Abstract
Objectives
To evaluate high dose tolerability and relative systemic dose potency between inhaled clinically equipotent dose increments of formoterol and terbutaline in children.
Methods
Twenty boys and girls (6–11 years-old) with asthma and normal ECGs were studied. Ten doses of formoterol (Oxis®) 4.5 µg (F4.5) or terbutaline (Bricanyl®) 500 µg (T500) were inhaled cumulatively via a dry powder inhaler (Turbuhaler®) over 1 h (three patients) or 2.5 h (17 patients) and compared to a day of no treatment, in a randomised, double-blind (active treatments only), crossover trial. Blood pressure (BP), ECG, plasma potassium, glucose, lactate, and adverse events were monitored up to 10 h to assess tolerability and relative systemic dose potency.
Results
Formoterol and terbutaline had significant β2-adrenergic effects on most outcomes. Apart from the effect on systolic BP, QRS duration and PR interval, the systemic effects were significantly more pronounced with terbutaline than with formoterol. Thus, mean minimum plasma potassium, was suppressed from 3.56 (95% confidence interval, CI: 3.48–3.65) mmol l−1 on the day of no treatment to 2.98 (CI: 2.90–3.08) after 10 × F4.5 and 2.70 (CI: 2.61–2.78) mmol l−1 after 10 × T500, and maximum Q-Tc (heart rate corrected Q-T interval [Bazett's formula]) was prolonged from 429 (CI: 422–435) ms on the day of no treatment, to 455 (CI: 448–462) ms after 10 × F4.5 and 470 (CI: 463–476) ms after 10 × T500. Estimates of relative dose potency indicated that F4.5 µg had the same systemic activity as the clinically less effective dose of 250 µg terbutaline. The duration of systemic effects differed marginally between treatments. Spontaneously reported adverse events (most frequently tremor) were fewer with formoterol (78% of the children) than with terbutaline (95%). A serious adverse event occurred after inhalation of 45 µg formoterol over the 1 h dosing time, that prompted the extension of dosing time to 2.5 h.
Conclusions
Multiple inhalations over 2.5 h of formoterol (4.5 µg) via Turbuhaler® are at least as safe as and associated with less systemic effects than multiple inhalations of the clinically equipotent dose of terbutaline (500 µg) in children with asthma.
Keywords: Children, formoterol, terbutaline, tolerability
Introduction
β2-adrenoreceptor agonists such as formoterol and terbutaline are widely used as bronchodilators in the treatment of asthma. Multiple inhalations of a short-acting β2-agonist are recommended for rapid relief of bronchospasm during acute asthma episodes [1]. The long-acting β2-agonist formoterol fumarate dihydrate (Oxis®, hereafter ‘formoterol’) 4.5 µg has a rapid onset of action and studies have indicated that this formoterol dose is therapeutically at least as effective as 500 µg of the short-acting terbutaline sulphate (Bricanyl®, hereafter ‘terbutaline’) in asthmatic adults [2–4]. Moreover, multiple inhalations of formoterol 4.5 µg had less pronounced systemic effects than multiple inhalations of terbutaline 500 µg in asthmatic adults [4, 5], suggesting that formoterol might have a more favourable balance between clinical and systemic effects than terbutaline.
The systemic effects of an inhaled drug depend on the amount of drug that is systemically absorbed and the metabolism of this drug to pharmacologically inactive substances. The systemic availability of an orally inhaled drug is the sum of drug absorbed from the oropharynx and gastrointestinal tract and drug absorbed from the intrapulmonary airways. The pharmacokinetics of many drugs are different in children than in adults. Compared with adults a higher proportion of orally inhaled nebulized budesonide is deposited in the oropharynx and a smaller proportion in the intrapulmonary airways in children [6]. Thus, conclusions based upon studies in adults cannot be directly applied to children. Therefore, the aim of this study was to assess the acute high-dose systemic effects of formoterol administered via the dry powder inhaler Turbuhaler in children and to compare these with the systemic effects measured after high doses of terbutaline also inhaled via Turbuhaler. As tolerance to the systemic effects develops during continued treatment with β2-agonists [5, 7], this study focused on a worst-case scenario – the tolerability of cumulatively administered high doses.
Methods
The study was performed in accordance with the Declaration of Helsinki [8] and approved by the local Ethics Committees in Kolding, Denmark, and Stockholm and Uppsala, Sweden. Before enrolment, children with asthma and their parents gave informed consent after verbal and written information.
Patients
Asthmatic children 6–11 years-old with asthma were recruited in Denmark and Sweden. Eligibility was assessed on the basis of medical history, physical examination, pulse and blood pressure, and laboratory tests. Holter monitoring was performed for at least 10 h prior to study to exclude patients with abnormal ECGs.
Protocol
Patients were assigned to inhale racemic formoterol (Oxis®, AstraZeneca, Sodertalje, Sweden) 18 + 9 + 9 + 9 µg and racemic terbutaline (Bricanyl®, AstraZeneca, Sodertalje, Sweden) 2000 + 1000 + 1000 + 1000 µg cumulatively at time zero, 0.5 h, 2 h, and 2.5 h via the dry-powder inhaler (Turbuhaler®, AstraZeneca, Sodertalje, Sweden), and a day of no treatment, double-blind (active treatments only), in a randomised, crossover fashion. (For the first three patients dosing was done over 1 h as follows: formoterol 18 + 18 + 9 µg and terbutaline 2000 + 2000 + 1000 µg cumulatively at time zero, 0.5 h, and 1 h.) Inhalation technique was rehearsed before administration of study drugs. Study days were separated by a washout period of at least one week. The double-blind design was chosen to minimize patient and study personnel bias regarding clinical evaluation of active treatment. The rational for an open day of no treatment rather than placebo was that lack of systemic β2-adrenoreceptor effects such as tremor would disclose placebo anyway.
Rescue medication with short-acting β2-agonists was withheld for at least 8 h and, based on previous experience from clinical studies with inhaled formoterol and salmeterol [9, 10], medication with long-acting β2-agonists was withheld for at least 48 h before and during study days. Intake of beverages containing caffeine, e.g. Coca Cola, was avoided completely for at least 36 h before and during study days. Intake of beverages containing sugar, and intake of food was restricted up to 3 h and 6 h, respectively, after the first dose. Water was allowed ad libitum. Strenuous activity was avoided for at least 12 h before and during study days.
Baseline assessments were done after a 15-min rest, before cumulative dosing was started or before the zero reference time on the day of no treatment. Subsequent measurements were performed 30 min after each dose increment (or imagined increment during the day of no treatment) and thereafter at several predetermined times up to 3 h (plasma glucose and lactate), and 10 h (pulse rate, blood pressure, ECG, and plasma potassium); scheduled assessment times are shown in Figure 1. A standard question, ‘Have you/the child had any health problems since the previous visit or last questioning?’ was addressed to each child or parent/legal guardian the morning before dosing, after completion of each 10-h assessment period, and via telephone within 2 weeks after the last treatment. Time of on- and off-set for typical class effects of β2-adrenoceptor stimulating drugs such as tremor, headache, and palpitations were recorded.
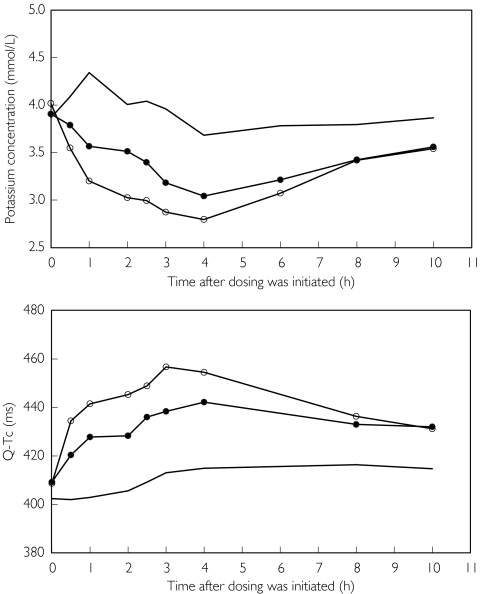
Figure 1.
Mean plasma potassium concentration and Q-Tc at scheduled assessment times, after a day of no treatment (no symbol), formoterol 10 × 4.5 µg (filled circles), and terbutaline 10 × 500 µg (open circles). Doses were given subdivided into four fractions at times zero, 0.5 h, 2 h and 2.5 h
Laboratory assessments and analyses
Single assessments of pulse rate and blood pressure (arteria radialis) were performed after a 5-min rest in the supine position, manually or by the oscillometric method using an electronic monitoring device (Omron® 705CP or 711, Scan Tech Medical, Columbia, SC, USA). Heart rate, QRS duration, RR-interval, PR-interval, Q-T interval, Q-Tc (heart-rate corrected Q-T), and assessments of sinus rhythm, extra systoles, conduction, and ST-T changes, were manually calculated from 12-lead ECGs (Siemens Megacart, Siemens-Elema AB, Solna, Sweden or equivalent) in a blinded fashion, by a cardiologist. QRS, RR, PR, Q-T, and ST-T are established tags referring to ECG tracing.
Venous blood was drawn from an indwelling catheter for measurement of plasma potassium, glucose, and lactate. The blood was centrifuged at ambient temperature within 1 h and the generated plasma was stored at −20 °C until analysed. The maximum interassay coefficient of variation in analyses of quality control samples was 1.08% for plasma potassium, 0.54% for plasma glucose, and 4.5% for plasma lactate.
Data analysis
Data are presented as unadjusted arithmetic mean values and ranges. An analysis of variance (anova) model with patient, treatment, and period as factors was used in the analysis of maximum/minimum and average values. The primary outcome variable was minimum plasma potassium concentration. To assess the relative dose potency, values at 30 min after each dose fraction were reduced to means using an anova model with patient, treatment, period, dose number and the interaction between treatment and dose number as factors. The relative dose potency was estimated as the horizontal shift between parallel lines fitted to these means plotted against logarithms of the cumulative doses of active treatments. Fieller's method was used to obtain 95% confidence intervals for the estimated relative dose potency [11]. Pre-dose measurements were used as covariates in the statistical analyses. Start of first adverse event (or change in a symptom reported before drug administration) was presented graphically with a Kaplan-Meier plot to explore if higher systemic dose potency would manifest itself as more rapidly occurring adverse events.
Results
Twenty patients were randomized and eligible for the safety evaluation [Table 1]. One patient was withdrawn because of a serious adverse event after inhalation of formoterol 45 µg over 1 h. This event prompted an extension of the originally planned dosing time of one hour to 2.5 h. Two other patients, who had received terbutaline 500 µg administered over 1 h, were also withdrawn after this serious adverse event, but were allowed to complete a day of no treatment. Hence, 19 patients were evaluable for the assessment of tolerability (formoterol day 18, terbutaline day 19, and no treatment day 19), and 17 patients, using the 2.5 h dosing regimen, were evaluable for the assessment of the relative systemic dose potency between formoterol and terbutaline. Thirteen patients were on regular treatment with an inhaled glucocorticosteroid (100–800 µg per day).
Table 1. Demographic characteristics.
| Age (year): mean (range) | 9.4 (6–11) |
| Sex ratio (boys): n (%) | 13 (65) |
| Weight (kg): mean (range) | 34 (27–49) |
| Height (cm): mean (range) | 140 (128–152) |
| Time since asthma diagnosis (year): mean (range) | 8 (0–11) |
| Patients who Inhaled glucocorticosteroids: n (%) – daily dose (mg): mean (range) | 13 (65) −292 (100–800) |
| Patients who used Short-acting β2: n (%) | 17 (85) |
| Patients who used Long-acting β2: n (%) | 4 (20) |
| FEV1 (L): mean (range) | 1.96 (1.47–2.31) |
| FEV1 (% predicted normal): mean (range) | 98.5 (81–117) |
Systemic effects
Active treatments decreased plasma potassium, PR-Interval, and diastolic blood pressure, and increased plasma glucose, plasma lactate, heart rate, systolic blood pressure, QRS duration and Q-Tc. The mean time profiles for plasma potassium concentration and Q-Tc are shown in Figure 1 and the individual minimum plasma potassium concentrations are shown in Figure 2. The analysis of minimum and maximum values are given in Table 2. All effects were statistically significant compared with no treatment except for diastolic blood pressure, which was statistically significant for terbutaline but not for formoterol. The systemic effects were significantly more pronounced after terbutaline than after formoterol except for systolic blood pressure, QRS duration and PR interval, for which active treatment effects were of similar magnitude. The conclusions based on analysis of average values (not shown) were similar to that based on maximum/minimum values.
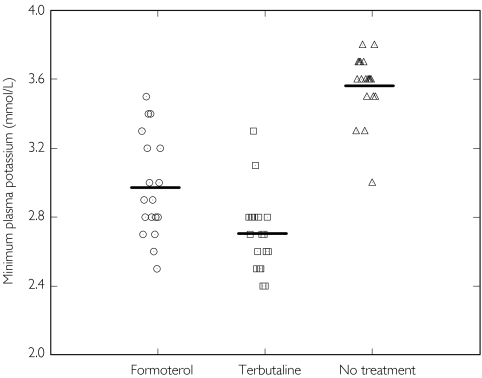
Figure 2.
Individual and mean minimum plasma potassium concentration after a day of no treatment (open triangles), formoterol 10 × 4.5 µg (open circles), and terbutaline 10 × 500 µg (open squares)
Table 2. Unadjusted mean maximum or minimum values and outcome of statistical analyses of mean differences between adjusted means after a day of no treatment, inhalation of formoterol 10 × 4.5 µg, or inhalation of terbutaline 10 × 500 µg via Turbuhaler. Statistically significant differences are in bold.
| Formoterol (n = 18) | Terbutaline (n = 19) | |||||
|---|---|---|---|---|---|---|
| Variable (extended normal range) | Day of no treatment (n = 19) Mean (range) | Mean (range) | Vs day of no treatment (95% CI) | Mean (range) | Vs day of no treatment (95% CI) | Formoterol vs. Terbutaline (95%) |
| Minimum plasma potassium, mmol/l (3.0–4.7) | 3.56 (3.0–3.8) | 2.98 (2.5–3.5) | −0.58 (−0.70, −0.46) | 2.71 (2.4-3.3) | −0.87 (−0.99, −0.75) | 0.29 (0.16, 0.41) |
| Maximum plasma glucose, mmol/l (3.63–9.0) | 5.52 (4.8–6.8) | 6.10 (5.2–8.8) | 0.58 (0.1, 1.07) | 6.75 (5.8–8.7) | 1.29 (0.82, 1.75) | −0.71 (−1.22, −0.20) |
| Maximum plasma lactate, mmol/l (0.5–2.5) | 1.57 (0.8–2.4) | 2.77 (2.1–4.3) | 1.21 (0.78, 1.64) | 3.97 (2.6–6.4) | 2.42 (2.01, 2.84) | −1.21 (−1.65, −0.78) |
| Maximum heart rate, beats per minute | 83.3 (63–97) | 94.5 (71–119) | 10.9 (4.9, 16.9) | 107.8 (75–133) | 24.4 (18.8, 29.9) | −13.5 (−19.1, −7.8) |
| Maximum systolic blood pressure, mmHg | 115.6 (98–130) | 120.9 (107–140) | 4.9 (2.0, 7.8) | 120.6 (106–145) | 4.9 (2.1, 7.7) | 0.0 (−2.9, 2.9) |
| Minimum diastolic blood pressure, mmHg | 59.2 (50–72) | 57.1 (48–68) | −2.2 (−5.1, 0.7) | 53.8 (40–68) | −5.5 (−8.5, −2.5) | 3.3 (0.4, 6.3) |
| Maximum Q-Tc, ms | 429 (386–464) | 453.3 (426–494) | 25.7 (16.1, 35.4) | 469.6 (437–503) | 40.8 (31.4, 50.1) | −15.0 (−24.1, −5.9) |
| Maximum QRS duration, ms | 85.0 (70–95) | 90.3 (80–100) | 5.8 (3.3, 8.2) | 90.8 (80–100) | 5.9 (3.5–8.2) | −0.1 (−2.6, 2.3) |
| Minimum PR interval, ms | 136.1 (110–155) | 130.3 (115–145) | −6.6 (−10.5, −2.7) | 125.5 (110–140) | −10.4 (−14.1, −6.7) | 3.8 (−0.2, 7.7) |
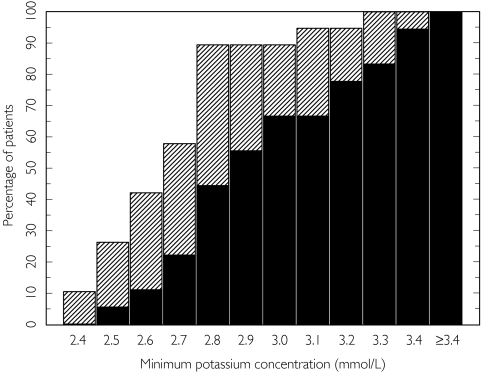
Values were more frequently outside normal ranges after 10 × T500 than after 10 × F4.5. The minimum plasma potassium was below 3.0 mmol l−1 in 10 of 18 patients after formoterol and 17 of 19 after terbutaline [Figure 3]. Maximum plasma glucose was generally below 9.0 mmol l−1 whereas plasma lactate was above the upper limit of 2.5 mmol l−1 in all patients after terbutaline and in 10 of 18 after formoterol. No clinically relevant treatment effects were seen on maximum heart rate and blood pressure. Prolonged Q-Tc values (above 445 ms and an increase of 50 ms or more) at one or more time point(s) were reported in four subjects after formoterol and in 11 subjects after terbutaline.
Figure 3.
Cumulative distribution of minimum plasma potassium concentrations by treatment. Formoterol (▪), terbutaline ( )
)
Except for the plasma lactate increase (monitoring stopped after 3 h), the systemic effects started to wear off during the observation period and the rate of disappearance was similar after the two active treatments. Normalization of plasma potassium and heart rate was temporarily dampened or even reversed by intake of food, especially the lunch (probably prandial effects mediated by insulin and increased gastrointestinal blood flow [12, 13]).
Adverse events
Spontaneously reported adverse events (most frequently tremor) were fewer with formoterol (78% of the children) than with terbutaline (95%). The serious adverse event after formoterol administration occurred two hours after inhalation of 45 µg over 1 h. The patient suffered from tremor, headache, nausea, vomiting, and somnolence. Plasma glucose dropped from 7.1 mmol l−1 to 5.2 mmol l−1, but the magnitude of the various systemic effects was not markedly different in this patient compared with the others.
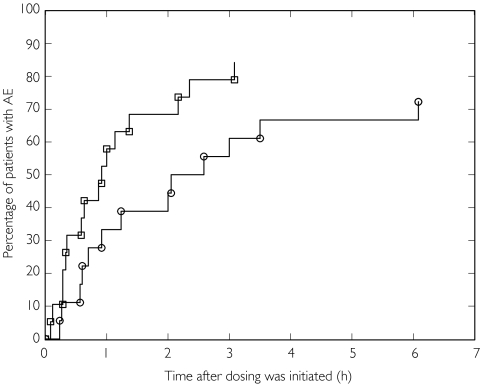
The time to first adverse event (mainly tremor) was shorter after inhalation of terbutaline (median 55 min) than after inhalation of formoterol (median 139 min); [Figure 4].
Figure 4.
(A) Kaplan-Meier plot of time to start of first adverse event by subject and treatment. Formoterol (○), terbutaline (□)
Relative systemic dose potency
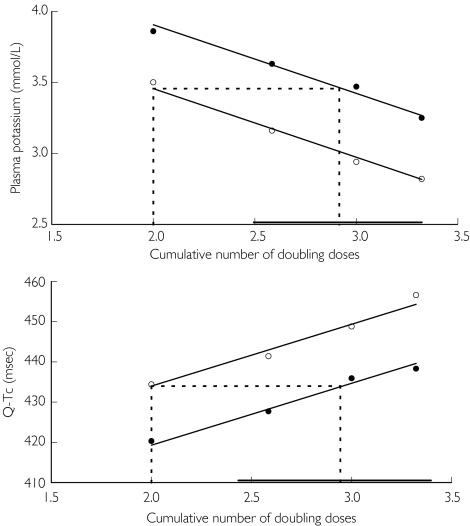
Inhalation of formoterol and terbutaline caused cumulative dose-dependent systemic responses on the various outcomes. The assumed log-linear and parallel cumulative dose–response to formoterol and terbutaline was particularly obvious for plasma potassium, plasma lactate, and QTc (data for plasma potassium and Q-Tc are shown in Figure 5). The mean estimates of the relative systemic dose-potency between formoterol and terbutaline, based on these three variables [Table 3], indicated that the systemic side-effect of F4.5 would correspond to that of 250 µg terbutaline. For plasma glucose the maximum effect was reached already after the second dose and for heart rate the dose–response curves were clearly not parallel. Therefore, these variables were not suitable for assessment of the relative dose -potency.
Figure 5.
Mean plasma potassium concentration and mean Q-Tc 30 min after each subdivided dose fraction vs. the cumulative number of doubling doses (2 doubling doses implies 22 = 4 inhalations, 2.585 doubling doses 22.585 = 6 inhalations, 3 doubling doses 23 = 8 inhalations, and 3.22 doubling doses 23.22 = 10 inhalations). Dotted lines indicate the required number of doubling formoterol doses to match 2 doubling doses (2000 µg) of terbutaline; the solid line, parallel to the x-axis, indicates the 95% confidence interval for the estimate. Dose fractions of formoterol were 18 + 9 + 9 + 9 µg (filled circles), and dose fractions of terbutaline were 2000 + 1000 + 1000 + 1000 µg (open circles)
Table 3. Estimates of relative systemic dose potency between formoterol and terbutaline after inhalation via Turbuhaler (n = 17) – statistical analyses of the horizontal shift in systemic cumulative dose–response curves.
| By number of inhalations | By µg | |||
|---|---|---|---|---|
| Effect | Mean | 95% confidence interval | Mean | 95% confidence interval |
| Plasma potassium decrease | 0.53 | (0.40, 0.64) | 59 | (45, 71) |
| Plasma lactate increase | 0.48 | (0.39, 0.56) | 53 | (43, 62) |
| Q-Tc prolongation | 0.52 | (0.34, 0.67) | 57 | (38, 74) |
Discussion
In spite of the possible differences between children and adults in deposition pattern of inhaled drugs the findings in the present study largely corroborated the findings with high doses of formoterol and terbutaline in asthmatic adults [4, 5]: Typically, systolic blood pressure, Q-Tc, plasma glucose and lactate concentrations were higher, and plasma potassium concentration was lower during active treatment compared with the day of no treatment. The effects were more pronounced after the terbutaline than after the formoterol administration.
In normal clinical practice 45 µg formoterol or 5000 µg terbutaline will only be administered over short time periods in children with very severe bronchoconstriction. Despite the observed statistically significant systemic effects of the administered doses the changes in the individual absolute values of the various variables were all of a magnitude that should not prevent clinicians from occasionally using such high doses in children with severe bronchial obstruction. Though our study was not conducted in children with severe bronchoconstriction there is no reason to believe that the systemic effects would be higher in such children. The major fraction of the systemically available drug (and hence the systemic effects) comes from absorption via the lungs and, if anything, lung deposition of an inhaled drug is reduced during episodes of severe bronchial obstruction. So it is possible that the systemic effects would be somewhat lower in such children.
The most significant safety finding was the one serious adverse event seen in one of the first three treated patients. This occurred after administration of 45 µg formoterol over the initial shorter one hour dosing period. That finding prompted an extension of the administration time from one to 2.5 h with both treatments, and no additional incidents were seen after this change. However, this does not allow firm conclusions about the time interval over which high doses should be administered. We do not know what caused this incidence. It might have been caused by the high dose of formoterol or other study-related events. The child had been fasting for more than 15 h and had not had any water because of nausea. Furthermore, having ECGs and multiple blood samples in a hospital setting may have caused a lot of stress and anxiety. These factors may have caused the incidence or contributed to its severity.
Q-Tc was used as safety surrogate marker of the potential arrhythmogenic effect of formoterol and terbutaline [14]. The Bazett formula, applied to correct for the increase in heart rate, may have overcorrected the Q-T interval [14] and therefore the clinical relevance, even for the highest Q-Tc of 503 ms, was uncertain. Occurrence of arrhythmias would have been more worrying, but no such incidents were observed during the study periods.
Studies in adults have suggested that, clinically, formoterol is at least 100 times as potent as terbutaline [2–4]. A study on the protection against exercise-induced bronchoconstriction in children essentially confirmed that relationship [15]. Therefore, we choose to compare a cumulated dose of formoterol of 45 µg with a cumulated dose of terbutaline of 5000 µg. The dose–response curves of several variables in the present study were log-linear and parallel for the two drugs. Therefore, accurate potency ratios could be calculated. Depending on the systemic effect variable formoterol was estimated to be between 53 and 59 times as potent as terbutaline on a µg-to-µg basis. This suggests that, systemically, 4.5 µg formoterol would be equivalent to 250 µg rather than 500 µg terbutaline to which it seems to be clinically equivalent. Our results corroborate previous findings in adults with asthma [4, 5]. The difference in systemic dose potency may explain the more rapid occurrence of adverse events during the administration of terbutaline compared with formoterol.
Clinically, inhaled and topically acting formoterol has a much longer duration of bronchodilatation than orally administered and systemically acting formoterol [16]. Therefore, the observed similar duration of systemically mediated metabolic and cardiovascular side-effects of inhaled formoterol and terbutaline at clinically equivalent doses was not surprising.
Conclusion
Multiple inhalations over 2.5 h of formoterol (4.5 µg) via Turbuhaler® are at least as safe as and associated with less systemic effects than multiple inhalations of the clinically equipotent dose of terbutaline (500 µg) in children with asthma.
Sponsorship
The study was sponsored by AstraZeneca Research & Development.
Acknowledgments
Quintiles (clinical laboratory tests); Magnus Simonsson (ECG evaluations); Tony Foucard, Pia Kalm-Stephens, Björn Nordlund (clinical performance); Cristina Mardell and Anita Ekdahl (study co-ordination); Agneta Hedlund (data management); Alf Andersson and Birgitta Overup (safety assessment); Lennart Jansson (scientific discussions)
References
- 1.Woodhead M. Guidelines on the management of asthma. Thorax. 1993;48:1–24. doi: 10.1136/thx.48.2_suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tattersfield A, Löfdahl C, Postma D, Eivindson A, Schreurs G, Rasidakis A, et al. Comparison of formoterol and terbutaline for as-needed treatment of asthma: a randomised trial. Lancet. 2001;357:257–61. doi: 10.1016/S0140-6736(00)03611-4. [DOI] [PubMed] [Google Scholar]
- 3.Ind P, Villasante C, Shiner R, Pietinalho A, Böszöményi Nagy G, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. Eur Respir J. 2002;20:859–66. doi: 10.1183/09031936.02.00278302. [DOI] [PubMed] [Google Scholar]
- 4.Malolepszy J, Böszöményi Nagy G, Selroos O, Larsson P, Brander R. Safety of formoterol Turbuhaler at cumulative dose of 90 ug in patients acute bronchial obstruction. Eur Respir J. 2001;18:928–34. doi: 10.1183/09031936.01.00251901. [DOI] [PubMed] [Google Scholar]
- 5.Tötterman K, Huhti L, Sutinen E, Backman R, Pietinalho A, Falck M, et al. Tolerability to high doses of formoterol and terbutaline via Turbuhaler for 3 days in stable asthmatic patients. Eur Respir J. 1998;12:573–9. doi: 10.1183/09031936.98.12030573. [DOI] [PubMed] [Google Scholar]
- 6.Agertoft L, Andersen A, Weibull E, Pedersen S. Systemic availability and pharmacokinetics of nebulised budesonide in preschool children. Arch Dis Child. 1999;80:241–7. doi: 10.1136/adc.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newnham D, Grove A, McDevitt D, Lipworth B. Subsensitivity of bronchodilator and systemic beta-2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax. 1995;50:497–504. doi: 10.1136/thx.50.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anonymous. 2002. World Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. World Association, Declaration of Helsinki http://www.wma.net/e/policy/b3.htm.
- 9.Palmqvist M, Persson G, Lazer L, Rosenborg J, Larsson P, Lötvall J. Inhaled dry-powder formoterol and salmeterol in asthmatic patients: Onset of action, duration of effect and potency. Eur Respir J. 1997;10:2484–9. doi: 10.1183/09031936.97.10112489. [DOI] [PubMed] [Google Scholar]
- 10.Rosenborg J, Larsson P, Rott Z, Böcskei C, Poczi M, Juhász G. Relative therapeutic index between inhaled formoterol and salbutamol in asthmatic patients. Respir H Med. 2002;96:412–7. doi: 10.1053/rmed.2002.1291. [DOI] [PubMed] [Google Scholar]
- 11.Fieller E. Some problems in biological assay. J R Statist Soc B. 1954;16:175–85. [Google Scholar]
- 12.Guyton A. Insulin, glucagon, and diabetes mellitus. In: AC G, editor. Textbook of Medical Physiology. 8. Philadelphia: W.B. Saunders Company; 1991. pp. 855–67. [Google Scholar]
- 13.Guyton A. General principles of gastrointestinal function – motility, nervous control, and blood circulation. In: AC G, editor. Textbook of Medical Physiology. 8. Philadelphia: W.B. Saunders Company; 1991. pp. 688–97. [Google Scholar]
- 14.Sha R. The significance of QT interval in drug development. Br J Clin Pharmacol. 2002;54:188–202. doi: 10.1046/j.1365-2125.2002.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grönneröd T, Von Berg A, Schwabe G, Soliman S. Formoterol via Turbuhaler® gave better protection than terbutaline against repeated exercise challenge for up to 12 hours in children and adolescents. Respir Med. 2000;94:661–7. doi: 10.1053/rmed.2000.0789. [DOI] [PubMed] [Google Scholar]
- 16.Löfdahl C, Svedmyr N. Formoterol fumarate, a new beta2-adrenoceptor agonist. Acute studies of selectivity and duration of effect after inhaled and oral administration. Allergy. 1989;44:264–71. doi: 10.1111/j.1398-9995.1989.tb01068.x. [DOI] [PubMed] [Google Scholar]