Abstract
Aim
St John's Wort (SJW) enhances CYP3A4 activity and decreases blood concentrations of CYP3A4 substrates. In this study, the effects of SJW on a benzodiazepine hypnotic, quazepam, which is metabolized by CYP3A4, were examined.
Methods
Thirteen healthy subjects took a single dose of quazepam 15 mg after treatment with SJW (900 mg day−1) or placebo for 14 days. The study was performed in a randomized, placebo-controlled, cross-over design with an interval of 4 weeks between the two treatments. Blood samples were obtained during a 48 h period and urine was collected for 24 h after each dose of quazepam. Pharmacodynamic effects were determined using visual analogue scales (VAS) and the digit symbol substitution test (DSST) on days 13 and 14.
Results
SJW decreased the plasma quazepam concentration. The Cmax and AUC0-48 of quazepam after SJW were significantly lower than those after placebo [Cmax; −8.7 ng ml−1 (95% confidence interval (CI) −17.1 to −0.2), AUC0-48; −55 ng h ml−1 (95% CI −96 to −15)]. The urinary ratio of 6β-hydroxycortisol to cortisol, which reflects CYP3A4 activity, also increased after dosing with SJW (ratio; 2.1 (95%CI 0.85–3.4)). Quazepam, but not SJW, produced sedative-like effects in the VAS test (drowsiness; P < 0.01, mental slowness; P < 0.01, calmness; P < 0.05, discontentment; P < 0.01). On the other hand, SJW, but not quazepam impaired psychomotor performance in the DSST test. SJW did not influence the pharmacodynamic profile of quazepam.
Conclusions
These results suggest that SJW decreases plasma quazepam concentrations, probably by enhancing CYP3A4 activity, but does not influence the pharmacodynamic effects of the drug.
Keywords: CYP3A4, quazepam, St. John's Wort
Introduction
St. John's Wort (SJW) is one of the most commonly used herbal medicines for the treatment of mild to moderate depression in the countries of the European Union and in the United States [1, 2]. The internet marketing sales of SJW were reported to be 235 million dollars in 2000 [3]. More than 2000 products that contain SJW are also consumed as dietary supplements or food products in Japan. SJW is a potent inducer of cytochrome P450 (CYP) 3A4 in the intestinal wall and liver [4], and it reduces the plasma concentrations of some CYP3A4 substrates, which, in turn, may influence the outcome of drug therapy [5, 6].
Hypnotic drugs are used for treating insomnia, which is one of the common symptoms of depressive patients and is involved in the diagnostic criteria for depression [7]. Therefore, it is likely that subjects with depressive states may simultaneously take SJW and a hypnotic drug. A recent epidemiological study demonstrated that more than 7% patients taking benzodiazepine hypnotics also used herbal preparations/supplements concomitantly [8]. The substantial overlap between use of benzodiazepines and herbal preparations/supplements such as SJW raises concern about unintended interactions. Drug interactions between SJW and midazolam [9] and alprazolam [10] have already been examined. Quazepam is a trifluoethylbenzodiazepine, and has significant effects on the induction and maintenance of sleep without major effects on sleep architecture [11, 12]. Quazepam is metabolized to 2-oxoquazepam, an active metabolite, which is further converted to other less active metabolites [13]. Because the metabolism of quazepam and 2-oxoquazepam is mediated by CYP3A4 and CYP2C9 [13], it is likely that SJW decreases plasma concentrations of quazepam and 2-oxoquazepam and consequently diminishes the pharmacodynamic effects of the drug. This study was undertaken to examine this hypothesis. The effect of SJW on the pharmacokinetics and pharmacodynamics of quazepam was evaluated in a double-blind, placebo-controlled, cross-over study in healthy subjects.
Methods
Subjects
To detect a difference of 30% in the area under the plasma concentration-time curve (AUC) of quazepam between with and without SJW with a power = 80% and α= 0.05, 12 subjects were required. Therefore, 13 healthy men [age mean ± s.d., 34 ± 6 years (range, 25–45); weight ± s.d., 66 ± 7 kg (range, 56–75)] were enrolled in this study. As the SJW-mediated induction of CYP3A4 activity is reported to differ between males and females [14], only male subjects were studied. Their biochemical and haematological functions were normal at screening. They were non-smokers and did not receive any continuous medications. Subjects were requested to abstain from grapefruit, grapefruit juice, herbal dietary supplements, and herbal tea during the study period. Caffeine-containing beverages, including coffee and green tea, were withheld from the night before the study day until the final blood sample. The study protocol was approved by the Ethics Committee of Jichi Medical School (Tochigi, Japan). All volunteers gave written informed consent.
Study design
A randomized, double-blind, cross-over design, with an interval of 4 weeks between treatments, was used in this study. In each phase, the subject took a 300 mg caplet of SJW (lot NO.265112) (TruNature, Carson, California, USA) or matching placebo orally three times a day for 14 days, according to a randomization schedule. The SJW caplet used in this study was labelled to be standardized to 0.3% hypericin. The dose of SJW was chosen on the basis of previous reports [4, 15]. Our previous study showed that 900 mg of SJW, which was the same caplet used in this study, for 14 days decreased the blood concentration of the CYP3A4 substrate, simvastatin [16]. In Japan the recommended dose of quazepam is 15–30 mg for the treatment of sleep disorders. Because 15 mg quazepam is used more often than 20 or 30 mg, we chose this dose for our study. Adverse symptoms were checked during repeated dosing with SJW or placebo. On day 14, a single oral dose of 15 mg quazepam (Mitsubishi Pharma Co. Ltd, Tokyo, Japan) was given to the subjects, with 150 ml water at 08:00. The subjects fasted overnight before the dose of quazepam and were allowed a meal 4 h afterwards. On days 7 and 14, we checked the number of remaining SJW caplets. CYP2C9 plays a role in the disposition of quazepam [13]. However, because polymorphisms in CYP2C9 are not common in the Asian population [17], genotyping for CYP2C9 was not performed in this study.
Blood and urine sampling
On day 14, blood samples (2 ml in each) were collected in heparinized tubes just before and at 0.5, 1, 2, 3, 4, 6, 8, 12, 24 and 48 h after the dose of quazepam. Urine was collected for 24 h. Plasma and urine samples were stored at −80 °C until analysis.
Pharmacodynamic measurements
The effect of SJW and/or quazepam on sedative-like self-rated moods was determined by visual analogue scale (VAS) and on psychomotor performance by the digit symbol substitution test (DSST) immediately before blood sampling at 0, 2, 4, 8 and 12 h after dosing on day 14. These tests were also performed at identical clock-times on day 13. The subjects had been fully trained to perform the tests before the start of the study. A 100-mm long horizontal VAS was used to measure sedative-like self-rated moods, which were the pairs of adjectives such as drowsy/alert, calm/nervous, mentally slow/quick-witted, and discontented/contented. In the DSST, the number of digits correctly substituted in 2 min was recorded.
Determination of quazepam and 2-oxoquazepam
Plasma concentrations of quazepam and 2-oxoquazepam were measured by a column-switching high performance liquid chromatography (HPLC) analysis, as described by Hikida et al.[18]. Aliquots of each plasma sample (1.5 ml), to which 0.1 ml of cisapride (800 ng ml−1) was added as an internal standard, were alkalinized with 500 kl of 0.5 m NaOH and then, 0.4 ml of water and 5 ml of toluene/chloroform (85 : 15, v/v) were added. The mixture was shaken vigorously for 15 min and then centrifuged at 2000 g for 10 min. A 4.5 ml portion of the organic layer was evaporated to dryness in vacuo at 45 °C. The residue was reconstituted with 0.8 ml of eluent A (see below) and used as an extract. A 0.5-ml portion of the extract was injected onto the column-switching HPLC system. The HPLC system consisted of a chromatography pump (LC-10 A; Shimadzu, Tokyo, Japan), an autoinjector (AS-8020, Toshoh Co., Tokyo, Japan), and an ultraviolet detector (SPD-10 A, Shimadzu, Tokyo, Japan). Column I (TSK-BSA-C8, 5 µm, Tosoh, 10 mm × 4.6 mm) was used for pretreatment and column II (STR-ODS II, 5 µm, Shimadzu, 150 mm × 4.6 mm) for the column oven module. Between 0 and 13 min after a sample injection, cisapride was separated from the interfering substances existing in the extract on column I with a mobile phase solvent (eluent A) consisting of acetnitrile/0.02 mol l−1 KH2P04 (13 : 87, v/v). Between 13 and 20 min after the injection, quazepam and 2-oxoquazepam retained on column I were eluted with a mobile phase (eluent B) consisting of acetnitrile/perchloric acid/0.02 mol l−1 KH2P04 (41 : 0.05: 58.95, v/v/v), and the effluent from column I was switched to column II. Quazepam and 2-oxoquazepam were separated on column II by eluting with a mobile phase solvent (eluent C) consisting of acetnitrile/0.02 mol l−1 KH2P04 (62.5 : 37.5, v/v) between 32.0 and 46.5 min. The mobile phase was pumped at a flow rate of 0.6 ml min−1. The absorbance of the effluent from column II was monitored at 254 nm for 2-oxoquazepam and 286 nm for quazepam. The limits of quantification for quazepam and 2-oxoquazepam were 0.5 ng ml−1. The coefficient of variation for intra- and inter reproducibility was better than 3.7% at 2, 20 and 40 ng ml−1.
Determination of cortisol and 6β-hydroxycortisol
Cortisol, 6β-hydroxycortisol and 6α-methylprednisolone were purchased from Sigma (St. Louis, MO, USA). O-methylhydroxylamine hydrochloride was purchased from Tokyo Chemical Industry (Tokyo, Japan) and N-(trimethylsilyl) imidazole (TMSI) from Nacalai Tesque (Kyoto, Japan). All other chemicals and solvents were of analytical grade. A 1.0-ml of urine sample with 6α-methylprednisolone (as an internal standard, 500 ng ml−1) was loaded onto a preconditioned Sep–Pak C18 cartridge (Waters, Milford, MA, USA). The cartridge was washed with 5 ml of distilled water and then eluted with 2 ml of ethyl acetate into a glass tube. The eluate was evaporated to dryness at 60 °C under reduced pressure. Derivatization was performed according to the general procedures as described previously [19]. The dried residue was dissolved into 100 µl of a 2% solution of O-methylhydroxylamine hydrochloride in pyridine. After 2 h at 60 °C, the pyridine was evaporated and 50 kl of TMSI was added. This was kept at 100 °C for 15 h to yield methyloxime-trimethylsilyl esters. Gas chromatography-mass spectrometry (GC-MS) analysis was carried out on a GCMS-QP5050A gas chromatography-mass spectrometer (Shimadzu, Kyoto, Japan). Gas chromatography was performed on an Ultra Ally 5 fused silica capillary column (30 m × 0.25 mm I.D., film thickness 0.25 µm, Frontier Laboratories Ltd, Fukushima, Japan) with splitless injection mode. Two µl of derivative were injected (injector:280 °C). The temperature program was as follows: the initial temperature 230 °C was held for 2 min, and then increased to 260 °C at 15 °C min−1. After a steady period for 1 min, the temperature was increased to 320 °C at 2 °C min−1, and held for 5 min. The selected ion monitoring was used for the detection of cortisol (m/z = 605), 6β-hydroxycortisol (m/z = 694) and 6α-methylprednisolone (m/z = 617). The quantifications of cortisol and 6β-hydroxycortisol were performed by measuring the peak-area ratios of these compounds and of the internal standard. The limit of quantification was 0.2 ng ml−1 for cortisol and 0.5 ng ml−1 for 6β-hydroxycortisol. The coefficient of variation for intra- and inter reproducibility was better than 5.9%.
Pharmacokinetic calculations
The pharmacokinetics were characterized by maximum plasma concentration (Cmax), time to maximum concentration (tmax), elimination half-life (t1/2), and area under the plasma concentration-time curve from 0 to 48 h after dosing (AUC0-48). Elimination rate constant (ke) was determined by a linear regression analysis of a log-linear phase of plasma drug concentration-time curve. The elimination half-life (t1/2) was calculated as follows: t1/2 = ln 2/ke. The AUC0–48 was calculated by the trapezoidal rule.
Statistical analysis
Data are expressed as the mean ± SE. Pharmacokinetic parameters were analysed by one-way anova. Pharmacodynamic effects [without (on day 13) vs. with (on day 14) quazepam] were analysed by repeated measures anova and pharmacodynamic effects (with vs. without SJW) were analysed by repeated measures anova of a cross-over design with the adjustment of Huynh-Feldt. Differences were considered to be statistically significant for P-values < 0.05. Calculations were performed by SAS software (SAS Institute Inc, Cary, NC, USA).
Results
All enrolled subjects took the full course of SJW and no adverse symptoms were observed during the study.
Plasma concentrations of quazepam and 2-oxoquazepam
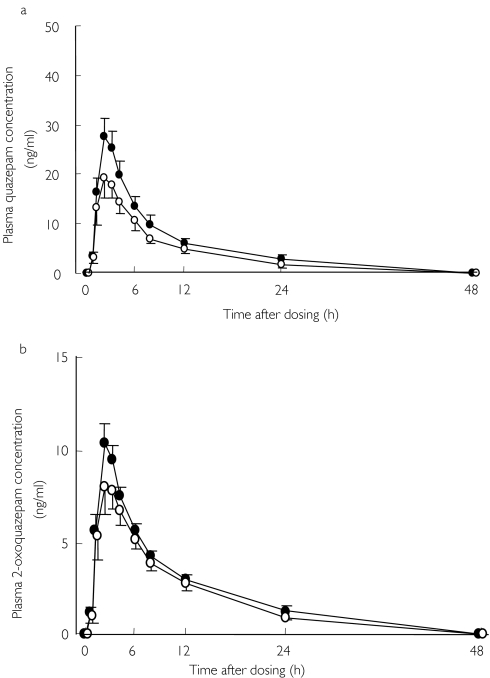
Plasma concentrations of quazepam and 2-oxoquazepam decreased after pretreatment with SJW (Figure 1a, b). The values of Cmax and AUC0–48 for quazepam with SJW were significantly lower than those after placebo (Table 1). The values of 2-oxoquazepam were also lower after SJW, but these differences did not reach statistical significance (Table 1). The ratio of 2-oxoquazepam to quazepam in the Cmax was significantly greater in the SJW period (placebo; 0.40 ± 0.04, SJW; 0.47 ± 0.04, P < 0.01). No significant differences were observed in the t1/2 or tmax (Table 1).
Figure 1.
Mean ± SE plasma concentrations of quazepam (1a) and 2-oxoquazepam (1b) after a single oral dose of 15 mg quazepam following pretreatment with placebo (•) or SJW (900 mg day−1 for 14 days) (○) in 13 healthy males
Table 1. Effect of St. John's Wort on the pharmacokinetics of quazepam and its metabolite, 2-oxoquazepam (Mean +/− SE, n = 13).
| Treatment | anova | |||
|---|---|---|---|---|
| Quazepam + placebo | Quazepam + SJW | Mean value of difference (95% CI) | P-value | |
| parameterquazepam | ||||
| Cmax (ng/ml) | 30.5 ± 3.9 | 21.8 ± 3.9 | −8.7 (−17.1 ∼−0.2) | P < 0.05 |
| tmax (h) | 2.2 ± 0.2 | 2.5 ± 0.2 | 0.3 (−0.3 ∼ 0.9) | NS |
| t1/2 (h) | 8.8 ± 0.5 | 8.4 ± 0.4 | −0.3 (−1.7 ∼ 1.0) | NS |
| AUC0-48 (ng h ml-1) | 217 ± 28.7 | 161 ± 25.2 | −55 (−96.0 ∼−15.0) | P < 0.05 |
| 2-oxoquazepam | ||||
| Cmax (ng ml-1) | 10.9 ± 1.0 | 9.1 ± 1.4 | −1.8 (−4.7 ∼ 1.2) | NS |
| tmax (h) | 2.2 ± 0.2 | 2.8 ± 0.3 | 0.6 (−0.1 ∼ 1.4) | NS |
| t1/2 (h) | 9.5 ± 0.5 | 9.1 ± 0.5 | −1.0 (−3.0 ∼ 1.0) | NS |
| AUC0-48 (ng h ml-1) | 92 ± 5.7 | 80 ± 9.5 | −12 (−32.6 ∼ 9.6) | NS |
Cmax, maximum plasma concentration; tmax, time to maximum concentration; t1/2, elimination half-life; AUC0-48, area under the plasma concentration-time curve from 0 to 48 h after dosing; CI, confidence interval; NS, not significant.
Urinary ratio of 6β-hydroxycortisol/cortisol (6β-OHC/C)
The ratio of 6β-OHC/C significantly increased after repeated dosing with SJW for 14 days (placebo; 9.4 ± 4.8, SJW; 18.4 ± 11.7: ratio; 2.1, 95% confidence interval, 0.85–3.4, P < 0.05).
Pharmacodynamics
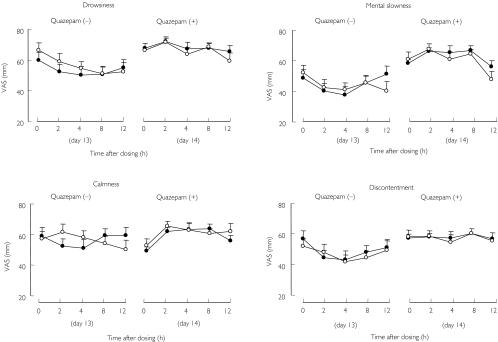
No significant change was detected in the VAS test with SJW alone (Figure 2). On the other hand, quazepam produced significant sedative-like effects (drowsiness; F-value 23.2, P < 0.01, mental slowness; F-value 8.1, P < 0.01, calmness; F-value 6.9, P < 0.05, discontentment; F-value 14.4, P < 0.01). SJW did not influence the subjective effects of quazepam.
Figure 2.
Mean ± SE subjective drug effects [based on scores from a 100-mm visual analogue scale (VAS)] before (on day 13) and after (on day 14) a single oral dose of 15 mg quazepam in 13 healthy males. Pretreatment with placebo (•); pretreatment with SJW (○) (900 mg day−1 for 14 days)
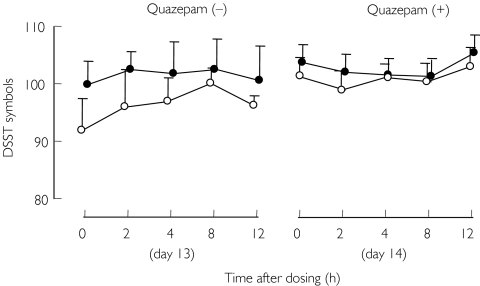
SJW significantly (F-value 4.9, P < 0.05) impaired psychomotor performance in the DSST while quazepam did not (Figure 3). A carry-over effect was detected in the DSST (F-value 16.5, P < 0.01). SJW did not influence performance after quazepam.
Figure 3.
Mean ± SE digit symbol substitution test (DSST) scores before (on day 13) and after (on day 14) a single oral dose of 15 mg quazepam in 13 healthy males. Pretreatment with placebo (•); pretreatment with SJW (○) (900 mg day−1 for 14 days)
Discussion
This study showed that the concomitant use of SJW decreased plasma concentrations of quazepam, although the effect was smaller than that for alprazolam [10]. Although the label stated that the SJW caplet contained 0.3% hypericin, this was not confirmed. However the same caplet of SJW was used by us in a previous study which showed that SJW substantially altered the pharmacokinetics of simvastatin [16]. In a previous report, SJW lowered the trough cyclosporin concentration in whole blood, but this returned to normal 7 days after discontinuation of SJW [20]. It therefore seems unlikely that the carry-over effect of SJW lasts for 4 weeks.
The baseline pharmacokinetic profiles of quazepam in Japanese male subjects were similar in this and other studies, except for t1/2[21]. The difference in the mean t1/2 (21.2 vs 8.8 h) may be due to the difference in the duration of the blood-sampling period.
Quazepam is a relatively weak benzodiazepine-receptor ligand and binds selectively to the type-1 benzodiazepine-receptor [22, 23]. An in vitro study showed that quazepam and 2-oxoquazepam are metabolized by CYP3A4 and CYP2C9 [13]. Because SJW induces the activity of CYP3A4, but not that of CYP2C9 [4, 15, 24], it is likely that the enhancement of the CYP3A4-mediated metabolism of quazepam and 2-oxoquazepam is involved in the SJW-related reductions in plasma concentrations of these two drugs.
CYP3A4 is the most abundantly expressed CYP (approximately 30% to 40% of the total CYP content in the human adult liver and small intestine) and plays a major role in the metabolic pathways of various drugs [25, 26]. SJW has been shown to induce hepatic and intestinal CYP3A4 activity [4, 15], probably through the activation of the pregnane X receptor, a human orphan nuclear receptor [27]. The activated CYP3A4, in turn, has enhanced the metabolism of several drugs such as indinavir and cyclosporin and diminished their efficacy during repeated dosing with SJW [5, 6]. In this study, the urinary ratio of 6β-hydroxycortisol to cortisol increased after dosing with SJW for 14 days, which indicates that the hepatic CYP3A4 activity was activated under the present study conditions [28]. Similar data have already been reported [15, 29]. However, because the elimination t1/2 of quazepam and 2-oxoquazepam were not significantly changed in this study, the degree of the SJW-mediated induction might be relatively small for hepatic CYP3A4. On the other hand, the ratio of Cmax of 2-oxoquazepam to quazepam, which might reflect CYP3A4 activity in the liver and intestine, significantly increased after the repeated dosing of SJW in this study. Therefore, although there is no evidence indicating intestinal metabolism of quazepam, we believe that SJW activated intestinal and hepatic CYP3A4 activity and consequently, enhanced the conversion of quazepam to 2-oxoquazepam.
SJW is also reported to induce P-glycoprotein expression in human subjects [4, 30]. Induction of P-glycoprotein would decrease the extent of absorption of the substrates for this transporter. Although benzodiazepines, such as flunitrazepam and midazolam, are not substrates for P-glycoprotein [31, 32], it is not known whether quazepam is a substrate for this transporter. The possibility that the SJW-related reduction in plasma quazepam concentration was caused by an induction of P-glycoprotein remains to be determined.
Meta-analyses of randomized clinical trials have demonstrated that SJW is as effective as standard antidepressants in the treatment of mild to moderate depression [1, 2]. Although SJW is reported to be as safe as, or possibly safer, than standard antidepressants, it also causes adverse effects including central nervous system (CNS)-related symptoms [33]. To our knowledge, well-controlled clinical trials, which were undertaken to examine the CNS-related symptoms caused by SJW, are limited [34]. In this study, SJW had no effect on the sedative-like self-rated moods, which agrees with previous data [34]. SJW impaired psychomotor performance measured by the DSST test although it has been reported that it did not impair cognitive function [34] as determined using the Block Board tapping test [35]. The diverse effects of SJW could be explained by the different methodologies used. In this study, a carry-over effect was detected in the DSST test. However, as the trial was performed with a cross-over design, the influence of such an effect on the data should be small. Further studies are needed to conclude that SJW impairs psychomotor function after repeated use.
The mechanism of the antidepressant activity of SJW is not fully understood, but inhibition of the GABA receptor-mediated response is involved [33, 36]. On the other hand, benzodiazepines including quazepam stimulate the GABA receptor-mediated response [37]. Therefore, the effect of SJW on the CNS seems to differ from that of benzodiazepines. In fact, the quantitative electroencephalogram showed that SJW increases slow wave activity while benzodiazepines enhance fast wave activity [34, 37]. These data led us to speculate that the pharmacodynamic effects of quazepam might be altered by the repeated dosing with SJW. In this study, quazepam 15 mg produced significant sedative-like effects. Although plasma concentrations of quazepam and 2-oxoquazepam decreased after repeated dosing with SJW, SJW did not reduce the sedative-like effects of quazepam.
In summary, this study showed that SJW decreased plasma concentrations of quazepam, probably by activating CYP3A4, but it did not diminish the pharmacodynamic effects of the drug.
Acknowledgments
The authors gratefully thank Dr T. Kotegawa of the Oita Medical University for his helpful suggestions and Ms. C. Fukushima, Ms. T. Kawaguchi, and Ms. M. Hojo for their expert assistance.
References
- 1.Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John's Wort for depression – an overview and meta-analysis of randomized clinical trials. BMJ. 1996;313:253–8. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaster B, Holroyd J. St John's Wort for depression. Arch Intern Med. 2000;160:152–6. doi: 10.1001/archinte.160.2.152. [DOI] [PubMed] [Google Scholar]
- 3.Morris CA, Avorn. J Internet Marketing Herbal Products JAMA. 2003;290:1505–9. doi: 10.1001/jama.290.11.1505. [DOI] [PubMed] [Google Scholar]
- 4.Durr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 5.Ruschitzka F, Meier PJ, Turina M, Luscher TF, Noll G. Acute heart transplant rejection due to Saint John's wort. Lancet. 2000;355:548–9. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 6.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's Wort. Lancet. 2000;355:547–8. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 7.Judd LL, Braff DL, Britton KT, Risch SC, Gillin JC, Grant I. Psychiatry and Medicine. In: Wilson JD, Brannwald E, Isselbacher KJ, Pefersdorf RG, Martin JB, Fanci AS, Root RK, editors. Harrison's Principles of. 12. New York: McGraw-Hill: Internal Medicine; 1991. p. 2139. P2123- [Google Scholar]
- 8.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone Survey. JAMA. 2002;287:337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 9.Dresser GK, Schwarz UI, Wilkinson GR, Kim RB. Coordinate induction of both cytochrome P4503A and MDR1 by St John's wort in healthy subjects. Clin Pharmacol Ther. 2003;73:41–50. doi: 10.1067/mcp.2003.10. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz JS, Donovan JL, DeVane CL, Taylor RM, Ruan Y, Wang JS, Chavin KD. Effect of St John's Wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290:1500–4. doi: 10.1001/jama.290.11.1500. [DOI] [PubMed] [Google Scholar]
- 11.Kales A. Quazepam: hypnotic efficacy and side effects. Pharmacotherapy. 1990;10:1–10. [PubMed] [Google Scholar]
- 12.Mendels J. Evaluation of the safety and efficacy of quazepam for the treatment of insomnia in psychiatric outpatients. J Clin Psychiatry. 1994;55:60–5. [PubMed] [Google Scholar]
- 13.Fujisaki H, Hirotsu K, Ogawa T, Mizuki K, Mizuta H, Arima N. Metabolism of quazepam and its metabolites in humans: Identification of metabolic enzymes and evaluation of drug interaction in vitro. Xenobio Metabol Dispos. 2001;16:558–68. (in Japanese with English summary) [Google Scholar]
- 14.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY. Cytochrome P450 phenotypic ratios for predicting herb–drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–87. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 15.Roby CA, Anderson GD, Kantor E, Dryer DA, Burstein AH. Clin Pharmacol Ther. Vol. 67. St John's Wort: effect on CYP3A4 activity; 2000. pp. 451–7. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto K, Ohmori M, Tsuruoka S, Nishiki K, Kawaguchi A, Harada K, Arakawa M, Sakamoto K, Masada M, Miyamori I. Different effects of St John's Wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther. 2001;70:317–26. doi: 10.1067/mcp.2001.120025. [DOI] [PubMed] [Google Scholar]
- 17.Nasu K, Kubota T, Ishizaki T. Genetic analysis of CYP2C9 polymorphism in a Japanese population. Pharmacogenetics. 1997;7:405–9. doi: 10.1097/00008571-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Hikida K, Inoue Y, Miyazaki T, Kojima N, Ohkura Y. Determination of bromperidol in serum by automated column-switching high-performance liquid chromatography. J Chromatogr. 1989;495:227–34. doi: 10.1016/s0378-4347(00)82624-9. [DOI] [PubMed] [Google Scholar]
- 19.Palermo M, Gomez-Sanchez C, Roitman E, Shackleton CHL. Quantitation of cortisol and related 3-oxo-4-ene steroids in urine using gas chromatography/mass spectrometry with stable isotope-labeled internal standards. Steroids. 1996;61:583–9. doi: 10.1016/s0039-128x(96)00118-9. [DOI] [PubMed] [Google Scholar]
- 20.Breidenbach T, Kliem V, Burg M, Radermacher J, Hoffmann MW, Klempnauer J. Profound drop of cyclosporin A whole blood trough levels caused by St. John's Wort (Hypericum perforatum) Transplantation. 2000;69:2229–30. doi: 10.1097/00007890-200005270-00052. [DOI] [PubMed] [Google Scholar]
- 21.Kanda H, Yasui-Furukori N, Fukasawa T, Aoshima T, Suzuki A, Otani K. Interaction study between fluvoxamine and quazepam. J Clin Pharmacol. 2003;43:1392–7. doi: 10.1177/0091270003258667. [DOI] [PubMed] [Google Scholar]
- 22.Miller LG, Galpern WR, Byrnes JJ, Greenblatt DJ. Benzodiazepine receptor binding of benzodiazepine hypnotics: receptor and ligand specificity. Pharmacol Biochem Behav. 1992;43:413–16. doi: 10.1016/0091-3057(92)90170-k. [DOI] [PubMed] [Google Scholar]
- 23.Wamsley JK, Hunt MA. Relative affinity of quazepam for type-1 benzodiazepine receptors in brain. J Clin Psychiatry. 1991;52(Suppl 9):15–20. [PubMed] [Google Scholar]
- 24.Wang Z, Gorski JC, Hamman MA, Huang SM, Lesko LJ, Hall SD. The effects of St John's wort (Hypericum perforatum) On Human Cytochrome P450 Activity. Clin Pharmacol Ther. 2001;70:317–26. [PubMed] [Google Scholar]
- 25.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A ontogeny and drug disposition. Clin Pharmacokinet. 1999;37:485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Benet LZ. The gut as a barrier to drug absorption. combined role of cytochrome P450 3A and P–glycoprotein. Clin Pharmacokinet. 2001;40::. doi: 10.2165/00003088-200140030-00002. 159, 68. [DOI] [PubMed] [Google Scholar]
- 27.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, St. Kliewer SA. John's Wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ged C, Rouillon JM, Pichard L, Combalbert J, Bressot N, Bories P, Michel H, Beaune P, Maurel P. The increase in urinary excretion of 6β-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989;28:373–87. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer S, Stormer E, Kerb R, Johne A, Brockmoller J, Roots I. Differential effects of Saint John's Wort (hypericum perforatum) on the urinary excretion of d-glucaric acid and 6β-hydroxycortisol in healthy volunteers. Eur J Clin Pharmacol. 2002;58:581–5. doi: 10.1007/s00228-002-0527-5. [DOI] [PubMed] [Google Scholar]
- 30.Hennessy M, Kelleher D, Spiers JP, Barry M, Kavanagh P, Back D, Mulcahy F, Feely J. St John's Wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol. 2002;53:75–82. doi: 10.1046/j.0306-5251.2001.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schinkel AH, Wagenaar E, Mol CAAM, van Deemter L. P-glycoprotein in the blood–brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–24. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takano M, Hasegawa R, Fukuda T, Yumoto R, Nagai J, Murakami T. Interaction with P-glycoprotein and transport of erythromycin, midazolam and ketoconazole in Caco-2 cells. Eur J Pharmacol. 1998;358:289–94. doi: 10.1016/s0014-2999(98)00607-4. [DOI] [PubMed] [Google Scholar]
- 33.Di Carlo G, Borrelli F, Ernst E, Izzo AA. St John's Wort: Prozac from the plant kingdom. Trends Pharmacol Sci. 2001;22:292–7. doi: 10.1016/s0165-6147(00)01716-8. [DOI] [PubMed] [Google Scholar]
- 34.Siepmann M, Krause S, Joraschky P, Muck-Weymann M, Kirch W. The effects of St John's Wort extract on heart rate variability, cognitive function and quantitative EEG. a comparison with amitriptyline and placebo in healthy men. Br J Clin Pharmacol. 2002;54:277–82. doi: 10.1046/j.1365-2125.2002.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smirni P, Villardita C, Zappala G. Influence of different paths on spatial memory performance in the Block-Tapping Test. J Clin Neuropsychol. 1983;5:355–9. doi: 10.1080/01688638308401184. [DOI] [PubMed] [Google Scholar]
- 36.Henderson L, Yue QY, Bergquist C, Gerden B, Arlett P. St John's Wort (Hypericum perforatum) : Drug Interactions and Clinical Outcome Br J Clin Pharmacol. 2002;54:349–56. doi: 10.1046/j.1365-2125.2002.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobbs WR, Rall TW, Verdoorn TA. In: The Pharmacological Basis of Therapeutics. 9. Hardman JG, Limbind LE, Molinoff PB, Ruddon RW, Goodman-Gilman A, editors. New York: McGraw-Hill; 1996. pp. p361–396. [Google Scholar]