Abstract
Objective
To evaluate the influence of renal impairment on the pharmacokinetics of desmopressin.
Methods
Twenty-four subjects were enrolled in the study, 18 with varying degrees of renal impairment and six healthy volunteers. Each subject received a single intravenous dose of 2 µg desmopressin. Blood and urine samples were collected for 24 h and assayed for desmopressin by radioimmunoassay. Plasma concentrations and the amounts of desmopressin excreted in the urine were analysed simultaneously by use of mixed effects modelling.
Results
Only mild adverse events were observed. Both the renal and the nonrenal clearance of desmopressin were found to vary with the creatinine clearance (CrCL). A decrease of 1.67% in the CrCL (corresponding to 1 ml min−1 from 60 ml min−1) was found to cause a 1.74% decrease in the renal clearance and a 0.93% decrease in the nonrenal clearance. The fall in renal clearance caused the amount of desmopressin excreted in urine to decrease from 47% in healthy subjects to 21% in the patients with severe renal impairment. The mean systemic clearance of desmopressin was 10 litres h−1 in healthy subjects and 2.9 litres h−1 in patients with severe renal impairment (difference −7.5 litres h−1, 95% CI [−11; −4.3] litres h−1). Correspondingly, the mean terminal half-life, was 3.7 h in healthy subjects and 10 h in patients with severe renal impairment (difference 6.7 h, 95% CI [4.0; 9.4] h).
Conclusion
Although desmopressin appears to be safe and well-tolerated by patients with impaired renal function, great caution should be exercised when titrating towards an efficient dosage regimen if patients with moderately or severely impaired renal function are to be treated with desmopressin at all.
Keywords: desmopressin, DDAVP, renal impairment, pharmacokinetics, urinary excretion, NONMEM, nonlinear mixed effects
Introduction
Vasopressin is a natural pituitary hormone with antidiuretic properties. The deamination of vasopressin at the N-terminal 1 position and replacement of 8-L-aginine results in a structural analogue called desmopressin. This drug acts on the V2 receptors located in the collecting ducts of the kidney. The desmopressin–V2 receptor complex initiates a cascade of intracellular events resulting in water channel proteins (the aqauporins) moving from the intracellular reservoir to the apical cell membrane, thereby increasing water reabsorption [1]. These events result in decreased urine production by the kidneys and hence decreased diuresis. Desmopressin has for many years been the treatment of choice for patients with central diabetes insipidus. In addition, desmopressin is the drug of choice for children with primary nocturnal enuresis [2–5].
Nocturia is a major contributor to a reduced quality of life in the elderly population [6]. It is well known that total urine output over 24 h does not change with increasing age [7, 8], but the circadian rhythm of urine output is altered in many elderly persons [9]. In the daytime, young adults excrete urine at a rate twice as high as during the night, whereas the day and night-time output in elderly people is similar [7]. Therefore, the increased need in the elderly population to void during the night is most likely to be caused by the urine production exceeding bladder capacity. It has been reported that more than 40% of 65–80 year olds have to get up twice or more during the night in order to void [10].
Desmopressin has been demonstrated to be an effective treatment of nocturia [11–15], and side-effects have only rarely been reported [16, 17]. However, a relatively large proportion of elderly people may have reduced renal function [18]. Previous reports have demonstrated that after intravenous (iv) dosing, 39% of desmopressin is excreted via the urine, whereas after an oral dose 65% of absorbed drug was found to be renally eliminated [19]. These results suggest that impaired renal function may change the pharmacokinetics of desmopressin, thereby increasing the risk of adverse events.
Only a few studies have investigated the pharmacokinetics of desmopressin in subjects with renal impairment [20, 21]. However, none of the studies present data on the renal excretion of desmopressin. The objectives of the present study were to: (i) investigate the correlation between creatine clearance (CrCL) and the renal clearance of desmopressin in populations ranging from healthy subjects to severely renally impaired patients, and (2) to compare the urinary excretion of a single iv dose of desmopressin in subjects with different degrees of renal impairment and healthy subjects.
Methods
Study design
The present study had a prospective, open-labelled, controlled, single intravenous administration design. Twenty-four subjects were enrolled, 18 with varying degrees of renal impairment and six with normal renal function. Their age ranged from 49 to 68 years, their body mass index (BMI) from 23 to 32 kg m−2, and their height from 152 to 184 cm. Subjects, none of whom were in dialysis, were stratified on the basis of their renal function to one of four groups: group 1 with normal renal function, group 2 with mild renal impairment, group 3 with moderate renal impairment and group 4 with severely impaired renal function. The groups were studied sequentially (beginning with group 2).
All subjects received a single iv dose of 2 µg desmopressin acetate (0.5 ml, 4 µg ml−1) given as an injection over 1 min. The maximum fluid intake allowed was 2 litres within the first 24 h post dosing. No other nutritional food restrictions were imposed in the study.
The protocol was approved by the Schwaben Independent Ethics Committee and the Bayerische Landesärztekammer (BLÄK) and conducted in accordance with the Helsinki Declaration [22] and ICH guidelines of Good Clinical Practice. Subjects gave written informed consent after they were given a full explanation of the procedure.
Protocol
Blood samples (7–10 ml) were drawn before dosing and at 5, 15, 30, 45 min and 1, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h after dosing. Blood samples, which were collected in 7–10 ml Vacutainer® tubes (Becton Dickinson, Franklin Lakes, NJ, USA), containing 0.05 ml K3EDTA, 0.38 mol l−1: 17% as an anticoagulant, were centrifuged within 45 min for 10 min at 1000 g. Plasma was separated and stored frozen (−70°C) pending analysis.
Urine was collected over the following time intervals: 0–3, 3–6, 6–9, 9–12, 12–15, 15–18, 18–21 and 21–24 h after dosing. Urine was stored at 2–8°C during each 3-h collecting period. After the volume was measured, an aliquot of approximately 50 ml was frozen within 60 min of collection and stored frozen (−70°C) pending analysis.
Analytical procedures
The methods used for the analysis of desmopressin were modified from that described by Lundin et al. [23]. The proteins in plasma samples (1 ml) were precipitated by the addition of ice-cold acetone (2 ml) followed by vortex mixing. After centrifugation (1800 g, 15 min at 2–8°C), the supernatant was extracted with 2 ml ice-cold petroleum. After centrifugation (250 g, 10 min at 2–8°C), the organic phase was discarded and the aqueous phase was repeatedly extracted. The aqueous phase was then evaporated to dryness (Savant Speedvac concentrator, Farmingdale, NY) and reconstituted in 0.5 ml buffer (0.1 m phosphate buffer, 0.05 m NaCl, 0.1% HAS, 0.01% Triton X-100). Duplicate assays were performed using a competitive radioimmunoassay based on 125I-labelled desmopressin as tracer. After a 48-h incubation period at 4°C, the bound fraction was separated from the free fraction using a charcoal suspension. The supernatant was counted for 5 min in a gamma-counter and the plasma concentration was calculated from a calibration curve obtained from the assay of 11 calibration samples prepared by spiking human blank plasma with desmopressin. The lower limit of quantification (LLOQ) was 2.5 pg ml−1 plasma. The inter-assay precision of the method, expressed as the coefficient of variation, was 18% at LLOQ and less that 11% at all other concentrations up to 100 pg ml−1. The accuracy, expressed as bias, was within 2% over the analytical range. The cross-reactivity of the method to various peptides was 0.03%.
Urine samples were assayed using solid phase extraction (SPE) prior to radioimmunoassay. Urine (0.5 ml), acidified and diluted with 2 ml 0.1 m HCl, was loaded on to a pre-solvated (1 ml methanol + 1 ml water) 100 mg Isolute™ C8 (IST, Mid Glamorgan, UK) SPE column. The column was washed with acetonitrile: 0.1% TFA in water (10:90) (2 ml) and desmopressin was eluted using acetonitrile: 0.1% TFA in water (60:40) (2 × 0.5 ml). After evaporation (to dryness) and reconstitution in 0.5 ml, desmopressin was determined by a radioimmunoassay procedure similar to that for plasma. The LLOQ was 20 pg ml−1 urine. The inter-assay precision of the method, expressed as the coefficient of variation, was less than 12% between 20 and 36 000 pg ml−1. The accuracy, expressed as bias, was within 15% over the same range. The specificity of the method was tested by the analysis of blank urine samples from six individuals. No desmopressin was detected in any of the samples.
Data analysis
Creatinine clearance was assessed from a 24-h urine collection period, using the following equation:
where Crurine = the creatinine concentration in urine (mg dl−1) [24]; UV = urine volume (ml); Δt = time interval of urine collection (min); BS = body surface (m2) calculated from the Gehan and George formula [25]; Cr = the creatinine concentration in serum (mg dl−1) [24]; 1.73 = correction factor for the normal body surface (m2).
The pharmacokinetic parameters for desmopressin were determined by nonlinear mixed effect modelling using the NONMEM program, version V [26], and the Fortran compiler Compaq Visual Fortran version 6.5. In the model-building process, the first-order (FO) estimation method was used initially, whereas in the final stage of model development, including final covariate selection, the conditional first-order method (FOCE) with the interaction option was used. Several models with a different number of compartments and input functions were tested. The clearance was modelled by splitting it into renal and nonrenal. Plasma and urine data were fitted simultaneously. Interindividual variability was modelled by an exponential error model and covariances between parameters were considered. Combined additive and proportional intra-individual error models were used separated for plasma and urinary data. Interindividual variability in the residual error magnitude as previously described [27] was included separately for plasma and urine data. The goodness of fit was evaluated by graphic analysis of predicted vs. observed concentrations (distribution of the points around the unity line), and by weighted residuals vs. predicted concentrations using Xpose 3.0 [28]. Individual parameter values were obtained as empirical Bayes estimates from the final population model. The objective function values were also compared.
In the first step, individual estimates of the pharmacokinetic parameters were obtained as empirical Bayes estimates based on a NONMEM fit using no covariates. In the second step, the individual pharmacokinetic parameter estimates were regressed on the covariates (CrCL, age, body weight, BMI and height) using a variety of models (linear, log-linear and sigmoidal relationships). The covariate resulting in the largest improvement in the population model was included in the model. After inclusion of the first covariate, the above procedure was repeated. The new set of individual random effect parameters was plotted against the available covariates, and their relationship evaluated by use of nonlinear regression methods. The covariates resulting in the largest improvement of the population model were then included in the model, and the process was repeated.
Results
No subjects were withdrawn due to adverse events, of which there were no serious or life-threatening episodes. Five subjects had a total of seven adverse events, but only two were considered to be possibly related to the study medication, these being headache and weight increase. The remaining adverse events were deemed as ‘unlikely to be related’ or ‘unrelated’ to the study medication. Several of the subjects were receiving concomitant medication, the most frequent of which was for the treatment of hypertension. None of the co-administered drugs was considered to have influence on the pharmacokinetics of desmopressin. The liver function of the patients studied was within the normal range. Thus, the mean ALTs from groups 1 (healthy), 2, 3 and 4 (severely impaired) were found to be 9, 11, 14 and 13 U litre−1, respectively.
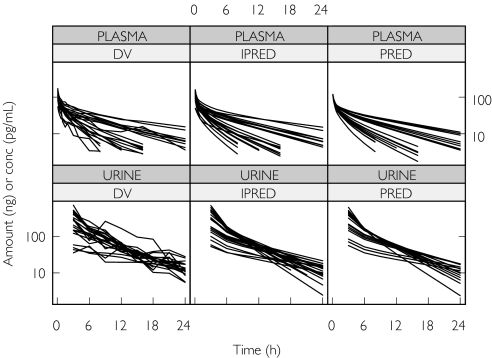
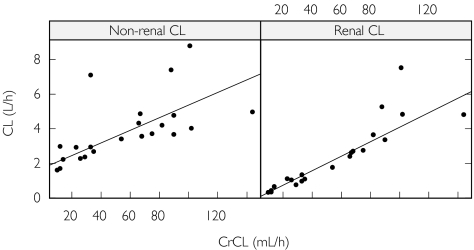
Two subjects were excluded from the pharmacokinetic analysis because they both had a plasma concentration vs. time profile that was incompatible with the route of administration (i.e. an absorption phase was observed in both cases, indicating extravascular administration). After iv administration of desmopressin, concentration plasma vs. time curves were best described by a three-compartmental model. Its parameters include the intercompartment flow rates (Q2 and Q3), the volumes of the central and the peripheral compartments (V1, V2 and V3), renal clearance (CLr) and nonrenal clearance (CLnr). The amount excreted in urine during any time interval was given by CLr multiplied by the area under the plasma concentration–time profile for the interval. Thus, it was possible to fit the plasma concentrations and the amounts excreted in urine simultaneously. The interindividual variability in the pharmacokinetic parameters could be divided into separate values for the clearance components and a single term for V1 and V3, which was positively correlated with the interindividual variability in nonrenal clearance. The intra-individual variation was best described by a combined additive and proportional error model for both the plasma concentration vs. time data and the amount excreted in the urine. Interindividual variability in residual error magnitude was significant for both plasma and urine data. Both the observed plasma concentrations and the amount excreted in the urine were well described by the model (Figure 1). Several covariate relationships were tested, but the only two that significantly improved model fit were the CrCL as a covariate on both Clr and on Clnr(Figure 2). A decrease of 1 ml min−1 (1.67%) in the CrCL at 60 ml min−1, was found to cause a decrease of 1.74% in the renal clearance and a decrease of 0.933% in the nonrenal clearance (Table 1).
Figure 1.
Goodness of fit plots after modelling the desmopressin plasma concentration vs. time data (upper panels) and the amount of desmopressin excreted in the urine (lower panels). The left panels (DV) are the observed values, the middle panels (IPRED) the individual predicted values and the right panels (PRED) are the population predictions
Figure 2.
Relationships between creatinine clearance (CrCL) and nonrenal clearance and renal clearance. The solid line defines the relationships estimated by the model
Table 1. Population pharmacokinetic parameters after intravenous administration of desmopressin to healthy subjects and subjects with varying degrees of renal impairment.
| Parameters | IIV | |||
|---|---|---|---|---|
| Estimate | RSE% | CV% | RSE%‡ | |
| CLr (litres h−1)* | 2.41 | 7 | 24 | 58 |
| CLnr (litres h−1)* | 3.91 | 7 | 30§ | 43 |
| V1 (litres) | 13.9 | 7 | 31§ | 45 |
| V2 (litres) | 12.5 | 8 | – | – |
| V3 (litres) | 11.7 | 8 | 31§ | 45 |
| Q2 (litres h−1) | 4.43 | 21 | – | – |
| Q3 (litres h−1) | 27.5 | 19 | – | – |
| CrCL effect on CLr (%)† | 1.74 | 2 | – | – |
| CrCL effect on CLnr (%)† | 0.933 | 12 | – | – |
| Add. Error on Cp (pg ml−1) | 0.27 | 59 | 43 | 34 |
| Prop. error on Cp (%) | 29 | 10 | 43 | 34 |
| Add. Error on urine (pg) | 4140 | 21 | 65 | 50 |
| Prop. error on urine (%) | 47 | 21 | 65 | 50 |
The renal and nonrenal clearance is estimated for a CrCL of 60 ml min−1 (corresponding to the median of the population).
Percentage change in CLr or CLnr per 1 ml change in CrCL.
Based on the corresponding variance estimate.
Estimated correlation between V1/V3 and CLnr was 0.86. CLr = renal clearance; CLnr = non-renal clearance; V1 = volume of the central compartment; V2 = volume of the peripheral compartment; V3 = volume of the peripheral compartment; Q2 = inter-compartmental flow between V1 and V2; Q3 = inter-compartmental flow between V1 and V3; Cp = plasma concentration; IIV = interindividual variability; RSE% = relative standard error; Add = additive error; Prop = proportional error.
Based on the quartiles for the CrCL, subjects were divided into four groups to compare the change in the pharmacokinetic parameters with that in the CrCL (Table 2). Desmopressin was rapidly distributed throughout the body with an initial half-life (t1/2λ1) of 0.14–0.18 h, and a second distribution half-life (t1/2λ2) of 1.0–1.3 h (Table 2). The distribution phases were not affected by changes in the CrCL. The fraction of drug excreted unchanged in the urine (fe) decreased with increasing renal impairment, resulting in a mean value of 47% in healthy subjects, dropping to 21% in the group with the lowest CrCL (Table 2). The elimination half-life, was 3.7 h in the group representing the upper quartile of the CrCL distribution, and 10 h in those in the lower quartile of the CrCL distribution.
Table 2. Individual and mean pharmacokinetic parameters after intravenous administration of 2 µg desmopressin to healthy subjects and to patients with varying degrees of renal impairment. The subjects were divided into four groups, based on the quantiles of the creatinine clearance (CrCL).
| ID | CrCL (ml min−1) | CL (litres h−1) | CLr (litres h−1) | Vss (litres) | t1/2( λ1) (h) | t1/2( λ2) (h) | t1/2(λ3) (h) | fe |
|---|---|---|---|---|---|---|---|---|
| Group 1 | ||||||||
| 24 | 88 | 13 | 5.3 | 46 | 0.18 | 1.2 | 3.5 | 0.42 |
| 5 | 90 | 8.1 | 3.4 | 34 | 0.12 | 1.0 | 4.0 | 0.41 |
| 17 | 90 | 7.0 | 3.4 | 33 | 0.12 | 1.0 | 4.3 | 0.48 |
| 23 | 101 | 16 | 7.5 | 51 | 0.20 | 1.2 | 3.1 | 0.46 |
| 21 | 102 | 8.8 | 4.8 | 31 | 0.10 | 0.86 | 3.6 | 0.55 |
| 19 | 144 | 9.8 | 4.8 | 35 | 0.13 | 0.99 | 3.6 | 0.49 |
| Mean | 103 | 10 | 4.9 | 38 | 0.14 | 1.0 | 3.7 | 0.47 |
| SD | 21 | 3.4 | 1.5 | 8.1 | 0.04 | 0.13 | 0.41 | 0.05 |
| CV% | 21 | 33 | 32 | 21 | 28 | 13 | 11 | 11 |
| Group 2 | ||||||||
| 3 | 66 | 6.7 | 2.4 | 46 | 0.19 | 1.3 | 5.6 | 0.36 |
| 2 | 67 | 7.5 | 2.6 | 43 | 0.17 | 1.2 | 4.8 | 0.35 |
| 1 | 68 | 6.2 | 2.7 | 38 | 0.14 | 1.2 | 5.1 | 0.43 |
| 4 | 75 | 6.5 | 2.7 | 31 | 0.10 | 0.94 | 4.4 | 0.43 |
| 6 | 82 | 7.8 | 3.6 | 34 | 0.12 | 1.0 | 4.1 | 0.47 |
| Mean | 72 | 6.9 | 2.8 | 38 | 0.15 | 1.1 | 4.8 | 0.41 |
| SD | 6.8 | 0.68 | 0.48 | 6.3 | 0.04 | 0.2 | 0.59 | 0.05 |
| CV% | 10 | 10 | 17 | 16 | 24 | 14 | 12 | 12 |
| Group 3 | ||||||||
| 8 | 29 | 3.1 | 0.76 | 33 | 0.12 | 1.1 | 8.2 | 0.24 |
| 7 | 33 | 8.4 | 1.3 | 80 | 0.38 | 1.7 | 7.3 | 0.16 |
| 12 | 33 | 3.9 | 0.96 | 36 | 0.14 | 1.2 | 7.3 | 0.25 |
| 14 | 35 | 3.8 | 1.1 | 38 | 0.15 | 1.2 | 7.7 | 0.29 |
| 9 | 54 | 5.2 | 1.8 | 33 | 0.12 | 1.1 | 5.4 | 0.34 |
| Mean | 37 | 4.9 | 1.2 | 44 | 0.18 | 1.3 | 7.2 | 0.26 |
| SD | 9.9 | 2.1 | 0.38 | 20 | 0.11 | 0.2 | 1.1 | 0.07 |
| CV% | 27 | 43 | 33 | 46 | 62 | 19 | 15 | 26 |
| Group 4 | ||||||||
| 16 | 10 | 1.9 | 0.32 | 42 | 0.17 | 1.4 | 16 | 0.17 |
| 18 | 12 | 2.1 | 0.35 | 30 | 0.10 | 1.1 | 11 | 0.17 |
| 22 | 12 | 3.4 | 0.42 | 47 | 0.20 | 1.4 | 10 | 0.12 |
| 15 | 14 | 2.9 | 0.65 | 32 | 0.12 | 1.1 | 8.8 | 0.23 |
| 11 | 23 | 4.0 | 1.1 | 42 | 0.17 | 1.3 | 8.1 | 0.27 |
| 13 | 26 | 3.3 | 1.0 | 36 | 0.14 | 1.2 | 8.4 | 0.31 |
| Mean | 16 | 2.9 | 0.65 | 38 | 0.15 | 1.3 | 10 | 0.21 |
| SD | 6.6 | 0.82 | 0.35 | 6.4 | 0.04 | 0.13 | 2.9 | 0.07 |
| CV% | 41 | 28 | 54 | 17 | 24 | 10 | 28 | 34 |
CrCL = creatinine clearance; CL = clearance; CLr = renal clearance; Vss = volume of distribution at steady state; t1/2λ1 = half-life for the first distribution phase; t1/2λ2 = half-life for the second distribution phase; t1/2λ3 = half-life for the terminal phase; fe = fraction of the dose excreted in the urine.
Discussion
Desmopressin was well tolerated by all the subjects. The pharmacokinetics of desmopressin was best described by a three-compartmental model. In previous reports, a two-compartmental model has best described the data in healthy subjects [29]. In renally impaired patients, the pharmacokinetics have been described by both a two- and three-compartmental model [21]. In vitro investigations in human kidney microsomes and crude kidney homogeneate have shown that desmopressin is not metabolized by this tissue, suggesting that it is mainly metabolized in the liver prior to biliary excretion [30]. The renal excretion of desmopressin has been reported to be 39% of an iv dose of desmopressin, whereas 65% of the absorbed fraction of an oral dose is eliminated via the urine [19]. These results imply that renal function may be important in the elimination of desmopressin.
As anticipated, the renal clearance of desmopressin was found to vary with creatinine clearance, however the nonrenal clearance of the drug was also found to vary with creatinine clearance. The reason for this is not clear, but an association between renal impairment and decreased hepatic metabolism has been observed in animal models. These reports suggest that hepatic function may be altered by renal function, because of an accumulation of uraemic toxins that may inhibit hepatic drug metabolism [31, 32]. Recent results from clinical studies have also suggested a correlation between renal function and hepatic function. Thus, patients with end-stage renal disease had an increased risk of toxicity from the drugs that were metabolized predominantly in the liver [33].
Previous reports have described the pharmacokinetics of desmopressin in renally impaired patients. In patients with severe kidney disease the clearance was found to be 1.62 litres h−1 and the half-life 9.7 h [21]. These values are similar to ours in the patients with the lowest CrCL. In another study in eight uraemic patients on chronic haemodialysis due to end-stage renal failure, the clearance of desmopressin was 5.88 litres h−1 and the half-life 3.3 h [20]. These values differ from those reported in the present study and by Ruzicka and colleagues [21], the most likely explanation being differences in the sensitivity of the assay for desmopressin.
The decrease in renal function resulted in a considerable (240%) increase in the terminal half-life (t1/2λ3) of desmopressin. Renal clearance is only partly responsible for desmopressin elimination, thus its total clearance will be less affected by renal function. The adjustment of the dose of renally excreted drugs is most important for those that have a narrow therapeutic range. In general, dosage reduction is recommended for patients in whom systemic clearance is reduced by 50%[34], because plasma concentrations are inversely correlated with this parameter. Desmopressin does not have a narrow therapeutic range, but adverse events may occur with patients at high doses of desmopressin, leading to prolonged antidiuretic effects (and increasing the risk of hyponatraemia) [16, 17]. Hence, although desmopressin appears to be safe and well-tolerated by patients with impaired renal function, great caution should be exercised when titrating towards an efficient dosage regimen, if patients with moderately or severely impaired renal function are to be treated with desmopressin at all.
References
- 1.Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frokiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol. 1999;10:647–63. doi: 10.1681/ASN.V103647. [DOI] [PubMed] [Google Scholar]
- 2.Asplund R, Sundberg B, Bengtsson P. Desmopressin for the treatment of nocturnal polyuria in the elderly: a dose titration study. Br J Urol. 1998;82:642–6. doi: 10.1046/j.1464-410x.1998.00849.x. [DOI] [PubMed] [Google Scholar]
- 3.Birkasova M, Birkas O, Flynn MJ, Cort JH. Desmopressin in the management of nocturnal enuresis in children: a double-blind study. Pediatrics. 1978;62:970–4. [PubMed] [Google Scholar]
- 4.Hilton P, Stanton SL. The use of desmopressin (DDAVP) in nocturnal urinary frequency in the female. Br J Urol. 1982;54:252–5. doi: 10.1111/j.1464-410x.1982.tb06969.x. [DOI] [PubMed] [Google Scholar]
- 5.Evans JH, Meadow SR. Desmopressin for bed wetting: length of treatment, vasopressin secretion, and response. Arch Dis Child. 1992;67:184–8. doi: 10.1136/adc.67.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asplund R, Aberg H. Health of the elderly with regard to sleep and nocturnal micturition. Scand J Prim Health Care. 1992;10:98–104. doi: 10.3109/02813439209014044. [DOI] [PubMed] [Google Scholar]
- 7.Kirkland JL, Lye M, Levy DW, Banerjee AK. Patterns of urine flow and electrolyte excretion in healthy elderly people. BMJ. 1983. pp. 1665–7. (Clin Res Ed) [DOI] [PMC free article] [PubMed]
- 8.Larsson G, Victor A. Micturition patterns in a healthy female population, studied with a frequency/volume chart. Scand J Urol Nephrol Suppl. 1988;114:53–7. [PubMed] [Google Scholar]
- 9.Asplund R, Aberg HE. Micturition habits of older people. Voiding frequency and urine volumes. Scand J Urol Nephrol. 1992;26:345–9. doi: 10.3109/00365599209181224. [DOI] [PubMed] [Google Scholar]
- 10.Brocklehurst JC, Fry J, Griffiths LL, Kalton G. Dysuria in old age. J Am Geriatr Soc. 1971;19:582–92. doi: 10.1111/j.1532-5415.1971.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 11.Cannon A, Carter PG, McConnell AA, Abrams P. Desmopressin in the treatment of nocturnal polyuria in the male. BJU Int. 1999;84:20–4. doi: 10.1046/j.1464-410x.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- 12.Chancellor MB, Atan A, Rivas DA, Watanabe T, Tai HL, Kumon H. Beneficial effect of intranasal desmopressin for men with benign prostatic hyperplasia and nocturia: preliminary results. Techn Urol. 1999;5:191–4. [PubMed] [Google Scholar]
- 13.Eckford SD, Carter PG, Jackson SR, Penney MD, Abrams P. An open, in-patient incremental safety and efficacy study of desmopressin in women with multiple sclerosis and nocturia. Br J Urol. 1995;76:459–63. doi: 10.1111/j.1464-410x.1995.tb07745.x. [DOI] [PubMed] [Google Scholar]
- 14.Eckford SD, Swami KS, Jackson SR, Abrams PH. Desmopressin in the treatment of nocturia and enuresis in patients with multiple sclerosis. Br J Urol. 1994;74:733–5. doi: 10.1111/j.1464-410x.1994.tb07116.x. [DOI] [PubMed] [Google Scholar]
- 15.Valiquette G, Herbert J, Maede-D’Alisera P. Desmopressin in the management of nocturia in patients with multiple sclerosis. A double-blind, crossover trial. Arch Neurol. 1996;53:1270–5. doi: 10.1001/archneur.1996.00550120082020. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein SA, Williford SL. Intranasal desmopressin-associated hyponatremia: a case report and literature review. J Fam Pract. 1997;44:203–8. [PubMed] [Google Scholar]
- 17.Odeh M, Oliven A. Coma and seizures due to severe hyponatremia and water intoxication in an adult with intranasal desmopressin therapy for nocturnal enuresis. J Clin Pharmacol. 2001;41:582–4. doi: 10.1177/00912700122010320. [DOI] [PubMed] [Google Scholar]
- 18.Mulder WJ, Hillen HF. Renal function and renal disease in the elderly: part I. European Journal of Internal Medicine. 2001. pp. 86–97. [DOI] [PubMed]
- 19.Fjellestad-Paulsen A, Hoglund P, Lundin S, Paulsen O. Pharmacokinetics of 1-deamino-8-D-arginine vasopressin after various routes of administration in healthy volunteers. Clin Endocrinol. 1993;38:177–82. doi: 10.1111/j.1365-2265.1993.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 20.Aunsholt NA, Vilhardt H, Schmidt EB. Plasma half-life of DDAVP in uraemic patients. Acta Pharmacol Toxicol. 1986;59:332–3. doi: 10.1111/j.1600-0773.1986.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 21.Ruzicka H, Bjorkman S, Lethagen S, Sterner G. Pharmacokinetics and antidiuretic effect of high-dose desmopressin in patients with chronic renal failure. Pharmacol Toxicol. 2003;92:137–42. doi: 10.1034/j.1600-0773.2003.920306.x. [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48:206–8. [PubMed] [Google Scholar]
- 23.Lundin S, Melin P, Vilhardt H. Plasma concentrations of 1-deamino-8-D-arginine vasopressin after intragastric administration in the rat. Acta Endocrinologica. 1985;106:179–83. doi: 10.1530/acta.0.1080179. [DOI] [PubMed] [Google Scholar]
- 24.Mazzachi BC, Peak MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffé creatine assays in plasma and serum and early morning urine. Clin Lab. 2000;46:53–5. 1–2. [PubMed] [Google Scholar]
- 25.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Report. 1970;54:225–35. [PubMed] [Google Scholar]
- 26.Beal SL, Sheiner LB. NONMEM Users Guide. San Francisco: University of California, NONMEM Project Group; 1998. [Google Scholar]
- 27.Karlsson MO, Jonsson EN, Wiltse CG, Wade JR. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm. 1998;26:207–46. doi: 10.1023/a:1020561807903. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson EN, Karlsson MO. Xpose – an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 29.Callreus T, Odeberg J, Lundin S, Hoglund P. Indirect-response modeling of desmopressin at different levels of hydration. J Pharmacokinet Biopharm. 1999;27:513–29. doi: 10.1023/a:1023238514015. [DOI] [PubMed] [Google Scholar]
- 30.Fjellestad-Paulsen A, d'Agay-Abensour L, Hoglund P, Rambaud JC. Bioavailability of 1-deamino-8-D-arginine vasopressin with an enzyme inhibitor (aprotinin) from the small intestine in healthy volunteers. Eur J Clin Pharmacol. 1996;50:491–5. doi: 10.1007/s002280050146. [DOI] [PubMed] [Google Scholar]
- 31.Patterson SE, Cohn VH. Hepatic drug metabolism in rats with experimental chronic renal failure. Biochem Pharmacol. 1984;33:711–6. doi: 10.1016/0006-2952(84)90451-9. [DOI] [PubMed] [Google Scholar]
- 32.Uchida N, Kurata N, Shimada K, Nishimura Y, Yasuda K, Hashimoto M, Uchida E, Yasuhara H. Changes of hepatic microsomal oxidative drug metabolizing enzymes in chronic renal failure (CRF) rats by partial nephrectomy. Jpn J Pharmacol. 1995;68:431–9. doi: 10.1254/jjp.68.431. [DOI] [PubMed] [Google Scholar]
- 33.Dowling TC, Briglia AE, Fink JC, Hanes DS, Light PD, Stackiewicz L, Karyekar CS, Eddington ND, Weir MR, Henrich WL. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther. 2003;73:427–34. doi: 10.1016/s0009-9236(03)00056-0. [DOI] [PubMed] [Google Scholar]
- 34.Bennett WM. Guide to drug dosage in renal failure. Clin Pharmacokinet. 1988;15:326–54. doi: 10.2165/00003088-198815050-00005. [DOI] [PubMed] [Google Scholar]