||||||
| No | 1.0 | 14.5 | ||||||||
| Yes | 0.94 | 0.87, 1.02 | 13.8 | |||||||
| Hospitalization | ||||||||||
| No | 1.0 | 1.0 | 13.9 | |||||||
| Yes | 1.28 | 1.12, 1.46 | 1.31† | 1.15, 1.50 | 17.4 | |||||
Variables significantly associated with therapy failure after antibiotic treatment (RR < > 1) were analysed in one multivariate model.
Significantly different from reference category. RR >1 indicates that the variable increases therapy failure after antibiotic therapy, while RR <1 indicates that the variable decreases therapy failure after antibiotic therapy.
Figure 1.
Percentage of female patients who did not return for a second antibiotic treatment after the initial antibiotic treatment. 3 days (—); 5 days (·····); 7 days (——)
Discussion
Our data indicate that treatment failure or the need to have further treatment within 31 days after the end of the initial treatment for urinary tract infections is not extraordinarily high. This result is in line with data from a study from the UK [11] in which 14% treatment failure was found. However, data from our study indicate that in certain groups of female patients, especially the older and recently hospitalized, the risk of treatment failure is not negligible. It might be assumed that older patients have a higher lifetime exposure to antibiotics which might lead to an increased resistance [14] and subsequently increased treatment failure rate. In another paper from the Netherlands it was indicated that resistance to quinolones increases independently of age [15]. Another suggestion might be that urinary tract infections in older patients have a more complicated nature, which might lead to a higher treatment failure percentage. However, data from different Dutch GP surveillance systems [16, 17] indicate that the incidence of uncomplicated urinary tract infections is high (>95% of all urinary tract infections in women), even in the elderly, compared with other more complicated types of urinary tract infections. Moreover, the exclusion of patients who had antibiotics within a half year before the initial treatment will lead to the removal of patients with complicated types of urinary tract infections from the study group.
The effects of previous hospitalization on treatment failure percentage can be explained in different ways. First, the overall condition of patients previously hospitalized may be inferior to the condition of nonhospitalized patients, leading to less therapy success. Additionally, the antibiotic treatment of patients in the hospital might lead to the development of resistant bacteria that in turn might be responsible for the following urinary tract infection. Our data indicate that the effect of previous hospitalization is most pronounced in patients who were hospitalized within 3 months before the index date. Moreover, we have some indications that the effect of previous hospitalization is more distinct in patients who had an initial therapy with norfloxacin; therapy failure rates in hospitalized and nonhospitalized patients are, respectively, 20.6% and 8.4%. In the Netherlands, patients in hospitals are often treated with fluoroquinolones, while treatment with trimethoprim or nitrofurantoin is less common [18]. This might explain the discrepancy of our results with the results of studies from the UK that did not find any relation between previous hospitalization and resistance to trimethoprim [12, 13].
A possible effect of the duration of therapy on treatment failure is important for daily practice. In particular, treatment with nitrofurantoin for 3 days appears to lead to more treatment failure, while the effect for trimethoprim is less evident. In a study from the UK a difference between the number of second consultations within 2 weeks after the first prescription of trimethoprim was shown for 3, 5 and 7 days of treatment [1]. This difference was not significant, probably due to the small number of prescriptions studied (n = 271). In another study from the UK [11], a difference in treatment failure between 3, 5 or 7 days of trimethoprim treatment was not found. Differences in results between our study and the two studies in the UK might be related to the fact that we used a pharmacy database, while in the two UK studies data from the GPs were used. A major advantage of a pharmacy database is that the pharmaceuticals are actually delivered to the patient, while in a GP database this information is not available. There are data from the UK indicating that the primary noncompliance, when patients fail to have their prescription dispensed, for anti-infective drugs for women is >6%[19, 20]. This may bias the outcome of a study on the effects of different treatment regimes on treatment failure rates.
Our results question the quality of evidence that supports the use of 3 days’ trimethoprim or nitrofurantoin treatment for urinary tract infection. For trimethoprim, most evidence is based on extrapolations of randomized controlled trials on cotrimoxazole, which are often of mediocre quality [1, 2, 21]. For nitrofurantoin, most evidence is based on information from one small clinical trial on 3 days’ treatment that demonstrates a very low cure rate of 61%[22], which is significantly lower than the cure rate after treatment with cotrimoxazole. Additionally, noncompliance that increases with the number of administrations per day [23] may limit the efficacy of these 3-day courses in daily practice. This is especially relevant in the case of nitrofurantoin, which needs be administered at least twice per day.
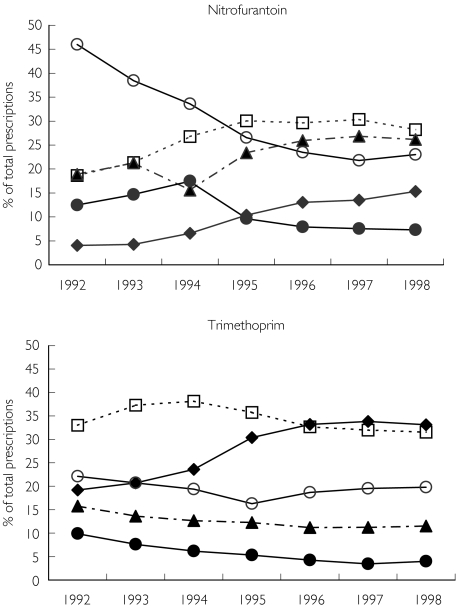
Results from the PHARMO database indicate that in more recent years GPs in the Netherlands have changed their behaviour concerning prescribing antibiotics for treatment of urinary tract infections. In particular, it was demonstrated that there has been an increase in the percentage of 3-day trimethoprim prescriptions from <20% in 1992 to 35% in 1998 and for nitrofurantoin from 4% in 1992 to 15% in 1998 (Figure 2). We believe that in most cases this change in daily practice, as suggested by the guidelines of the NHG, is reasonable. However, a GP should evaluate carefully the background of the patient, including age, history of urinary tract infections, previous hospitalizations and previous adverse effects [19]. In the case of a patient with previous hospitalization the treatment failure rate after 3 days’ nitrofurantoin treatment was 24%, while this percentage was 15% after 7 days’ trimethoprim treatment. So, in these cases it might be suggested that the GP requests antibiotic susceptibility testing after one treatment failure or after a recent hospitalization instead of the advice of the Dutch College of General Practitioners to do antibiotic susceptibility testing only after two treatment failures [3]. However, we believe that a relationship between treatment failure and antibiotic susceptibility in daily practice for these types of infections should be established more clearly before giving such advice. For these patients a GP should look for an alternative antimicrobial therapy that could decrease the chance of therapy failure.
Figure 2.
Changes in the duration (in days) of the prescriptions for nitrofurantoin and trimethoprim between 1992 and 1998. 3 days (♦); 5 days (□); 7 days (▴); >= 10 days (○); others (•)
Acknowledgments
We thank all pharmacists (U-Expo), medical specialists, and staff members of the hospitals participating the PHARMO system. We thank Dr A. L. M. Kerremans from the Elkerliek Hospital, Helmond, and Dr A. J. de Neeling from the National Institute of Public Health and the Environment, Bilthoven, for reviewing this manuscript.
References
- 1.Lipman T, Price D. Decision making, evidence, audit, and education: case study of antibiotic prescribing in general practice. Br Med J. 2000;320:1114–18. doi: 10.1136/bmj.320.7242.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norrby SR. Short-term treatment of uncomplicated lower urinary tract infections in women. Rev Infect Dis. 1990;12:458–67. doi: 10.1093/clinids/12.3.458. [DOI] [PubMed] [Google Scholar]
- 3.Wiersma TJ, Timmermans AE. [Summary of the ‘Urinary tract infections’ guideline (first revision) of the Dutch College of General Practitioners] Ned Tijdschr Geneeskd. 2001;145:735–9. [PubMed] [Google Scholar]
- 4.Boel CHE, van de Ven MJGT. Sensitivity of uropathogens in the general population of the Netherlands. Int J Antimicrob Agents. 2001;17(Suppl. 1):S94. [Google Scholar]
- 5.Overbeek BP, Overdijk CJ, Dikhoff JH, Goettsch WG. [High sensitivity of Escherichia coli to antimicrobial agents used for first-line treatment of urinary tract infections; results of an examination of feces of healthy subjects in Friesland] Ned Tijdschr Geneeskd. 2001;145:688–91. [PubMed] [Google Scholar]
- 6.Ferry S, Burman LG, Holm SE. Clinical and bacteriological effects of therapy of urinary tract infection in primary health care: relation to in vitro sensitivity testing. Scand J Infect Dis. 1988;20:535–44. doi: 10.3109/00365548809032503. [DOI] [PubMed] [Google Scholar]
- 7.Herings RM. in The Netherlands Dissertation. Utrecht: Utrecht University; 1993. PHARMO: a record linkage system for postmarketing surveillance of prescription drugs. [Google Scholar]
- 8.Herings RM, Klungel OH. An epidemiological approach to assess the economic burden of NSAID- induced gastrointestinal events in The Netherlands. Pharmacoeconomics. 2001;19:655–65. doi: 10.2165/00019053-200119060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Mantel-Teeuwisse AK, Klungel OH, Verschuren WM, Porsius AJ, de Boer A. Time trends in lipid lowering drug use in the Netherlands. Has the backlog of candidates for treatment been eliminated? Br J Clin Pharmacol. 2002;53:379–85. doi: 10.1046/j.1365-2125.2002.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Vijver DA, Roos RA, Jansen PA, Porsius AJ, de Boer A. Start of a selective serotonin reuptake inhibitor (SSRI) and increase of antiparkinsonian drug treatment in patients on levodopa. Br J Clin Pharmacol. 2002;54:168–70. doi: 10.1046/j.1365-2125.2001.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrenson RA, Logie JW. Antibiotic failure in the treatment of urinary tract infections in young women. J Antimicrob Chemother. 2001;48:895–901. doi: 10.1093/jac/48.6.895. [DOI] [PubMed] [Google Scholar]
- 12.Steinke DT, Seaton RA, Phillips G, MacDonald TM, Davey PG. Prior trimethoprim use and trimethoprim-resistant urinary tract infection: a nested case–control study with multivariate analysis for other risk factors. J Antimicrob Chemother. 2001;47:781–7. doi: 10.1093/jac/47.6.781. [DOI] [PubMed] [Google Scholar]
- 13.Steinke DT, Seaton RA, Phillips G, MacDonald TM, Davey PG. Factors associated with trimethoprim-resistant bacteria isolated from urine samples. J Antimicrob Chemother. 1999;43:841–3. doi: 10.1093/jac/43.6.841. [DOI] [PubMed] [Google Scholar]
- 14.Arstila T, Huovinen S, Lager K, Lehtonen A, Huovinen P. Positive correlation between the age of patients and the degree of antimicrobial resistance among urinary strains of Escherichia coli. J Infect. 1994;29:9–16. doi: 10.1016/s0163-4453(94)94925-5. [DOI] [PubMed] [Google Scholar]
- 15.Goettsch W, van Pelt W, Nagelkerke N, et al. Increasing resistance to fluoroquinolones in Escherichia coli from urinary tract infections in the Netherlands. J Antimicrob Chemother. 2000;46:223–8. doi: 10.1093/jac/46.2.223. [DOI] [PubMed] [Google Scholar]
- 16.Van der Velden J, De Bakker DH, Claessens AAMC, Schellevis FG. Een nationale studie naar ziekten en verrichtingen in de huisartspraktijkBasisrapport: morbiditeit in de huisartspraktijk. Utrecht: NIVEL; 1991. [Google Scholar]
- 17.Van de Lisdonk AH, Van den Bosch WJHM, Huygen FJA, Lagro-Janssen ALM. Ziekten in de huisartspraktijk. Utrecht: Wetenschappelijke Uitgeverij Bunge; 1994. [Google Scholar]
- 18.Janknegt R, Oude Lashof A, Gould IM, van der Meer JW. Antibiotic use in Dutch hospitals 1991–1996. J Antimicrob Chemother. 2000;45:251–6. doi: 10.1093/jac/45.2.251. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald TM. The economic evaluation of antibiotic therapy: relevance to urinary tract infection. J Antimicrob Chemother. 1994;33(Suppl. A):137–45. doi: 10.1093/jac/33.suppl_a.137. [DOI] [PubMed] [Google Scholar]
- 20.Beardon PH, McGilchrist MM, McKendrick AD, McDevitt DG, MacDonald TM. Primary non-compliance with prescribed medication in primary care. Br Med J. 1993;307:846–8. doi: 10.1136/bmj.307.6908.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg RN, Reilly PM, Luppen KL, Weinandt WJ, Ellington LL, Bollinger MR. Randomized study of single-dose, three-day, and seven-day treatment of cystitis in women. J Infect Dis. 1986;153:277–82. doi: 10.1093/infdis/153.2.277. [DOI] [PubMed] [Google Scholar]
- 22.Hooton TM, Winter C, Tiu F, Stamm WE. Randomized comparative trial and cost analysis of 3-day antimicrobial regimens for treatment of acute cystitis in women. JAMA. 1995;273:41–5. [PubMed] [Google Scholar]
- 23.Sclar DA, Tartaglione TA, Fine MJ. Overview of issues related to medical compliance with implications for the outpatient management of infectious diseases. Infect Agents Dis. 1994;3:266–73. [PubMed] [Google Scholar]