Abstract
Aims
Gemfibrozil markedly increases the plasma concentrations and blood glucose-lowering effects of repaglinide, but the effects of other fibrates on repaglinide pharmacokinetics are not known. Our aim was to investigate the effects of bezafibrate and fenofibrate on the pharmacokinetics and pharmacodynamics of repaglinide.
Methods
In a randomized, three-phase cross-over study, 12 healthy subjects received 400 mg bezafibrate, 200 mg fenofibrate or placebo once daily for 5 days. On day 5, a single 0.25 mg dose of repaglinide was ingested 1 h after the last pretreatment dose. The concentrations of plasma repaglinide, bezafibrate and fenofibrate and blood glucose were measured up to 7 h postdose.
Results
During the bezafibrate and fenofibrate phases, the total area under the concentration-time curve [AUC(0,∞)] of repaglinide was 99% (95% confidence interval of the ratio to the control phase 73, 143%) and 99% (85, 127%) of the corresponding value during the placebo (control) phase, respectively. Bezafibrate and fenofibrate had no significant effect on the peak concentration (Cmax) of repaglinide. The mean half-life of repaglinide was 1.3 h in all phases. The blood glucose-lowering effect of repaglinide was not affected by bezafibrate or fenofibrate. The AUC(0,8 h) values for bezafibrate and fenofibrate varied 3.0-fold and 4.4-fold between individual subjects, respectively. Neither bezafibrate nor fenofibrate affected the pharmacokinetic variables of repaglinide.
Conclusions
Bezafibrate and fenofibrate do not affect the pharmacokinetics or pharmacodynamics of repaglinide.
Keywords: bezafibrate, fenofibrate, interaction, repaglinide
Introduction
Repaglinide, a short-acting meglitinide analogue [1], is a novel antidiabetic agent used to normalize postprandial glucose concentrations in patients with type II diabetes [2]. It acts by enhancing glucose-stimulated insulin release from the pancreas, and its efficacy is dependent on the residual β-cell function of pancreatic islets [3, 4]. Repaglinide undergoes marked first-pass metabolism, resulting in an oral bioavailability of approximately 60%[5]. Repaglinide is completely metabolized mainly by CYP3A4 and CYP2C8 [6], and inactive metabolites are excreted primarily into faeces [7].
A recent study has revealed a clinically important interaction between gemfibrozil and repaglinide [8]. Gemfibrozil (600 mg administered twice daily) caused on average an 8-fold increase in the AUC(0,∞) of repaglinide, and greatly increased and prolonged its glucose-lowering effect. This interaction was further magnified when itraconazole and gemfibrozil were given together with repaglinide. The mechanism of the gemfibrozil–repaglinide interaction may involve inhibition of CYP2C8. However, as gemfibrozil is a relatively weak inhibitor of CYP2C8 in vitro[9], other mechanisms are possible [10–12].
Diabetic patients have an increased risk of cardiovascular morbidity and mortality [13], and benefit from active anti-atherosclerotic medical treatment [14]. Statins are most commonly used for the treatment of lipid abnormalities in diabetic patients. However, fibrates are also frequently used, because they correct low HDL cholesterol and high triglyceride concentrations, which are often associated with diabetes [15]. Thus, it is important to know whether fibrates other than gemfibrozil affect the pharmacokinetics of repaglinide. Accordingly, we have studied the effects of bezafibrate and fenofibrate on the plasma concentrations and blood glucose-lowering effect of repaglinide.
Methods
Subjects
Twelve healthy non-smoking male subjects (age range 21–26 years; weight range 58–100 kg) participated (Table 1) after giving their written informed consent. Before entering the study, the subjects were ascertained to be healthy by a medical history, a physical examination, and routine laboratory tests. None of the subjects used continuous medication, and grapefruit juice and any medication were not allowed for 2 weeks before the study. The sample size was chosen so that a possible clinically significant pharmacokinetic drug interaction could be verified statistically without the use of an unnecessarily large group of healthy subjects. The number of subjects was estimated to be sufficient to detect a 40% change in the AUC(0,∞) of repaglinide with a power of 80% (alpha-level 5%).
Table 1. Characteristics of the subjects and pharmacokinetic data for bezafibrate and fenofibrate.
| Bezafibrate | Fenofibrate | |||||
|---|---|---|---|---|---|---|
| subject | age (years) | Cmax (mg l–1) | AUC(0,8 h) (mg l–1 h) | Cmax (mg l–1) | ||
| 1 | 25 | 75 | 21.4 | 5.6 | 38.0 | 5.6 |
| 2 | 23 | 88 | 9.8 | 3.9 | 47.8 | 8.1 |
| 3 | 26 | 73 | 21.4 | 5.7 | 52.5 | 8.4 |
| 4 | 21 | 77 | 19.7 | 5.0 | 35.1 | 5.0 |
| 5 | 24 | 80 | 26.5 | 6.3 | 72.0 | 11.2 |
| 6 | 25 | 100 | 13.4 | 3.4 | 36.0 | 5.2 |
| 7 | 21 | 74 | 19.5 | 4.8 | 82.6 | 11.6 |
| 8 | 24 | 69 | 29.6 | 8.2 | 79.7 | 12.2 |
| 9 | 23 | 58 | 9.8 | 3.4 | 43.5 | 7.9 |
| 10 | 22 | 73 | 13.6 | 5.1 | 19.6 | 2.9 |
| 11 | 26 | 65 | 12.8 | 3.3 | 85.4 | 11.4 |
| 12 | 25 | 85 | 19.5 | 4.8 | 41.8 | 6.5 |
| Mean ± SD | 23.8 ± 1.8 | 76.4 ± 11.0 | 18.1 ± 6.3 | 4.9 ± 1.4 | 52.8 ± 21.7 | 8.0 ± 3.1 |
AUC(0,8 h), area under the concentration-time curve after the last pretreatment dose up to 8 h. Cmax, the peak concentration in plasma.
Study design
The study protocol was approved by the Ethics Committee for Studies in Healthy Subjects and Primary Care of the Hospital District of Helsinki and Uusimaa and the Finnish National Agency for Medicines. A randomized, placebo-controlled, cross-over study with three phases and a wash-out period of 2 weeks was carried out. The subjects received either placebo, 400 mg bezafibrate (one Bezalip 400 mg slow-release tablet, Roche, Mannheim, Germany) or 200 mg fenofibrate (one Lipanthyl 200 mg capsule, Fournier S.A., Fontaine Les Dijon, France) at 08.00 h for 5 days. On day 5, following an overnight fast, 0.25 mg repaglinide (one half of a NovoNorm 0.5 mg tablet, Novo Nordisk A/S, Bagsvaerd, Denmark) was ingested with 150 ml water at 09.00 h, 1 h after the last pretreatment dose. The timing of repaglinide administration was chosen to ensure adequate absorption of the fibrates, thus maximizing the extent of any possible interaction. Food intake on day 5 was identical in all phases. Subjects were served a light standard breakfast at precisely 15 min after repaglinide administration, standard snacks rich in carbohydrates precisely 1 h and 2 h after repaglinide, a standard warm meal after 3 h and a standard light meal after 7 h. The breakfast was eaten within 10 min, the snacks within 5 min. The breakfast contained approximately 370 kcal energy, 70 g carbohydrates, 8 g protein and 6 g fat. The snacks were identical and contained about 200 kcal energy, 45 g carbohydrates, 2 g protein and 1 g fat each. During the days of repaglinide administration, the subjects were under direct medical supervision and blood glucose concentrations were monitored throughout the day. Additional carbohydrates, glucose solution for intravenous use and glucagon for intramuscular use were available, but they were not needed.
Blood sampling and determination of blood glucose concentrations
Blood samples (10 ml each) were drawn from a cannulated forearm vein prior to, and 20, 40, 60, 80, and 100 min and 2, 2.5, 3, 4, 5, and 7 h after the administration of repaglinide. Blood samples were collected into tubes containing ethylenediaminetetraacetic acid (EDTA). Glucose concentrations were measured immediately after each blood sampling using the glucose oxidase method (Precision G Blood Glucose Testing System, Medisense, Bedford, MA). Plasma was separated within 30 min after blood sampling, and was stored at −70 °C until analysis. The between-day coefficient of variation (CV) for blood glucose was 3.2% at 3.1 mmol l−1, 3.6% at 5.8 mmol l−1 and 2.7% at 17.2 mmol l−1 (n = 3).
Determination of plasma repaglinide concentrations
Each plasma sample (0.5 ml) was placed into a 10 ml glass tube and 0.5 ml of 0.1 mol l−1 potassium phosphate solution (pH 4.0), and 0.6 g of solid sodium chloride salt were added. The mixture was shaken for 20 min with 6 ml diethyl ether and centrifuged for 5 min at 2000 g. The organic layer (5 ml) was transferred into another tube and evaporated at 30 °C to dryness under nitrogen stream. The residue was dissolved in 0.1 ml of the mobile phase and transferred into an autosampler vial.
The concentrations of repaglinide were quantified by use of PE SCIEX API 3000 liquid chromatography-tandem mass spectrometry system (Sciex Division of MDS Inc, Toronto, Ontario, Canada). Chromatography was performed on a Symmetry C8 column (150 × 2.1 mm I.D., 3.5 µm particle size) (Waters Corp., Milford, Massachusetts, USA) using gradient elution. The mobile phase consisted of 10 mmol l−1 ammonium formate (pH 4.0, adjusted with 99% formic acid) and acetonitrile. The mass spectrometer was operated in the turbo ion spray mode with positive ion detection and the ion transition monitored was m/z 453 to m/z 230. This transition represents the product ion of the [M + H]+ ion. The limit of quantification for repaglinide was 0.05 ng ml−1 and the between-day CV was 10.0% at 0.1 ng ml−1 and 8.5% at 2.0 ng ml−1 (n = 12).
Determination of plasma bezafibrate and fenofibrate
Plasma bezafibrate and fenofibrate concentrations on day 5 were measured by HPLC with ultraviolet detection [16]. The limit of quantification for bezafibrate was 0.1 mg l−1, and the between-day CV was 8.9% at 0.2 mg l−1, 1.9% at 4.4 mg l−1 and 4.7% at 10.2 mg l−1 (n = 5). For fenofibrate, the limit of quantification was 0.1 mg l−1, and the between-day CV was 11.0% at 0.3 mg l−1, 2.5% at 3.5 mg l−1 and 2.3% at 8.8 mg l−1 (n = 7).
Pharmacokinetics
The pharmacokinetics of repaglinide were characterized by the peak concentration in plasma (Cmax), the time to Cmax (tmax), the area under the concentration-time curve from 0 to 7 h [AUC(0,7 h)] and from 0 h to infinity [AUC(0,∞)], and the elimination half-life (t1/2,z). Values for Cmax and tmax were taken directly from the raw data. For each subject, the terminal log-linear part of the concentration-time curve was identified visually, and the elimination rate constant (λz) was determined from these data using linear regression analysis. The t1/2,z was calculated from the equation t1/2,z = ln2/λz. AUC values were determined using the linear trapezoidal rule for the rising phase of the plasma repaglinide concentration-time curve and the log-linear trapezoidal rule for the descending phase, with extrapolation to infinity, when appropriate, by dividing the last measured concentration by λz. The pharmacokinetics of bezafibrate and fenofibrate were characterized by Cmax and AUC (0,8 h). All pharmacokinetic calculations were performed using the program MK-Model, version 5.0 (Biosoft, Cambridge, UK).
Pharmacodynamics
The pharmacodynamics of repaglinide were characterized by baseline, mean and minimum blood glucose concentrations. The baseline and minimum values were taken directly from the original data, and the mean concentration was calculated by dividing the area under the blood glucose concentration-time curve from 0 to 7 h by the corresponding time interval.
Statistical analysis
Results are expressed as mean values ± SD. The pharmacokinetic and pharmacodynamic variables between the placebo and bezafibrate or fenofibrate phases (the AUC and Cmax of repaglinide after log-transformation) were compared using repeated-measures analysis of variance (anova) and a paired t-test with the Bonferroni correction. For all variables, except tmax, 95% confidence intervals (CI) were calculated on the mean differences between the placebo and bezafibrate or fenofibrate phases. The tmax values were compared with Friedman's two-way anova followed by the Wilcoxon signed-rank test with the Bonferroni correction. The analyses were performed using the statistical program Systat for Windows, V6.0.1 (SPSS Inc., Chicago, Ill). Differences were considered statistically significant when P was < 0.05.
Results
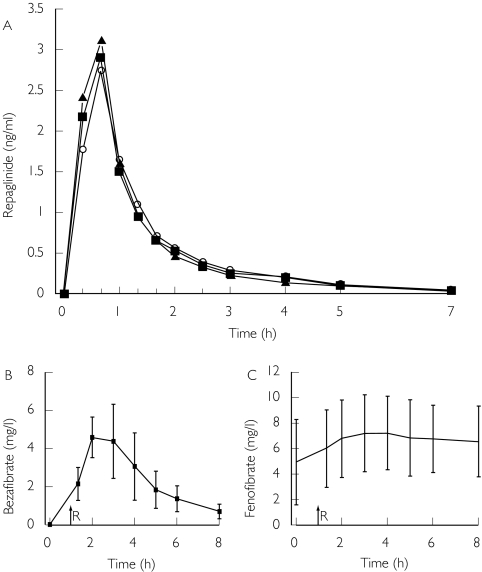
Bezafibrate and fenofibrate had no statistically significant effects on any of the pharmacokinetic variables for repaglinide (Figure 1, Table 2). In the bezafibrate and fenofibrate phases, the AUC(0,∞) for repaglinide was 99.1% (95% confidence interval of the ratio to the control phase 73, 143%) and 99.4% (85, 127%) of the corresponding value during the placebo (control) phase, respectively. The t1/2,z of repaglinide was 1.3 ± 0.4 h, 1.3 ± 0.3 h and 1.3 ± 0.3 h in the placebo, bezafibrate and fenofibrate phases, respectively. The mean Cmax values of repaglinide were slightly but nonsignificantly higher during the bezafibrate (3.0 ± 1.4 ng ml−1) and fenofibrate (3.3 ± 1.6 ng ml−1) phases than during the placebo phase (2.8 ± 1.4 ng ml−1). The AUC(0,7 h) values of repaglinide differed from the AUC(0,∞) values by less than 0.1% and are not presented.
Figure 1.
(A) Mean plasma concentrations of repaglinide in 12 healthy volunteers after a single 0.25 mg oral dose of repaglinide, alone or with 400 mg bezafibrate or 200 mg fenofibrate once daily for 5 days. ○, repaglinide alone; ▪, repaglinide with bezafibrate; ▴, repaglinide with fenofibrate. For clarity, only mean values are presented. Mean (± SD) plasma concentrations of (B) bezafibrate and (C) fenofibrate after the last dose, in their respective phases. Time zero in Figures B and C refers to administration of the fibrates, i.e. 1 h before the administration of repaglinide (R)
Table 2. Pharmacokinetic and pharmacodynamic data for repaglinide after a single 0.25 mg oral dose of the drug in 12 healthy volunteers, alone (placebo) or with 400 mg bezafibrate or 200 mg fenofibrate once daily for 5 days.
| Variable | Placebo phase | Bezafibrate phase | Fenofibrate phase |
|---|---|---|---|
| Repaglinide pharmacokinetics | |||
| Cmax (ng ml−1) | 2.8 ± 1.4 | 3.0 ± 1.4 | 3.3 ± 1.6 |
| 95% CI* | 78, 154% | 96, 154% | |
| tmax (min) | 40 (20–40) | 40 (20–40) | 40 (20–40) |
| t1/2,z (h) | 1.3 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.3 |
| 95% CI* | 77, 123% | 69, 115% | |
| AUC(0, ∞) (ng ml−1 h) | 3.7 ± 2.0 | 3.6 ± 1.8 | 3.7 ± 1.8 |
| 95% CI* | 73, 143% | 85, 127% | |
| Blood glucose | |||
| Baseline concentration (mmol l−1) | 4.8 ± 0.4 | 4.7 ± 0.6 | 4.9 ± 0.4 |
| 95% CI* | 83, 106% | 94, 108% | |
| Mean concentration (0–7 h) (mmol l−1) | 4.6 ± 0.5 | 4.6 ± 0.5 | 4.7 ± 0.4 |
| 95% CI* | 96, 107% | 96, 109% | |
| Minimum concentration (mmol l−1) | 3.5 ± 0.5 | 3.8 ± 0.5 | 3.6 ± 0.3 |
| 95% CI* | 97, 117% | 94, 109% |
Values shown are means ± SD; tmax data are given as median (range). Baseline concentration: fasting concentration on day 5 before repaglinide administration.
95% confidence interval of the ratio to the control phase (% of control).
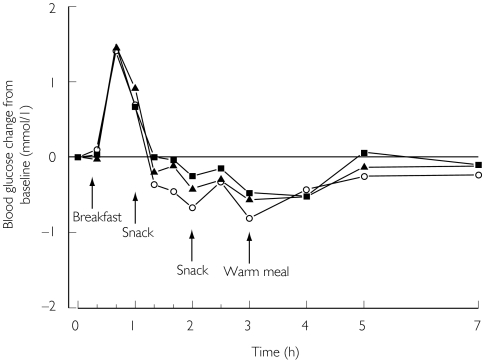
Bezafibrate and fenofibrate did not change the effect of repaglinide on the blood glucose concentrations, when compared with placebo. Neither baseline, mean nor minimum blood glucose concentrations were changed significantly by bezafibrate or fenofibrate (Figure 2, Table 2). None of the subjects had symptomatic hypoglycaemia.
Figure 2.
Mean change in blood glucose concentrations in 12 healthy volunteers after a single 0.25 mg oral dose of repaglinide, alone or with 400 mg bezafibrate or 200 mg fenofibrate once daily for 5 days. ○, alone; ▪, after bezafibrate; ▴, after fenofibrate. For clarity, only mean values are presented
The mean plasma concentrations of bezafibrate and fenofibrate are presented in Figure 2. The AUC(0,8 h) values of bezafibrate and fenofibrate varied 3.0-fold and 4.4-fold and Cmax values varied 2.5-fold and 4.2-fold between subjects, respectively (Table 1).
Discussion
Tight glycaemic control in type II diabetes is important in the prevention of microvascular disease [17, 18]. Management of dyslipidaemia, and the prevention of macrovascular disease is less often emphasized, although equally as important for hyperlipidaemic diabetic patients [14, 19, 20]. The prevalence of hypertriglyceridaemia and low HDL cholesterol concentrations is twice as high in diabetic as in nondiabetic groups [21]. Therefore, it is likely that many diabetic patients benefit from treatment with fibrates, and it is important to characterize the interaction potential of different fibrates with drugs that are commonly used in this patient group.
In this study, we found that administration of the usual therapeutic doses of bezafibrate and fenofibrate had no significant effect on the pharmacokinetics and pharmacodynamics of repaglinide in healthy subjects. These fibrates did not influence the AUC, Cmax or t1/2,z of repaglinde. Furthermore, blood glucose concentration remained unchanged. This lack of interaction is clearly at variance with the potent effect of gemfibrozil on the pharmacokinetics of repaglinide [8]. For safety reasons, only a small, 0.25 mg dose of repaglinide was used in the present study in healthy subjects, who are more sensitive than diabetic patients to the pharmacodynamic effects of the drug. However, as the pharmacokinetics of repaglinide are linear, it is reasonable to assume that the present findings can be extrapolated to normal therapeutic doses (0.5–4 mg) of the drug. Previous studies have shown that repaglinide (0.5–4 mg) improves insulin secretion and reduces prandial hyperglycaemia dose-dependently [22].
The AUC and Cmax values for bezafibrate and fenofibrate, as well as their interindividual variation, were comparable with those found in previous studies [23, 24]. Based on individual plasma concentrations, compliance to bezafibrate and fenofibrate therapy was good, indicating that the lack of an interaction was not caused by a failure to take bezafibrate or fenofibrate.
CYP3A4 and CYP2C8 mainly metabolize repaglinide in vitro[6]. However, in vivo, inhibitors of CYP3A4 have caused only moderate increases in the plasma concentrations of repaglinide. Thus, clarithromycin [25] and itraconazole [8] increased the AUC(0,∞) of repaglinide by about 40%. On the other hand, gemfibrozil causes an 8-fold increase in the AUC(0,∞) of repaglinide and greatly enhances and prolongs its glucose-lowering effect [8]. In diabetic patients using both gemfibrozil and repaglinide, several cases of serious hypoglycaemia have been reported [26]. The combination of itraconazole and gemfibrozil causes an even larger increase in the AUC(0,∞) of about 20-fold and greatly enhances the effects of repaglinide [8]. Gemfibrozil inhibits CYP2C8 in vitro[9] and in vivo[27], but does not affect CYP3A4, at least not in vitro[28]. Gemfibrozil is also known to inhibit other CYP enzymes [10], UDP-glucuronosyltransferases [11], and certain transport proteins in vitro[12]. Thus, there are several possible mechanisms for the gemfibrozil–repaglinide interaction. Trimethoprim, a selective but not very potent CYP2C8 inhibitor [29], increases the AUC(0,∞) of repaglinide on average by 61%[30]. Furthermore, a polymorphism in the CYP2C8 gene is associated with decreased plasma concentrations of repaglinide [31]. Thus, CYP2C8 seems to have a central role in the metabolism of repaglinide in humans in vivo, whereas the contribution of CYP3A4 is probably of limited significance.
Gemfibrozil greatly increases the plasma concentrations of several statins [24, 25, 32, 33], whereas bezafibrate and fenofibrate appear devoid of this effect [33]. Both repaglinide and some of the statins (e.g. cerivastatin) or their active acid metabolites (e.g. simvastatin acid) are substrates of CYP2C8 [9, 34, 35]. Furthermore, the plasma concentrations of the CYP2C8 substrate rosiglitazone are increased by gemfibrozil [36].
The present finding that neither bezafibrate nor fenofibrate interact adversely with repaglinide has important therapeutic consequences. Many diabetic patients using oral hypoglyacemic agents have hypertriglyceridaemia and low HDL cholesterol concentrations, and may be prescribed fibrates. However, because of the potentially hazardous interaction between gemfibrozil and repaglinide, the concomitant use of these drugs is discouraged or even contraindicated in some countries (EU). In contrast, bezafibrate and fenofibrate can be used together with repaglinide due to the lack of interaction.
In conclusion, the administration of bezafibrate or fenofibrate does not increase the plasma concentrations of repaglinide or change its blood glucose-lowering effects in healthy subjects.
Acknowledgments
We thank Mrs Kerttu Mårtensson, Mrs Eija Mäkinen-Pulli and Mrs Lisbet Partanen for skilful technical assistance.
This study was supported by grants from the Helsinki University Central Hospital Research Fund, the National Technology Agency (Tekes), and the Sigrid Juselius Foundation, Finland.
References
- 1.Hatorp V. Clinical pharmacokinetics and pharmacodynamics of repaglinide. Clin Pharmacokinet. 2002;41:471–83. doi: 10.2165/00003088-200241070-00002. [DOI] [PubMed] [Google Scholar]
- 2.Culy CR, Jarvis B. Repaglinide: a review of its therapeutic use in type 2 diabetes mellitus. Drugs. 2001;61:1625–60. doi: 10.2165/00003495-200161110-00008. [DOI] [PubMed] [Google Scholar]
- 3.Gromada J, Dissing S, Kofod H, Frokjaer-Jensen J. Effects of the hypoglycaemic drugs repaglinide and glibenclamide on ATP-sensitive potassium-channels and cytosolic calcium levels in beta TC3 cells and rat pancreatic beta cells. Diabetologia. 1995;38:1025–32. doi: 10.1007/BF00402171. [DOI] [PubMed] [Google Scholar]
- 4.Dabrowski M, Wahl P, Holmes WE, Ashcroft FM. Effect of repaglinide on cloned beta cell, cardiac and smooth muscle types of ATP-sensitive potassium channels. Diabetologia. 2001;44:747–56. doi: 10.1007/s001250051684. [DOI] [PubMed] [Google Scholar]
- 5.Hatorp V, Oliver S, Su CA. Bioavailability of repaglinide, a novel antidiabetic agent, administered orally in tablet or solution form or intravenously in healthy male volunteers. Int J Clin Pharmacol Ther. 1998;36:636–41. [PubMed] [Google Scholar]
- 6.Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol. 2003;56:305–14. doi: 10.1046/j.0306-5251.2003.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Heiningen PN, Hatorp V, Kramer Nielsen K, et al. Absorption, metabolism and excretion of a single oral dose of 14C-repaglinide during repaglinide multiple dosing. Eur J Clin Pharmacol. 1999;55:521–5. doi: 10.1007/s002280050667. [DOI] [PubMed] [Google Scholar]
- 8.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46:347–51. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang JS, Neuvonen M, Wen X, Backman JT, Neuvonen PJ. Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos. 2002;30:1352–6. doi: 10.1124/dmd.30.12.1352. [DOI] [PubMed] [Google Scholar]
- 10.Wen X, Wang JS, Backman JT, Kivistö KT, Neuvonen PJ. Gemfibrozil is a potent inhibitor of human cytochrome P450 2C9. Drug Metab Dispos. 2001;29:1359–61. [PubMed] [Google Scholar]
- 11.Prueksaritanont T, Tang C, Qiu Y, Mu L, Subramanian R, Lin JH. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab Dispos. 2002;30:1280–7. doi: 10.1124/dmd.30.11.1280. [DOI] [PubMed] [Google Scholar]
- 12.Seral C, Carryn S, Tulkens PM, Van Bambeke F. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J Antimicrob Chemother. 2003;51:1167–73. doi: 10.1093/jac/dkg223. [DOI] [PubMed] [Google Scholar]
- 13.Resnick HE, Shorr RI, Kuller L, Franse L, Harris TB. Prevalence and clinical implications of American Diabetes Association-defined diabetes and other categories of glucose dysregulation in older adults: the health, aging and body composition study. J Clin Epidemiol. 2001;54:869–76. doi: 10.1016/s0895-4356(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 14.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis. epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 15.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 16.Masnatta LD, Cuniberti LA, Rey RH, Werba JP. Determination of bezafibrate, ciprofibrate and fenofibric acid in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996;687:437–42. doi: 10.1016/s0378-4347(96)00254-x. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 18.Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001;24:942–5. doi: 10.2337/diacare.24.5.942. [DOI] [PubMed] [Google Scholar]
- 19.Taskinen MR. Controlling lipid levels in diabetes. Acta Diabetol. 2002;39(Suppl 2):S29–34. doi: 10.1007/s005920200023. [DOI] [PubMed] [Google Scholar]
- 20.Taskinen MR. Diabetic dyslipidemia. Atheroscler Suppl. 2002;3:47–51. doi: 10.1016/s1567-5688(01)00006-x. [DOI] [PubMed] [Google Scholar]
- 21.Garg A, Grundy SM. Management of dyslipidemia in NIDDM. Diabetes Care. 1990;13:153–69. doi: 10.2337/diacare.13.2.153. [DOI] [PubMed] [Google Scholar]
- 22.Owens DR, Luzio SD, Ismail I, Bayer T. Increased prandial insulin secretion after administration of a single preprandial oral dose of repaglinide in patients with type 2 Diabetes. Diabetes Care. 2000;23:518–23. doi: 10.2337/diacare.23.4.518. [DOI] [PubMed] [Google Scholar]
- 23.Abshagen U, Bablok W, Koch K, et al. Disposition pharmacokinetics of bezafibrate in man. Eur J Clin Pharmacol. 1979;16:31–8. doi: 10.1007/BF00644963. [DOI] [PubMed] [Google Scholar]
- 24.Harvengt C, Desager JP. [Pharmacokinetics of fenofibrate in man (author's transl) ] Nouv Presse Med. 1980;9:3725–7. [PubMed] [Google Scholar]
- 25.Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001;70:58–65. doi: 10.1067/mcp.2001.116511. [DOI] [PubMed] [Google Scholar]
- 26.Novo Nordisk A/S. Prandin Tablets Package Insert. NDA 20,741. 2003.
- 27.Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–91. doi: 10.1067/mcp.2002.128469. [DOI] [PubMed] [Google Scholar]
- 28.Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–9. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 29.Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos. 2002;30:631–5. doi: 10.1124/dmd.30.6.631. [DOI] [PubMed] [Google Scholar]
- 30.Niemi M, Kajosaari L, Neuvonen M, Backman JT, Neuvonen PJ. The CYP2C8 inhibitor trimethoprim increases the plasma concentrations of repaglinide. Br J Clin Pharmacol. 2004. pp. 441–7. [DOI] [PMC free article] [PubMed]
- 31.Niemi M, Leathart JB, Neuvonen M, Backman JT, Daly AK, Neuvonen PJ. Polymorphism in CYP2C8 is associated with reduced plasma concentrations of repaglinide. Clin Pharmacol Ther. 2003;74:380–7. doi: 10.1016/S0009-9236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- 32.Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther. 2003;73:538–44. doi: 10.1016/S0009-9236(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 33.Kyrklund C, Backman JT, Kivistö KT, Neuvonen M, Laitila J, Neuvonen PJ. Plasma concentrations of active lovastatin acid are markedly increased by gemfibrozil but not by bezafibrate. Clin Pharmacol Ther. 2001;69:340–5. doi: 10.1067/mcp.2001.115542. [DOI] [PubMed] [Google Scholar]
- 34.Mück W. Clinical pharmacokinetics of cerivastatin. Clin Pharmacokinet. 2000;39:99–116. doi: 10.2165/00003088-200039020-00002. [DOI] [PubMed] [Google Scholar]
- 35.Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003;56:120–4. doi: 10.1046/j.1365-2125.2003.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niemi M, Backman JT, Granfors M, Laitila J, Neuvonen M, Neuvonen PJ. Gemfibrozil considerably increases the plasma concentrations of rosiglitazone. Diabetologia. 2003;46:1319–23. doi: 10.1007/s00125-003-1181-x. [DOI] [PubMed] [Google Scholar]