Abstract
The pharmaceutical industry continues to look for ways to reduce drug candidate attrition throughout the drug discovery and development process. A significant cause of attrition is due to safety issues arising either as a result of animal toxicity testing or in the clinical programme itself. A factor in the assessment of safety during early drug development is the pharmacokinetic profile of the compound. This allows safety data to be considered in the light of systemic drug exposure and therefore permits a quantitative assessment. This is particularly applicable when assessing the risk of a new chemical entity (NCE) in relation to safety parameters such as QT interval prolongation, where free plasma concentrations have been shown to be predictive of this property in relation to potency in preclinical testing. Prior to actual human exposure it is therefore important to be able to predict reliably the pharmacokinetic behaviour of an NCE in order to place such safety findings into a quantitative risk context. The emerging science of pharmacogenetics is likely to further our ability to assess the risk of NCEs to populations and individuals due to genetic variance. The drug metabolizing enzyme CYP2D6 has been recognized as providing the potential to result in widely differing systemic drug exposure in the patient population due to polymorphic expression. Further knowledge is likely to add to our understanding of population differences in exposure and response and aid in the identification of risk factors. One potential strategy for improving the effectiveness of the drug discovery process is to obtain clinical pharmacokinetic data more rapidly in order to assess more accurately the potential for both efficacy and safety of an NCE. Whilst procedures and technologies are available that allow this on the microdose scale, it is important that we recognize potential limitations of these approaches in order that they can be applied beneficially.
Keywords: drug discovery, drug safety, pharmacodynamics, pharmacokinetics
Introduction
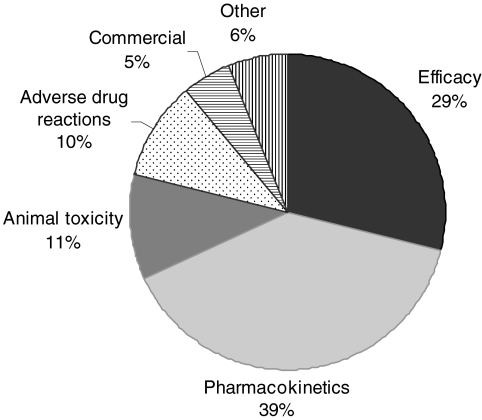
The cost and time taken to bring new medicines to the market has continued to rise over recent times [1, 2] whilst the number of new drug approvals has declined [3]. In order to try to maximize return on investment the pharmaceutical industry is generally looking to improve success rates and reduce candidate attrition during the drug development process. Early termination of drug development programmes that will ultimately fail is seen as an approach that leads to overall cost reduction [2]. In order to achieve this it is important to understand the root causes of attrition that have led to drug development failure in the past. A previously published survey on the causes of failure in drug development (Figure 1) indicated that inappropriate pharmacokinetics were a major cause [4]. Inappropriate pharmacokinetic behaviour includes such factors as low bioavailability due to high extraction or poor absorption characteristics, short elimination half-life leading to short duration of action and excessive variability due to genetic or environmental factors. This observation has led to an increased emphasis on pharmacokinetic input to the drug discovery process throughout the pharmaceutical industry [5]. Much progress has been made in developing tools for the prediction of drug absorption [6–10], drug clearance [11–14] and drug–drug interactions [15–18], in addition to the scaling of pharmacokinetic parameters from animals to man [19–22]. This increased consideration of the suitability of the pharmacokinetic profile has led to a reduction in the early termination of programmes due to pharmacokinetic failings [23]. This in turn has highlighted the other causes for compounds being considered unsuitable for drug development. Such reasons include inadequate safety and efficacy. Both of these aspects can be partially addressed by extending the prediction of pharmacokinetic behaviour to include the pharmacodynamic profile of the drug candidate. Preclinical pharmacodynamic studies [24] and the identification of appropriate safety and efficacy biomarkers [25, 26] provide avenues to increase the confidence in rationale and safety of new drug molecules. In combination, or as an alternative to such approaches, consideration of the required drug exposure providing the desired and/or undesired pharmacological effect can contribute to a quantitative assessment of the potential safety risk of a novel agent.
Figure 1.
The reasons for failure of drug development programmes by the seven UK-based pharmaceutical companies in the period 1964–1985 [4]
Free drug exposure as a basis for safety consideration
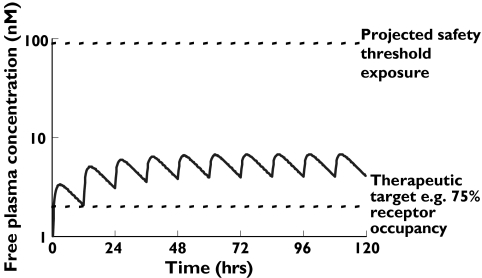
The free concentration of drug in plasma, i.e. the concentration that is not bound to plasma protein, is generally regarded as the concentration that is available to interact with target receptors or ion channels [27, 28]. This is especially true for receptors and ion channels where the binding site is accessed extracellularly and thus is exposed to the free drug concentration in plasma [29]. Therefore when novel molecules are being assessed for their potential suitability as drug development candidates it is important to consider the target therapeutic free drug concentration that is expected to be required for efficacy. This can then be considered against the expected pharmacokinetic properties of the compound to ascertain if the agent is likely to be able to provide efficacious exposure on a realistic dose regime. Target therapeutic free drug concentrations can often be estimated from in vitro pharmacology studies, which may be supported by studies in animal models of the disease. A concentration providing 75% receptor occupancy has been demonstrated as predictive of the therapeutic active concentration for many G-protein coupled receptor antagonists [29], although the degree of occupancy required may vary depending on the disease target. If available, additional data from preclinical pharmacokinetic–pharmacodynamic (PK/PD) studies may be used to refine the prediction of compound exposure required for efficacy. Having established target therapeutic levels of a compound and made estimates of the projected clinical pharmacokinetic profile, this can be extended to assess the potential safety risk of the novel agent in situations where plasma free concentration is considered relevant to compound safety. This is illustrated in Figure 2, where relatively crude pharmacokinetic predictions have been applied to maintain free drug exposure above a therapeutic threshold and compared with projected exposure which is estimated to pose a safety concern.
Figure 2.
Modelled pharmacokinetic profile of a novel chemical entity with consideration of free drug exposure relative to projected efficacy levels (based on, e.g. receptor occupancy) and safety threshold (based on, e.g. level at which QT prolongation is anticipated)
QT prolongation and PK/PD
The nonspecific pharmacology of many molecules to induce the form of ventricular tachycardia known as torsades de pointes (TDP) has become a major focus in the identification and development of new drug candidates [30–34]. Due to the potentially fatal outcome of TDP and the number of drug withdrawals and restrictions to use that have occurred as a consequence, it is clearly desirable to identify the risk and avoid developing compounds that may be associated with TDP. Prolongation of the QT interval has been demonstrated to be predictive of the risk of TDP and was exemplified in the case of terfenadine [33]. Various in vitro and in vivo systems for assessing QT prolongation have been developed [31, 34] and these have been applied to the selection of compounds with minimal risk. The ability of compounds to inhibit HERG potassium currents in recombinant cell systems has been extensively used in the early assessment of compounds likely to prolong the QT interval [35]. The close correlation between free plasma concentrations associated with QT prolongation in both dog and man and the concentration associated with inhibition of the HERG channel in vitro have been demonstrated for terfenadine, terodiline, cisapride and E-4031 [36]. A comprehensive analysis of available in vitro and in vivo data relating to QT prolongation [37] demonstrated the dependence of QT prolongation on free plasma concentrations and lent support to the application of a 30-fold safety multiple between therapeutic activity and concentration causing QT prolongation. This can be further refined by the incorporation of a pharmacokinetic component to provide greater assurance that clinical exposure at proposed therapeutic doses will not approach free plasma concentrations expected to cause this adverse pharmacology (as illustrated in Figure 2). Hence combining the predicted pharmacokinetic and pharmacodynamic properties of a new compound enables a rational decision to be made regarding the potential safety risk and hence ability to develop the agent.
In reality, such considerations of safety in relation to pharmacokinetics are little different from the comparisons made of systemic drug exposure in toxicology studies and in clinical use. However, undertaking these analyses in a predictive manner allows a risk assessment to be made prior to actual human exposure.
Limitations to the application of systemic drug exposure to the assessment of safety
The above example regarding the potential to cause QT prolongation demonstrates how systemic exposure can be used to assess quantitatively the safety risk of a novel agent. Systemic exposure is often of primary consideration during early drug development and has become a prime purpose of toxicokinetic studies [38]. Whilst no formal requirements exist, drug development programmes are likely to proceed with far greater confidence when systemic exposure in the toxicology species at a no adverse effect dose greatly exceeds what is expected or is observed in the clinical studies. Clearly when the limiting safety factor can be linked to free drug exposure, as in the case of QT prolongation, this can be sensibly applied. However, the actual relevance of systemic exposure (free or total) needs to be carefully considered, as many limiting safety findings are unrelated to systemic drug concentration. Obvious examples are hepatic toxicities. The chemical insult that occurs at the liver following oral dosing is often unrelated to systemic exposure because the extent of first-pass extraction may result in low systemic exposure even though the liver has been exposed to the whole dose. This can become particularly important when comparing across species. Marked variation in extent of hepatic extraction may mean that the livers of two species are exposed to the whole oral dose but there may be orders of magnitude difference in the systemic exposure. Such is often the case when comparing rodents and man. Hepatic extraction may be close to 100% in rodents and any resultant toxicity may be associated with low systemic exposure which bears no relation to the chemical insult imposed on the liver. Where hepatic extraction is lower a similar systemic exposure in man will be achieved at a fraction of the dose and similar fraction of the chemical insult to the liver. Rodent-specific induction of drug-metabolizing enzymes by xenobiotics can exacerbate the rodent–human differences in systemic exposure for a given dose of compound [39, 40]. In such cases careful consideration needs to be applied as to the relevance of systemic drug exposure in toxicology studies (toxicokinetic data) to the assessment of safety. Other toxicities may occur that are independent of systemic drug exposure. Examples include the species-specific renal toxicity of efavirenz in rats, which may be due to glutathione conjugate formation unique to this species [41]. It is thus always important to attempt to understand the aetiology of the limiting drug safety finding in order to establish the relevance (and limitation) of applying a concentration–effect relationship. It is also important to appreciate the potential for interspecies differences in pharmacodynamic response, which may limit the extrapolation of toxicity data across species [42].
Pharmacogenetics and safety assessment
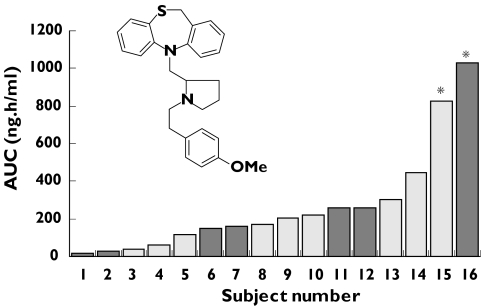
Pharmacogenetics has been defined as the use of biological markers (DNA, RNA or protein) to predict the efficacy of a drug and the likelihood of the occurrence of an adverse event in individual patients [43]. Pharmacogenetic-guided drug discovery provides the potential for developing treatments designed for individuals or specific subpopulations with minimized adverse effects [44]. Several drug-metabolizing enzymes have been shown to be polymorphic as a consequence of single nucleotide polymorphisms (SNPs), gene deletions and gene duplications. In recent years, pharmaceutical companies have increasingly aimed to screen out compounds that are substrates solely for a polymorphic enzyme in order to avoid the wider intersubject variability in exposure, and hence safety and efficacy, that is associated with such agents [45]. The CYP2D6 enzyme is the most studied of the polymorphic drug-metabolizing enzymes [46]. The structure of the active site and hence structural requirements for substrates of CYP2D6 have been characterized leading to a well-defined pharmacophore template model [47, 48]. Such a model allows the early identification of potential CYP2D6 substrates based on chemical structure alone. Such was the case for a calcium channel antagonist (UK-84,149, Figure 3) under investigation at around the time the pharmacophore model was being developed. Because, at this time, the science around CYP2D6 polymorphic metabolism was not fully recognized, this compound was continued to early clinical studies. However, given the emerging pharmacophore model together with additional in vitro data confirming the CYP2D6 dependence, the panel of volunteers in the first in human study were genotyped for CYP2D6 expression. Two individuals were characterized as poor metabolizers for CYP2D6 (PMs) and were included in the study. The resulting pharmacokinetic data demonstrated a markedly higher exposure in these individuals (Figure 3) and development of the compound was immediately terminated [49]. The current comprehensive knowledge about CYP2D6 polymorphic metabolism provides the opportunity to routinely avoid compounds shown to have a high dependence on this enzyme for clearance. The excessive intersubject variability in exposure provides an increased safety risk for such molecules and it is often possible to modify structures to reduce such dependence prior to nomination for development [45].
Figure 3.
Variation in area under the plasma concentration–time curve (AUC) following a single oral dose of the CYP2D6 substrate and calcium channel antagonist (UK-84,149) to human volunteers [49] Individuals characterised as poor metabolizers for CYP2D6 indicated by asterisks
Polymorphic expression of the cytochrome P450 enzyme, CYP2C19, is also responsible for excessive intersubject variability [45, 50, 51] and, like CYP2D6, dependence on CYP2C19 metabolism is seen as an undesirable safety risk. The increased availability of SNP screening results in increasing identification of polymorphisms and hence need for consideration of clinical consequence. The enzyme CYP2C9 has two common variant alleles (*2 and *3); however, unlike CYP2D6 and CYP2C19, these retain enzymic activity albeit at a reduced rate [52, 53]. Thus CYP2C9 polymorphisms have only a modest impact on pharmacokinetics and generally no significant effect on therapeutic outcome, as demonstrated for the angiotensin II receptor antagonist, irbesartan [54]. Conversely, losartan, another selective angiotensin II receptor antagonist, has been shown to possess reduced efficacy in individuals with reduced catalytic activity of CYP2C9 due to reduced formation of the active metabolite [55]. In the case of a narrow therapeutic index drug such as warfarin, CYP2C9 genotype has been shown to correlate with the titrated dose in a population of 200 patients [56]. Patients homozygous for the wild-type *1 allele, which has the highest catalytic activity, had the highest titrated dose, whilst *3 homozygotes, which have the lowest catalytic activity, also had the lowest titrated dose. Obviously compounds with therapeutic indices as low as warfarin are the exception and in general it would appear that CYP2C9 pharmacogenetics are not critical to the ability to develop CYP2C9 substrates.
The relevance of pharmacogenetics for other proteins involved in drug disposition is yet to be fully realised. The drug transporter P-glycoprotein, which is increasingly recognized as playing a significant role in the pharmacokinetic profile of xenobiotics [57], is a case in point. Twenty-eight SNPs for P-glycoprotein have been identified; however, results from studies investigating the consequence are equivocal and no clear clinical outcome has been demonstrated [58].
Making the drug development process more efficient
It is clear that consideration of the pharmacokinetic profile of a new chemical entity can be extremely beneficial to the assessment of its safety and efficacy. Generally it is possible to make fairly robust predictions of the pharmacokinetic profile in man using in vitro systems and preclinical pharmacokinetic studies as previously discussed. However, the science is incomplete and there remain occasions where in vitro and in vivo data obtained on a molecule during the drug discovery process may be ambiguous or even contradictory. A number of options are then available. The risk may be considered too great to warrant further investigation or chemical effort may be switched to an alternate series which may provide more predictable pharmacokinetic properties. Alternatively, the decision may be taken to progress the compound to man to obtain actual clinical pharmacokinetic data on which to base a decision on suitability for development. Assuming some knowledge of the predicted dynamics relating to safety and efficacy, single-dose clinical pharmacokinetic data can provide a very clear decision on suitability of a particular compound for further development.
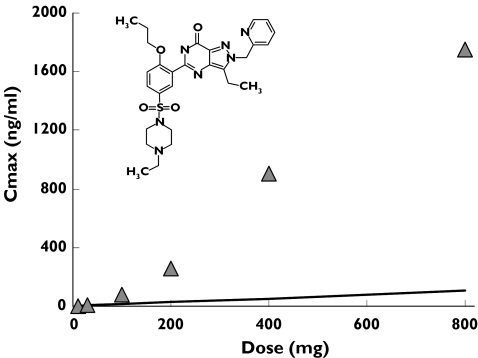
Human microdosing has been proposed as one approach to allow rapid evaluation of human pharmacokinetics utilizing ultrasensitive technology, such as accelerator mass spectrometry (AMS), to allow quantification of drug exposure after extremely low single-dose administration [59]. As the microdoses are not expected to have pharmacological activity, abbreviated safety packages have been proposed to support these limited clinical programmes. Indeed, the European regulatory bodies have issued guidance on such nonclinical safety packages and human microdoses [60]. Whilst some success has been achieved through this approach [61–63], it is important that the limitations of such studies are fully appreciated. First, the cases where preclinical drug metabolism tools fail to provide robust predictions of the human pharmacokinetics are often those where the science governing the drug disposition is poorly understood [64] and therefore the predictability of microdose pharmacokinetic data to pharmacologically relevant doses is highly questionable. Second, such approaches may potentially impact on drug discovery but do not speed up the drug development process, as successful readout from a microdose study will then require a full safety package to support a standard clinical drug development programme at appropriate doses. Finally, the use of AMS can only readily be applied to the measurement of total radioactivity [61, 62] without extensive sample preparation, hence the presence of metabolites may confound interpretation of pharmacokinetic data. However the increasing sensitivity that can be obtained from state-of-the-art mass spectrometric detection [65] does make specific analysis possible even at microdose levels for some compounds. Careful consideration therefore needs to be given to identify appropriate drug development programmes which might benefit from such an approach [63]. The growing appreciation of the role of drug transporters, such as P-glycoprotein, in drug disposition provides several examples which demonstrate the lack of dose linearity which would clearly invalidate a microdose approach to drug candidate selection. The greater ability of the drug discovery process to remove drug candidates with high metabolic lability has led to several examples where the pharmacokinetic profile is dependent on P-glycoprotein. These compounds show markedly nonproportional absorption across dose ranges studied in first clinical studies resulting in super-proportional increases in AUC such that the initial low doses would not predict the pharmacokinetics at therapeutic levels. An experimental PDE5 inhibitor (UK-343,664) provides an example of this nonproportional pharmacokinetics [65] as shown in Figure 4. For this compound there was an estimated 14-fold change in bioavailability between doses of 10 and 800 mg. Other examples in which P-glycoprotein involvement results in nonproportional pharmacokinetics cover a range of therapeutic classes from α-adrenoreceptor antagonists [66] and NK2-antagonists [67] to β-adrenoreceptor antagonists [68].
Figure 4.
Mean Cmax (n = 8 or 9) from single oral solution doses of 10, 30, 100, 200, 400 and 800 mg of the PDE5 inhibitor (UK-343,664) to human volunteers. The solid line shows the line of identity based on the 10-mg dose [66]
Summary
In order to reduce the cost of overall drug development it is essential that resources be focused on those compounds most likely to succeed. The utility of pharmacokinetic predictions (or at least estimations) in the drug discovery phase has resulted in fewer compounds failing as a result of inappropriate clinical pharmacokinetics. This application of pharmacokinetic prediction is now being extended to the pharmacodynamics of novel compounds in order to gain better understanding of their efficacy and safety profiles. The application of linking pharmacokinetics and pharmacodynamics in the risk assessment for QT prolongation is a notable example. The growing knowledge of pharmacogenetics has identified additional risk factors for individuals and specific groups and continued growth in this area is likely to improve the ability to discover successful drugs for both the wider and specific populations. Clearly pharmacokinetics is key to the overall interpretation of the safety profile; however, it is important that the link between systemic exposure and effect is established in order to allow appropriate consideration. In situations where pharmacokinetic predictions are ambiguous or not possible, there are opportunities to change the drug development paradigm to obtain information more rapidly. However, it is important to realise that this may only permit the rejection of compounds to be achieved more rapidly and the application of such methods is not universal.
Acknowledgments
The author would like to extend thanks to Dennis Smith, Barry Jones, Rob Webster and Don Nichols for their contributions to many of the discussion points within this manuscript.
References
- 1.Grabowski H, Vernon J, DiMasi JA. Returns on research and development for 1990s new drug introductions. Pharmacoeconomics. 2002;20(Suppl. 3):11–29. doi: 10.2165/00019053-200220003-00002. [DOI] [PubMed] [Google Scholar]
- 2.Di Masi JA. The value of improving the productivity of the drug development process. Pharmacoeconomics. 2002;20(Suppl. 3):1–10. doi: 10.2165/00019053-200220003-00001. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, Smith A. New drug approvals for 2002. Nat Rev Drug Discov. 2003;2:95–6. doi: 10.1038/nrd1014. [DOI] [PubMed] [Google Scholar]
- 4.Prentis RA, Lis Y, Walker SR. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985) Br J Clin Pharmacol. 1988;25:387–96. doi: 10.1111/j.1365-2125.1988.tb03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer AM. New horizons in drug metabolism, pharmacokinetics and drug discovery. Drug News Perspect. 2003;16:57–62. [PubMed] [Google Scholar]
- 6.Stewart BH, Chan OH, Lu RH, et al. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humans. Pharm Res. 1995;12:693–9. doi: 10.1023/a:1016207525186. [DOI] [PubMed] [Google Scholar]
- 7.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 8.Yee S. In vitro permeability across caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man –fact or myth. Pharm Res. 1997;14:763–6. doi: 10.1023/a:1012102522787. [DOI] [PubMed] [Google Scholar]
- 9.Irvine JD, Takahashi L, Lockhart K, et al. MDCK (Madin-Darby canine kidney) cells: a tool for membrane permeability screening. J Pharm Sci. 1999;88:28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- 10.van de Waterbeemd H, Smith DA, Beaumont K, Walker DK. Property-based design: optimisation of drug absorption and pharmacokinetics. J Med Chem. 2001;44:1313–33. doi: 10.1021/jm000407e. [DOI] [PubMed] [Google Scholar]
- 11.Houston JB. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol. 1994;47:1469–79. doi: 10.1016/0006-2952(94)90520-7. [DOI] [PubMed] [Google Scholar]
- 12.Obach RS, Baxter JG, Liston TE, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283:46–58. [PubMed] [Google Scholar]
- 13.Lave T, Coassolo P, Reigner B. Prediction of hepatic metabolic clearance based on interspecies allometric scaling techniques and in vitro–in vivo correlations. Clin Pharmacokinet. 1999;36:211–31. doi: 10.2165/00003088-199936030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Naritomi Y, Terashita S, Kimura S, Suzuki A, Kagayama A, Sugiyama Y. Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab Dispos. 2001;29:1316–24. [PubMed] [Google Scholar]
- 15.Schmider J, von Moltke LL, Shader RI, Harmatz JS, Greenblatt DJ. Extrapolating in vitro data on drug metabolism to in vivo pharmacokinetics: evaluation of the pharmacokinetic interaction between amitriptyline and fluoxetine. Drug Metab Rev. 1999;31:545–60. doi: 10.1081/dmr-100101935. [DOI] [PubMed] [Google Scholar]
- 16.Weaver RJ. Assessment of drug–drug interactions: concepts and approaches. Xenobiotica. 2001;31:499–538. doi: 10.1080/00498250110060950. [DOI] [PubMed] [Google Scholar]
- 17.Venkatakrishnan K, von Moltke LL, Obach RS, Greenblatt DJ. Drug metabolism and drug interactions: application and clinical value of in vitro models. Curr Drug Metab. 2003;4:423–59. doi: 10.2174/1389200033489361. [DOI] [PubMed] [Google Scholar]
- 18.Williams JA, Hurst SI, Bauman J, et al. Reaction phenotyping in drug discovery: moving forward with confidence? Curr Drug Metab. 2003;4:527–34. doi: 10.2174/1389200033489235. [DOI] [PubMed] [Google Scholar]
- 19.Ings RMJ. Interspecies scaling and comparisons in drug development and toxicokinetics. Xenobiotica. 1990;20:1201–31. doi: 10.3109/00498259009046839. [DOI] [PubMed] [Google Scholar]
- 20.Ritschel WA, Vachharajani NN, Johnson RD, Hussain AS. The allometric approach for interspecies scaling of pharmacokinetic parameters. Comp Biochem Physiol. 1992;103C:249–53. doi: 10.1016/0742-8413(92)90003-p. [DOI] [PubMed] [Google Scholar]
- 21.Mahmood I, Balian JD. The pharmacokinetic principles behind scaling from preclinical results to phase 1 protocols. Clin Pharmacokinet. 1999;36:1–11. doi: 10.2165/00003088-199936010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Mahmood I. Interspecies scaling: role of protein binding in the prediction of clearance from animals to humans. J Clin Pharmacol. 2000;40:1439–46. [PubMed] [Google Scholar]
- 23.Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov. 2003;2:566–80. doi: 10.1038/nrd1130. [DOI] [PubMed] [Google Scholar]
- 24.Greig NH, De Micheli E, Holloway HW, et al. The experimental Alzheimer drug phenserine: preclinical pharmacokinetics and pharmacodynamics. Acta Neurol Scand Supplement. 2000;176:74–84. doi: 10.1034/j.1600-0404.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 25.Coburn WA. Biomarkers in drug discovery and development: from target identification through drug marketing. J Clin Pharmacol. 2003;43:329–41. doi: 10.1177/0091270003252480. [DOI] [PubMed] [Google Scholar]
- 26.Tugwood JD, Hollins LE, Cockerill MJ. Genomics and the search for novel biomarkers in toxicology. Biomarkers. 2003;8:79–92. doi: 10.1080/1354750031000070103. [DOI] [PubMed] [Google Scholar]
- 27.Sjoqvist F. Blood binding and drug monitoring. In: Tillement J, Lindenlaub E, editors. Protein Binding and Drug Transport. Stuttgart: FK Schattauer; 1985. pp. 429–41. [Google Scholar]
- 28.Smith DA. Pharmacokinetics and pharmacodynamics in toxicology. Xenobiotica. 1997;27:513–25. doi: 10.1080/004982597240479. [DOI] [PubMed] [Google Scholar]
- 29.Smith DA, Jones BC, Walker DK. Design of drugs involving the concepts and theories of drug metabolism and pharmacokinetics. Med Res Rev. 1996;16:243–66. doi: 10.1002/(SICI)1098-1128(199605)16:3<243::AID-MED2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR. Torsades de pointes occurring in association with terfenadine use. J Am Med Assoc. 1990;264:2788–90. [PubMed] [Google Scholar]
- 31.Crumb W, Cavero I. QT interval prolongation by non-cardiovascular drugs: issues and solutions for novel drug development. Pharm Sci Tech Today. 1999;2:270–80. doi: 10.1016/s1461-5347(99)00172-8. [DOI] [PubMed] [Google Scholar]
- 32.De Ponti F, Poluzzi E, Montanaro N. Organising evidence on QT prolongation and occurrence of torsades de pointes with non-antiarrhythmic drugs: a call for consensus. Eur J Clin Pharmacol. 2001;57:185–209. doi: 10.1007/s002280100290. [DOI] [PubMed] [Google Scholar]
- 33.Cavero I, Mestre M, Guillon JM, Crumb W. Drugs that prolong QT interval as an unwanted effect: assessing their likelihood of inducing cardiac dysrhythmias. Exp Opin Pharmacother. 2000;1:947–73. doi: 10.1517/14656566.1.5.947. [DOI] [PubMed] [Google Scholar]
- 34.Netzer R, Ebneth A, Bischoff U, Pongs O. Screening lead compounds for QT interval prolongation. Drug Disc Today. 2001;6:78–84. doi: 10.1016/s1359-6446(00)01602-0. [DOI] [PubMed] [Google Scholar]
- 35.Leishman DJ, Helliwell R, Wakerall J, Wallis RM. Effects of E-4031, cisapride, terfenadine and terodiline on cardiac repolarisation in canine Purkinje fibre and HERG channels expressed in HEK293 cells. Br J Pharmacol. 2000;133:130. [Google Scholar]
- 36.Webster R, Allan G, Anto-Awuakye K, et al. Pharmacokinetic/ pharmacodynamic assessment of the effects of E4031, cisapride, terfenadine and terodiline on monophasic action potential duration in dog. Xenobiotica. 2001;31:633–50. doi: 10.1080/00498250110054632. [DOI] [PubMed] [Google Scholar]
- 37.Webster R, Leishman D, Walker D. Towards a drug concentration effect relationship for QT prolongation and torsades de pointes. Curr Opin Drug Discov Devel. 2002;5:116–26. [PubMed] [Google Scholar]
- 38.Smith DA, Humphrey MJ, Charuel C. Design of toxicokinetic studies. Xenobiotica. 1990;20:1187–99. doi: 10.3109/00498259009046838. [DOI] [PubMed] [Google Scholar]
- 39.Swales K, Plant N, Ayrton A, Hood S, Gibson G. Relative receptor expression is a determinant in xenobiotic-mediated CYP3A induction in rat and human cells. Xenobiotica. 2003;33:703–16. doi: 10.1080/0049825031000121626. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Song L, Zhang H, Matoney L, LeCluyse E, Yan B. Dexamethasone differentially regulates expression of carboxylesterase genes in humans and rats. Drug Metab Dispos. 2000;28:186–91. [PubMed] [Google Scholar]
- 41.Mutlib AE, Gerson RJ, Meunier PC, et al. The species-dependent metabolism of efavirenz produces a nephrotoxic glutathione conjugate in rats. Toxicol Appl Pharmacol. 2000;169:102–13. doi: 10.1006/taap.2000.9055. [DOI] [PubMed] [Google Scholar]
- 42.Monro A. Drug toxicokinetics: scope and limitations that arise from species differences in pharmacodynamic and carcinogenic responses. J Pharmacokin Biopharm. 1994;22:41–57. doi: 10.1007/BF02353409. [DOI] [PubMed] [Google Scholar]
- 43.Roses AD. Pharmacogenetics and the practice of medicine. Nature. 2000;405:857–65. doi: 10.1038/35015728. [DOI] [PubMed] [Google Scholar]
- 44.Ozdemir V, Shear NH, Kalow W. What will be the role of pharmacogenetics in evaluating drug safety and minimising adverse effects? Drug Safety. 2001;24:75–85. doi: 10.2165/00002018-200124020-00001. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues AD, Rushmore TH. Cytochromep450 pharmacogenetics in drug development: in vitro studies and clinical consequences. Curr Drug Metab. 2002;3:289–309. doi: 10.2174/1389200023337522. [DOI] [PubMed] [Google Scholar]
- 46.Tucker GT. Clinical implications of genetic polymorphism in drug metabolism. J Pharm Pharmacol. 1994;46(Suppl. 1):417–24. [PubMed] [Google Scholar]
- 47.Strobl GR, von Kruedener S, Stockigt J, et al. Development of a pharmacophore for inhibition of human liver cytochrome P-450 2D6: molecular modelling and inhibition studies. J Med Chem. 1993;36:1136–45. doi: 10.1021/jm00061a004. [DOI] [PubMed] [Google Scholar]
- 48.Islam SA, Wolf CR, Lennard MS, et al. A three-dimensional molecular template for substrates of human cytochrome P450 involved in debrisoquine 4-hydroxylation. Carcinogenesis. 1991;12:2211–9. doi: 10.1093/carcin/12.12.2211. [DOI] [PubMed] [Google Scholar]
- 49.Smith DA, Jones BC. Variability in drug response as a factor in drug design. Curr Opin Drug Discov Devel. 1999;2:33–41. [PubMed] [Google Scholar]
- 50.Chong E, Ensom MH. Pharmacogenetics of the proton pump inhibitors: a systematic review. Pharmacotherapy. 2003;23:460–71. doi: 10.1592/phco.23.4.460.32128. [DOI] [PubMed] [Google Scholar]
- 51.Jan MW, Zum Brunnen TL, Kazmi YR. Pharmacokinetics of fluvoxamine in relation to CYP2C19 phenotype and genotype. Drug Metabol Drug Interact. 2002;19:1–11. doi: 10.1515/dmdi.2002.19.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Crespi CL, Miller VP. The R144C change in CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH:cytochrome P450 oxidoreductase. Pharmacogenetics. 1997;7:203–10. doi: 10.1097/00008571-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Takanashi K, Tainaka H, Kobayashi K, et al. CYP2C9 Ile359 and Leu359 variants. Pharmacogenetics. 2000;10:95–104. doi: 10.1097/00008571-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Wen SY, Wang H, Sun OJ, Wang SQ. Rapid detection of the known SNPs of CYP2C9 using oligonucleotide microarray. World J Gastroenterol. 2003;9:1342–6. doi: 10.3748/wjg.v9.i6.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekino K, Kubota T, Okada Y, et al. Effect of the single CYP2C9*3 allele on pharmacokinetics and pharmacodynamics of losartan in healthy Japanese subjects. Eur J Clin Pharmacol. 2003;59:589–92. doi: 10.1007/s00228-003-0664-5. [DOI] [PubMed] [Google Scholar]
- 56.Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–8. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 57.Ayrton A, Morgan P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica. 2001;31:469–97. doi: 10.1080/00498250110060969. [DOI] [PubMed] [Google Scholar]
- 58.Sakaeda T, Nakamura T, Okumura K. Pharmacogenetics of MDR1 and its impact on the pharmacokinetics and pharmacodynamics of drugs. Pharmacogenomics. 2003;4:397–410. doi: 10.1517/phgs.4.4.397.22747. [DOI] [PubMed] [Google Scholar]
- 59.Lappin G, Garner RC. Big physics, small doses. the use of AMS and PET in human microdosing of development drugs. Nat Rev Drug Discov. 2003;2:233–40. doi: 10.1038/nrd1037. [DOI] [PubMed] [Google Scholar]
- 60.European Agency for the Evaluation of Medicinal Products (EMEA) London: EMEA; 2003. Position Paper on Non-clinical Safety Studies to Support Clinical Trials with a Single Microdose. CPMP/SWP/2599/02. [Google Scholar]
- 61.Kaye B, Garner RC, Mauthe RJ, Freeman SPHT, Turteltaub KW. A preliminary evaluation of accelerator mass spectrometry in the biomedical field. J Pharm Biomed Anal. 1997;16:541–3. doi: 10.1016/s0731-7085(97)00104-0. [DOI] [PubMed] [Google Scholar]
- 62.Young G, Ellis W, Ayrton J, Hussey E, Adamkiewicz B. Accelerator mass spectrometry (AMS): recent experience of its use in a clinical study and the potential future of the technique. Xenobiotica. 2001;31:619–32. doi: 10.1080/00498250110052724. [DOI] [PubMed] [Google Scholar]
- 63.Sarapa N. Early human microdosing to reduce attrition in clinical drug development. Am Pharmaceut Outsourcing. 2003;1:1–5. [Google Scholar]
- 64.Smith DA, Johnson DE, Park BK. Use of microdosing to probe pharmacokinetics in humans –is it too much for too little? Curr Opin Drug Discov Devel. 2003;6:39–40. [Google Scholar]
- 65.Papac DI, Shahrokh Z. Mass spectrometry innovations in drug discovery and development. Pharm Res. 2001;18:131–45. doi: 10.1023/a:1011049231231. [DOI] [PubMed] [Google Scholar]
- 66.Abel S, Beaumont KC, Crespi CL, et al. Potential role for P-glycoprotein in the non-proportional pharmacokinetics of UK-343,664 in man. Xenobiotica. 2001;31:665–76. doi: 10.1080/00498250110052779. [DOI] [PubMed] [Google Scholar]
- 67.Beaumont K, Harper A, Smith D, Bennett J. The role of P-glycoprotein in determining the oral absorption and clearance of the NK2 antagonist, UK-224,671. Eur J Pharm Sci. 2000;12:41–50. doi: 10.1016/s0928-0987(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 68.Gramatté T, Oertel R, Terhaag B, Kirch W. Direct demonstration of small intestinal secretion and site dependent absorption of the β-blocker talinolol in humans. Clin Pharmacol Ther. 1996;59:541–9. doi: 10.1016/S0009-9236(96)90182-4. [DOI] [PubMed] [Google Scholar]