Abstract
Aim
The herbal medicine St. John's Wort (SJW) causes substantial decreases in the plasma concentrations of a range of co-administered drugs. Therefore, we evaluated the extent of systemic exposure to hyperforin and hypericin, two of the main constituents of SJW, in patients on admission and during hospital stay, and compared the results with known use of SJW as documented in the drug chart and detected in additional interviews.
Methods
One hundred and fifty patients aged ≥ 18 years and admitted, between August 2000 and February 2002, to an internal medicine ward of a large German university hospital were included. Hyperforin and hypericin was determined in plasma by a sensitive liquid chromotography/mass spectometry (LC/MS/MS) method. To assess undeclared use of SJW the data were compared to information obtained from drug charts and from up to three interviews that had a particular focus on intake of herbal medicines and self-medication during hospitalization.
Results
Hyperforin was detected in 12 patients (plasma concentration on the first day of hospitalization = 12–100 ng ml−1 in five patients and <3 ng ml−1 in seven), and hypericin in five patients (0.5–4.3 ng ml−1). Nine patients (6%) were taking/had taken SJW without the knowledge of the medical team and the pharmacist, who conducted the additional interviews, and 11 (7.3%) were taking/had taken SJW without the knowledge of the medical team alone. Seven of these patients were treated concurrently with drugs that can interact with SJW.
Conclusions
Unrecognized use of SJW is frequent and may have an important influence on the effectiveness and safety of drug therapy during hospital stay.
Keywords: Hypericum, self-medication, hospitalisation
Introduction
More than half of inpatient medication histories are incomplete with regard to prescription drugs [1], however the extent of medication unknown to the treating physician may be even higher, because physicians only rarely inquire about the use of over-the-counter (OTC) drugs [2]. In particular, the intake of herbal medicines may be frequently unrecognized. Jones et al. [3] found that during a routine drug history, one in seven patients did not disclose that they were taking herbal medicines. This information was revealed only in an additional interview. In another study, half of outpatients reported that their doctor or pharmacist was unaware that they were taking St John's Wort (SJW) [4]. Drug screening reveals a higher frequency of undisclosed exposure than an interview. In elderly patients hospitalized with hip fractures caused by falls, 41% tested positive for benzodiazepines, whereas only 18% indicated use by history or prescription records [5]. Similarly, 21% of patients admitted to a pain clinic did not mention at interview that they were consuming drugs [6].
Detailed information on concurrent drug use is important because exposure to unknown drugs may hamper individualization of therapy and compromise drug safety. The herbal OTC medicine SJW is a potent inducer of the human cytochrome P450 enzymes CYP3A4, CYP2C9, and CYP1A2, as well as the efflux transporter P-glycoprotein [7–9]. Consequently, SJW may cause substantial changes in dose requirements of co-administered drugs, because otherwise it may cause therapeutic failure, as exemplified by transplant graft rejections during ciclosporin treatment [10] and a case of unwanted pregnancy despite oral contraception [11], a drug interaction confirmed by two pharmacokinetic studies [12, 13]. It takes several days after discontinuation of SJW before blood concentrations of affected drugs return to pretreatment concentrations [14], therefore undeclared co-medication with SJW before hospitalization may complicate drug therapy even if intake of SJW is discontinued. Thus we evaluated the extent of undisclosed exposure to hyperforin and hypericin, the two main constituents of SJW [15], in patients on admission and during hospital stay by comparing the results of liquid chromatography/mass spectometry (LC/MS/MS) determination with information in the patients’ chart and from up to three interviews.
Methods
After approval by the Ethics Committee of the Medical Faculty of Heidelberg, 234 patients aged ≥18 years who were admitted to an internal medicine ward between August 2000 and February 2002, were asked to participate. Exclusion criteria were (1) inability to speak German or English or (2) a state of health precluding an interview and/or blood collection. One hundred and fifty patients (64%) were enrolled after giving written informed consent. All patients underwent up to three semistructured interviews by a pharmacist, the number of which depended on the length of stay on the ward. The first interview was conducted on the second day of hospitalization, in which the pharmacist solicited information on pre-admission drug history with a particular focus on intake of herbal medicines. If the patient was still on the same ward, a second interview was held on days 3–5 and a third between days 7 and 11, the subject of which was self-medication by the patient during hospitalization. To document the drug therapy as comprehensively as possible, patients were asked to specify all drugs taken before hospitalization (first interview) and on their own initiative during hospitalization (second and third interviews). In addition, a predefined list of diseases that may be treated with OTC drugs was presented to the subjects, which included headache, rheumatologic disorders, infections, allergies, insomnia, oedema of the leg, abdominal discomfort, and restlessness. Prescription data were extracted from the patients’ charts. Information was obtained on the previous drug history and drugs prescribed during hospitalization.
Blood samples were collected on the first day of hospitalization and at the time of subsequent interviews. Collection tubes (MonovetteTM/NH4+-heparinate, Sarstedt, Nuembrecht, Germany) were protected from light immediately to avoid degradation of hyperforin and hypericin [16] using brown coloured containers (ref. no. 78/898.300, Sarstedt). Centrifuged plasma was stored at −20 °C until analysis.
Plasma hyperforin and hypericin concentrations were determined by HPLC coupled to tandem mass spectrometry (LC/MS/MS), and validated according to the requirements of the FDA [17]. Plasma samples were extracted with ethyl acetate/n-hexane (7/3 v/v) in the dark, evaporated to dryness in a stream of nitrogen and reconstituted in HPLC eluent. Samples were analysed isocratically on a Kromasil RP-18 column. The analytes were detected by MS/MS in the selected reaction monitoring mode using an electrospray ion source. The lower limit of quantification (LOQ) was 0.035 ng ml−1 for hyperforin and 0.05 ng ml−1 for hypericin. The accuracy of the method varied between −4.4% and +10.6% and the inter-assay precision (coefficient of variation) ranged from 5% to 11% for both analytes.
Results
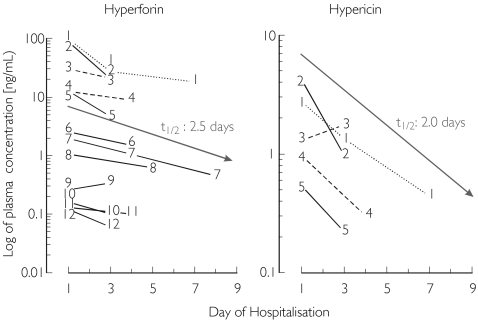
Forty-seven women and 103 men with a mean age of 60 ± 13 years (mean ± SD; range: 19–85) were included in the study. Twelve patients were positive for hyperforin and/or hypericin (Figure 1). One of these patients indicated that he was taking SJW (two tablets per day of Jarsin®, Lichtwer Pharma GmbH, Berlin, Germany, containing 300 mg SJW dry extract for over 1 year) in both the routine drug history and the interview, and two admitted taking SJW in the interview only (patient 3: one tablet per day Johanniskraut forte containing 200 mg SJW dry extract for 6 months; patient 4: one tablet per day of Felis®, Biocur Arzneimittel GmbH, Barleben, Germany, 650 containing 650 mg SJW dry extract for 1 year and Hyperforat®, Dr. Gustav Klein, Zell an Harmersbach, Germany, containing 40 mg SJW at an unknown dosage). Thus, nine out of 150 (6%) subjects were taking SJW without the knowledge of the research team, and 11 (7.3%) were taking SJW without the knowledge of the medical team treating the patient. Seven of these patients were also being treated with drugs whose plasma concentrations may be expected to be decreased by SJW (Table 1).
Figure 1.
Plasma concentrations in 12 patients out of 150 admitted to an internal medicine ward, who tested positive for hyperforin and/or hypericin, the two main constituents of the herbal medicine St. John's Wort (SJW). Patient 1 had admitted taking SJW during both a routine drug history and interviews by a pharmacist inquiring about drug history before and during hospitalization, with a particular focus on intake of herbal medicines and self-medication. Patients 3 and 4 had admitted taking SJW in the interviews only. No other patient reported recent intake of SJW
Table 1. Co-medication of drugs whose metabolism or P-glycoprotein-mediated transport can be induced by SJW, in patients with detectable hyperforin and/or hypericin in their plasma.
| Hyperforin plasma concentration [ng ml−1] (day of hospitalization) | Hypericin plasma concentration [ng ml−1] (day of hospitalization) | ||||||
|---|---|---|---|---|---|---|---|
| Patient | Drugs | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 |
| 3 | Amlodipine (C), atorvastatin (C) | 30.2 (1) | 21.0 (3) | – | 1.3 (1) | 1.8 (3) | – |
| 6 | Diltiazem (C), theophylline (A: case report + B1) | 2.6 (1) | 1.5 (4) | – | <LOQ (1) | <LOQ (4) | – |
| 7 | Atorvastatin (C) | 2.0 (1) | 1.1 (4) | 0.5 (9) | <LOQ (1) | <LOQ (4) | <LOQ (9) |
| 8 | Atorvastatin (C), conjugated oestrogens (A), Diazepam (B1 + B2) | 1.0 (1) | 0.6 (5) | – | <LOQ (1) | <LOQ (5) | – |
| 9 | Atorvastatin (C), diazepam (B1 + B2) | 0.3 (1) | 0.3 (3) | – | <LOQ (1) | <LOQ (3) | – |
| 10 | Simvastatin (A), amlodipine (C) | 0.2 (1) | 0.1 (3) | – | <LOQ (1) | <LOQ (3) | – |
| 11 | Diazepam (B1 + B2) | 0.1 (1) | 0.1 (3) | – | <LOQ (1) | <LOQ (3) | – |
< LOQ = below limit of quantification (0.05 ng ml−1); –= discharge of the patient from model ward before third sampling time; A = interaction established between this drug and SJW; B1 = interaction established between this drug and rifampicin; B2 = interaction established between other representatives of this chemical and SJW; C = substrate of CYP enzymes or P-glycoprotein that are induced by SJW.
Of the patients who tested negative for both hyperforin and hypericin, one patient admitted to taking SJW some time before admission, however she could not remember the time of the last intake. All the other patients who tested negative did not mention intake of SJW either in the routine drug history or in any of the interviews. No patients were prescribed SJW during hospitalization. Accordingly there were no false negative results.
Discussion
Substantial dose increases of substrates of CYP3A4 and P-glycoprotein are required to compensate for the marked pharmacokinetic changes induced by concurrent use of SJW. Although additional ingredients of SJW may contribute to these interactions in vitro evidence convincingly suggests the involvement of hypericin as an inducer of P-glycoprotein [18] and hyperforin as a potent ligand of the pregnane X receptor [19], which is a key transcriptional regulator of important ABC drug transporters and CYP3A4 [20]. In vivo, a relevant part of the interaction can be explained by hyperforin [21]. We thus sought to determine both hyperforin and hypericin. In the present study, every 14th patient admitted to an internal medicine ward was exposed to hyperforin that was not detected from a routine drug history. Even if an additional interview was performed by a pharmacist, every 17th patient had hyperforin present that was unrecognized by the medical team.
In addition, seven of the 11 patients with exposure to SJW that was unknown to the medical team were treated with drugs known to interact with SJW. In the two patients with hyperforin plasma concentrations of more than 10 ng ml−1, hypericin was also detectable. These concentrations are roughly comparable to those observed in the patients with known intake of SJW before hospitalization, suggesting recent or continued use of the herbal remedy. In vitro, the half-maximally effective concentration for activation of the pregnane X receptor by hyperforin is 12 ng ml−1[19], a value similar to the plasma concentrations found in several of our patients. However, because SJW is administered orally, intraluminal and portal venous concentrations are expected to be substantially higher than the corresponding systemic steady state concentrations [22]. Thus, local concentrations at the major sites of interaction (the gut and liver) are likely to reach those required for induction.
In most patients plasma hyperforin and hypericin concentrations were falling, which indicates that SJW intake was not continued during hospitalization. Because the concentration of hyperforin was not decreasing in patient 9, continued intake of SJW cannot be excluded. The patient did not admit to self-medication at interview. Based on published maximal concentrations, half-lives [23–27], and the LOQ of our method, hypericin may be detected up to about 12 days and hyperforin about 5 days after discontinuation of SJW.
Hyperforin concentration measured on the first day of admission varied by a factor of 800. This might be explained by (1) different time spans between the last intake of SJW and hospitalization, (2) use of different commercially available products containing variable amounts of hyperforin [28], and (3) consumption of teas containing SJW, but hypericum containing teas are not available in our hospital. Finally (4), hyperforin and/or hypericin can be added to food and beverages as flavouring agents [29]. However, the achievement of significant tissue concentrations of these compounds is unlikely, because only very small amounts have been found in foods to which SJW was added [30].
No attempt was made to demonstrate induction of CYP3A and to measure the activities of CYP and ABC drug transporters. However, SJW has been shown by others to induce CYP3A activity consistently [31].
In conclusion, this study has revealed that self-medication with nonprescription medicines may remain undisclosed even after several attempts by trained people to complete drug histories. This finding suggests that many patients are taking medication that their physicians are unaware of. The potential for drug interactions in these circumstances may have an important influence on both the effectiveness and safety of drug therapy.
Acknowledgments
We are grateful to Monika Maurer for her excellent technical assistance. This study was supported by BMBF grant #01EC9902 from the German Federal Ministry for Education and Research.
References
- 1.Lau HS, Florax C, Porsius AJ, De Boer A. The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br J Clin Pharmacol. 2000;49:597–603. doi: 10.1046/j.1365-2125.2000.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hocking G, deMello WF. Taking a ‘drugs’ history. Anaesthesia. 1997;52:904–5. doi: 10.1111/j.1365-2044.1997.197-az0332.x. [DOI] [PubMed] [Google Scholar]
- 3.Jones HA, Metz JM, Devine P, Hahn SM, Whittington R. Rates of unconventional medical therapy use in patients with prostate cancer: standard history versus directed questions. Urology. 2002;59:272–6. doi: 10.1016/s0090-4295(01)01491-1. [DOI] [PubMed] [Google Scholar]
- 4.Beckman SE, Sommi RW, Switzer J. Consumer use of St. John's Wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy. 2000;20:568–74. doi: 10.1592/phco.20.6.568.35152. [DOI] [PubMed] [Google Scholar]
- 5.Schwab M, Roder F, Ammon S, Thon KP, Mörike K. Increased number of hip fractures. Lancet. 1999;353:2160. doi: 10.1016/S0140-6736(05)75600-2. [DOI] [PubMed] [Google Scholar]
- 6.Berndt S, Maier C, Schütz HW. Polymedication and medication compliance in patients with chronic non-malignant pain. Pain. 1993;52:331–9. doi: 10.1016/0304-3959(93)90167-N. [DOI] [PubMed] [Google Scholar]
- 7.Henderson L, Yue QY, Bergquist C, Gerden B, Arlett P. St John's Wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54:349–56. doi: 10.1046/j.1365-2125.2002.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dürr D, Stieger B, Kullak-Ublick GA, et al. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 9.Hennessy M, Kelleher D, Spiers JP, et al. St John's Wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol. 2002;53:75–82. doi: 10.1046/j.0306-5251.2001.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruschitzka F, Meier PJ, Turina M, Luscher TF, Noll G. Acute heart transplant rejection due to Saint John's Wort. Lancet. 2000;355:548–9. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz UI, Büschel B, Kirch W. Unwanted pregnancy on self-medication with St John's Wort despite hormonal contraception. Br J Clin Pharmacol. 2003;55:112–3. doi: 10.1046/j.1365-2125.2003.t01-1-01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall SD, Wang Z, Huang SM, et al. The interaction between St John's Wort and an oral contraceptive. Clin Pharmacol Ther. 2003;74:525–35. doi: 10.1016/j.clpt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Pfrunder A, Schiesser M, Gerber S, Haschke M, Bitzer J, Drewe J. Interaction of St John's Wort with low-dose oral contraceptive therapy: a randomized controlled trial. Br J Clin Pharmacol. 2003;56:683–90. doi: 10.1046/j.1365-2125.2003.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandelbaum A, Pertzborn F, Martin-Facklam M, Wiesel M. Unexplained decrease of cyclosporin trough levels in a compliant renal transplant patient. Nephrol Dial Transplant. 2000;15:1473–4. doi: 10.1093/ndt/15.9.1473. [DOI] [PubMed] [Google Scholar]
- 15.Barnes J, Anderson LA, Phillipson JD. St John's Wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53:583–600. doi: 10.1211/0022357011775910. [DOI] [PubMed] [Google Scholar]
- 16.Bilia AR, Bergonzi MC, Morgenni F, Mazzi G, Vincieri FF. Evaluation of chemical stability of St. John's Wort commercial extract and some preparations. Int J Pharm. 2001;213:199–208. doi: 10.1016/s0378-5173(00)00660-8. [DOI] [PubMed] [Google Scholar]
- 17.Shah VP, Midha KK, Dighe S, et al. Analytical methods validation: Bioavailability, bioequivalence and pharmacokinetic studies. J Pharm Sci. 1992;81:309–12. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- 18.Perloff MD, von Moltke LL, Stormer E, Shader RI, Greenblatt DJ. Saint John's Wort: an in vitro analysis of P-glycoprotein induction due to extended exposure. Br J Pharmacol. 2001;134:1601–8. doi: 10.1038/sj.bjp.0704399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore LB, Goodwin B, Jones SA, et al. St. John's Wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–90. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 21.Bauer S, Mai I, Johne A, Uehleke B, Roots I. Effect of hypericum extracts with different hyperforin content on the pharmacokinetics of cyclosporin A. Int J Clin Pharmacol Ther. 2003;41:557. [Google Scholar]
- 22.von Moltke LL, Greenblatt DJ, Schmider J, Wright CE, Harmatz JS, Shader RI. In vitro approaches to predicting drug interactions in vivo. Biochem Pharmacol. 1998;55:113–22. doi: 10.1016/s0006-2952(97)00239-6. [DOI] [PubMed] [Google Scholar]
- 23.Staffeldt B, Kerb R, Brockmoller J, Ploch M, Roots I. Pharmacokinetics of hypericin and pseudohypericin after oral intake of the hypericum perforatum extract LI 160 in healthy volunteers. J Geriatr Psychiatry Neurol. 1994;7(Suppl 1):S47–53. doi: 10.1177/089198879400700113. [DOI] [PubMed] [Google Scholar]
- 24.Kerb R, Brockmoller J, Staffeldt B, Ploch M, Roots I. Single-dose and steady-state pharmacokinetics of hypericin and pseudohypericin. Antimicrob Agents Chemother. 1996;40:2087–93. doi: 10.1128/aac.40.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson JM, Feinman L, Liebes L, Ostrow N, Koslowski V, Tobia A, Cabana BE, Lee D, Spritzler J, Prince AM. Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John's Wort plant, in patients with chronic hepatitis C virus infection. Antimicrob Agents Chemother. 2001;45:517–24. doi: 10.1128/AAC.45.2.517-524.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biber A, Fischer H, Romer A, Chatterjee SS. Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers. Pharmacopsychiatry. 1998;31(Suppl 1):36–43. doi: 10.1055/s-2007-979344. [DOI] [PubMed] [Google Scholar]
- 27.Agrosi M, Mischiatti S, Harrasser PC, Savio D. Oral bioavailability of active principles from herbal products in humans. A study on Hypericum perforatum extracts using the soft gelatin capsule technology. Phytomedicine. 2000;7:455–62. doi: 10.1016/S0944-7113(00)80029-X. [DOI] [PubMed] [Google Scholar]
- 28.Wurglics M, Westerhoff K, Kaunzinger A, et al. Comparison of German St. John's Wort products according to hyperforin and total hypericin content. J Am Pharm Assoc. 2001;41:560–6. doi: 10.1016/s1086-5802(16)31280-3. [DOI] [PubMed] [Google Scholar]
- 29.Council Directive 88/388/EEC of 22 June 1988 on the approximation of the laws of the Member States relating to flavourings for use in foodstuffs and to source materials for their production. [on 21 March 2004]. Available from: URL: http://europa.eu.International/comm/food/fs/sfp/addit_flavor/flav09_en.pdf.
- 30.Ang CY, Cui Y, Chang HC, Luo W, Heinze TM, Lin LJ, Mattia A. Determination of St. John's Wort components in dietary supplements and functional foods by liquid chromatography. J AOAC Int. 2002;85:1360–9. [PubMed] [Google Scholar]
- 31.Sugimoto K, Ohmori M, Tsuruoka S, et al. Different effects of St John's Wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther. 2001;70:518–24. doi: 10.1067/mcp.2001.120025. [DOI] [PubMed] [Google Scholar]