Saquinavir is a selective HIV protease inhibitor with limited systemic bioavailability (4% for the hard gelatin capsule (Invirase®), and about 12% for the soft gelatin capsule (Fortovase®) [1]) as a result of extensive phase I metabolism by CYP3A in the gut and liver. Moreover, saquinavir bioavailability is characterized by substantial interindividual variability [2], a feature which is common to other CYP3A substrates [3] and which could only be partly explained by drug interactions with inducers and/or inhibitors of CYP3A4 or by disease [4–6]. Unlike other CYP enzymes (e.g. CYP2D6, CYP2C19), there is no evidence for a CYP3A4 null allele. However, polymorphically expressed CYP3A5 might account for some of the pharmacokinetic variability seen with CYP3A substrates [7], because many of them are also substrates of CYP3A5 as well as CYP3A4 [8]. If expressed, CYP3A5 is present mainly in the gut and contributes to only a minor extent to hepatic drug metabolism [9]. Hence, CYP3A5 might be expected to modulate the bioavailability and metabolism of its substrates, whereas systemic clearance would not be affected to any extent. This hypothesis is supported by the recent finding of a significantly higher oral clearance (CL/F) of the protease inhibitor indinavir in patients expressing CYP3A5 [10], which may be due to a larger systemic clearance (CL), a decreased bioavailability (F) or by a combination of both.
During the course of two different drug interaction studies we have characterized the plasma and urine pharmacokinetics of saquinavir and the urine pharmacokinetics of its hydroxylated metabolites after administration of Invirase® to 7 [5] and Fortovase® to 12 healthy individuals, respectively [6]. The concentrations of parent compound and metabolites were determined by LC/MS/MS [11] and because of their identical mass, the concentrations of the two hydroxy metabolites are reported as the sum of M2 and M3. The ratios of the area of saquinavir to M2 and M3 were calculated assuming identical MS responses and recovery based on the close structural similarities of parent compound and metabolites [12]. Both studies were approved by the Ethics Committee of the Medical Faculty of Heidelberg and all participants gave written informed consent, including consent for the analysis of genetic variants of drug metabolizing enzymes. To evaluate the potential role of CYP3A5 in saquinavir pharmacokinetics, we determined a newly described CYP3A5 polymorphism (A6986G in intron 3 of the CYP3A5 gene, CYP3A5*3 allele), which is associated with the production of an inoperable truncated protein [13]. Genotyping was performed by direct sequencing of purified PCR products (20 ng) containing the polymorphic region using the dideoxy chain termination method (DYEnamic ET Kit, Amersham Biosciences, Freiburg, Germany) on a MegaBACE500 sequencer (Amersham Biosciences, Freiburg, Germany).
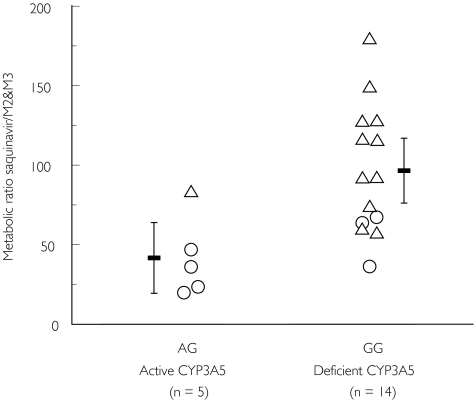
Fourteen participants were homozygote carriers of the CYP3A5*3 allele (lacking functional CYP3A5), and five participants (four given Invirase®) were heterozygous for the CYP3A5*1 allele (expressing functional CYP3A5). There was no correlation between the CYP3A5*3 polymorphism and the plasma pharmacokinetics (tmax, Cmax, AUC, t1/2,z) of saquinavir, but we found a statistically significant (P = 0.01, Mann–Whitney U-test) association with the 24 h urinary metabolic ratio of saquinavir to its hydroxy metabolites M2 and M3 (Figure 1). The mean (95% CI) metabolic ratio in individuals with active CYP3A5 was 41.6 (19.4, 63.8) and 96.5 (76.1, 117) in individuals deficient of functional CYP3A5.
Figure 1.
Association of the A6986G polymorphism of CYP3A5 (CYP3A5*3) with the metabolic ratio of saquinavir to its M2 and M3 hydroxymetabolites in urine. Data are shown as mean (± 95%CI) and individual metabolic ratios (○: participants receiving Invirase®; ▵: participants receiving Fortovase®)
Our findings indicate that saquinavir is substantially metabolized by CYP3A5 in humans expressing a functional enzyme, but that other enzymes are also involved. In accordance with its preferential expression in the gut, the absence of CYP3A5 had no significant influence on the elimination half-life of saquinavir. Because bioavailability differences between Invirase® and Fortovase® are substantial, and only one individual expressed CYP3A5 in the Fortovase® group, these data lack the power to assess the influence of the polymorphism on bioavailability. To this end a larger population should be investigated and should include sufficient numbers of subjects who do and do not express CYP3A5.
Acknowledgments
This work was supported by grant 01EC9902 from the German Ministry for Education and Research (BMBF).
References
- 1.Figgitt DP, Plosker GL. Saquinavir soft-gel capsule. An updated review of its use in the management of HIV infection. Drugs. 2000;60:481–516. doi: 10.2165/00003495-200060020-00016. [DOI] [PubMed] [Google Scholar]
- 2.Wacher VJ, Silverman JA, Zhang Y, Benet LZ. Role of P-glycoprotein and cytochrome P4503A in limiting oral absorption of peptides and peptidomimetics. J Pharm Sci. 1998;87:1322–30. doi: 10.1021/js980082d. [DOI] [PubMed] [Google Scholar]
- 3.Backman JT, Kivistö KT, Olkkola KT, Neuvonen PJ. The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol. 1998;54:53–8. doi: 10.1007/s002280050420. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Facklam M, Burhenne J, Ding R, et al. Dose-dependent increase of saquinavir bioavailability by the pharmaceutic aid cremophor EL. Br J Clin Pharmacol. 2002;53:576–81. doi: 10.1046/j.1365-2125.2002.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fröhlich M, Burhenne J, Martin-Facklam M, et al. Oral contraception does not alter single dose saquinavir pharmacokinetics in women. Br J Clin Pharmacol. 2004;57:244–52. doi: 10.1111/j.1365-2125.2003.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikus G, Schmidt L, Burhenne J, et al. Reduction of saquinavir exposure by co-administration of loperamide: a two–way pharmacokinetic interaction. Clin Pharmacokinet. 2004 doi: 10.2165/00003088-200443140-00004. in press. [DOI] [PubMed] [Google Scholar]
- 7.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–94. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 8.Patki KC, Von Moltke LL, Greenblatt DJ. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes P450: role of CYP3A4 and CYP3A5. Drug Metab Dispos. 2003;31:938–44. doi: 10.1124/dmd.31.7.938. [DOI] [PubMed] [Google Scholar]
- 9.Westlind-Johnsson A, Malmebo S, Johansson A, et al. Comparative analysis of CYP3A expression in human liver suggests only a minor role for CYP3A5 in drug metabolism. Drug Metab Dispos. 2003;31:755–61. doi: 10.1124/dmd.31.6.755. [DOI] [PubMed] [Google Scholar]
- 10.Anderson PL, Lamba J, Schuetz E, Fletcher CV. 11th Conference on Retroviruses and Opportunistic Infections. San Francisco: Abstract; 2004. CYP3A5 and MDR1 (P-gp) polymorphisms in HIV–infected adults: associations with indinavir concentrations and antiviral effects; p. 169. [Google Scholar]
- 11.Burhenne J, Riedel K-D, Martin-Facklam M, Mikus G, Haefeli WE. Highly sensitive determination of saquinavir in biological samples with liquid chromatography/tandem mass spectrometry. J Chromatogr B. 2003;784:233–42. doi: 10.1016/s1570-0232(02)00803-6. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimmons ME, Collins JM. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4. Potential contribution to high first-pass metabolism. Drug Metab Dispos. 1997;25:256–66. [PubMed] [Google Scholar]
- 13.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]