Abstract
Aims
To investigate the possibility that (S)-methadone influences therapeutic and adverse responses to rac-methadone maintenance treatment, by examining how subjective and physiological responses among rac-methadone maintenance patients vary in relation to relative exposure to (S)- vs. (R)-methadone.
Methods
Mood states (Profile of Mood States), opioid withdrawal (Methadone Symptoms Checklist), physiological responses (pupil diameter, heart rate, respiration rate, blood pressure), and plasma concentrations (CP) of (R)- and (S)-methadone were measured concurrently 11–12 times over a 24-h interdosing interval in 55 methadone maintenance patients. Average steady-state plasma concentrations (Cav) and pharmacodynamic responses were calculated using area under the curve (AUC). Linear regression was used to determine whether variability in pharmacodynamic responses was accounted for by (S)-methadone Cav controlling for (R)-methadone Cav and rac-methadone dose. Ratios of (S)-:(R)-methadone using AUCCP and trough values were correlated with pharmacodynamic responses for all subjects and separately for those with daily rac-methadone doses ≥100 mg.
Results
(S)-methadone Cav accounted for significant variability in pharmacodynamic responses beyond that accounted for by (R)-methadone Cav and rac-methadone dose, showing positive associations (partial r) with the intensity of negative mood states such as Tension (0.28), Fatigue (0.31), Confusion (0.32), and opioid withdrawal scores (0.30); an opposite pattern of relationships was evident for (R)-methadone. The plasma (S)-:(R)-methadone AUCCP ratio (mean ± SD 1.05 ± 0.21, range 0.65–1.51) was not significantly related to pharmacodynamic responses for the subjects as a whole but showed significant positive associations (r) with the intensity of negative mood states such as Total Mood Disturbance (0.61), Tension (0.69), Fatigue (0.65), Confusion (0.64), Depression (0.49) and heart rate (0.59) for the ≥100-mg dose range.
Conclusions
These findings agree with previous evidence that (S)-methadone is associated with a significant and potentially adverse profile of responses distinct from that of (R)-methadone. Individual variability in relative (S)- vs. (R)-methadone exposure may be associated with variability in response to rac-methadone maintenance treatment.
Keywords: enantiomeric ratio, methadone, mood, stereoisomers, withdrawal
Introduction
Methadone is a synthetic opioid commonly used as a maintenance pharmacotherapy for opioid dependence. It is normally administered orally once daily as a racemic mixture of the (R)- and (S)-methadone enantiomers. These enantiomers display different pharmacokinetic profiles [1–5], such that the ratio of each in plasma changes over a 24-h interdosing interval [4] and also varies considerably between individuals [6]. Knowledge of how variation in relative exposure to (R)- and (S)-methadone may influence the therapeutic response of patients maintained on rac-methadone is presently incomplete.
Methadone primarily acts on the mu (µ) opioid receptor; both enantiomers have low affinity for delta (δ) and kappa (κ) receptors [7–9]. Relative to (S)-methadone, (R)-methadone shows at least 10-fold greater affinity for the µ opioid receptor in vitro[7–9] and produces opioid agonist effects (e.g. analgesia, euphoria, sedation, miosis, respiratory depression) with correspondingly greater potency in vivo[10–12]. Although (R)-methadone is believed to account for most if not all of the therapeutic effects of methadone maintenance treatment (e.g. suppression of opioid withdrawal and cravings), rac-methadone is normally used due to its lower production cost and evidence that it produces similar therapeutic outcomes when compared with (R)-methadone alone [13–16].
Despite lacking strong opioid effects, (S)-methadone may be a clinically important determinant of therapeutic and adverse responses to rac-methadone. Administration of (S)-methadone alone in high oral doses (50–1000 mg) has been found to produce mild opioid effects (e.g. physical dependence, withdrawal suppression, respiratory depression, miosis), but is also associated with adverse subjective effects (e.g. effects disliked and described as not opioid-like) [17] and symptomatic complaints (e.g. nervousness, confusion, hallucinations) [11, 12, 17] inconsistent with µ opioid receptor activation, which may increase with chronic dosing [17]. These findings suggest that (S)-methadone may contribute significantly to the adverse but not the therapeutic effects of rac-methadone during maintenance treatment for opioid dependence. To this extent, treatment responses may vary between individuals as a consequence of individual variability in relative exposure to (S)- vs. (R)-methadone. This study investigated the possibility that (S)-methadone influences therapeutic response to rac-methadone maintenance treatment by examining how subjective and physiological responses among rac-methadone maintenance patients vary in relation to relative plasma concentrations of (S)- vs. (R)-methadone
Methods
Subjects
Subjects were derived from four separate investigations conducted between 1997 and 2003, two of which have been reported in part previously [18, 19] and each of which used the same subject selection criteria. For individuals who had participated in more than one of these studies, only data from the first such occasion have been included, yielding a sample size of 55. All subjects were opioid-dependent volunteers receiving methadone maintenance treatment on an outpatient basis. Inclusion criteria required subjects to be aged between 18 and 65 years and to have been maintained on once-daily oral rac-methadone for more than 6 weeks without a dose change in the preceding 4 weeks. Exclusion criteria included poor venous access, significant medical or psychiatric illness, elevated liver enzymes (aspartate aminotransferase and alanine aminotransferase greater than three times the upper limit of normal range), pregnant or lactating, and the consumption of concomitant medications known to interfere with methadone pharmacokinetics (e.g. enzyme inducers, enzyme inhibitors, monoamine oxidase inhibitors). Table 1 summarizes demographic and treatment variables and urinalysis results (described below) for the subjects as a whole and separately for each study cohort (numbered chronologically according to order of commencement). The sample (all Caucasian) included subjects self-reporting both adequate (‘holders’, n = 26) and inadequate (‘nonholders’, n = 29) withdrawal suppression whilst maintained on methadone prior to commencing the study. Ethical approval to conduct these investigations was obtained from the Royal Adelaide Hospital Research Ethics Committee (Studies 1–3) or the University of Western Australia Human Research Ethics Committee (Study 4). All subjects provided written informed consent prior to participating.
Table 1.
Demographic and treatment details for 55 methadone maintenance patients as a whole and according to study cohort
| Variable | Overall | Study 1 | Study 2 | Study 3 | Study 4 |
|---|---|---|---|---|---|
| N | 55 | 18 | 16 | 12 | 9 |
| Male : female (n) | 34 : 21 | 11 : 7 | 7 : 9 | 10 : 2 | 6 : 3 |
| Age (years) | 34 ± 8 | 38 ± 7 | 36 ± 7 | 34 ± 9 | 30 ± 8 |
| (mean ± SD, range) | (21–48) | (21–45) | (21–45) | (23–48) | (22–46) |
| Daily dose (mg) | 80 ± 48 | 65 ± 34 | 83 ± 38 | 72 ± 33 | 113 ± 84 |
| (mean ± SD, range) | (7.5–300) | (7.5–130) | (30–150) | (25–100) | (48–300) |
| Weight (kg) | 73 ± 16 | 74 ± 11 | 71 ± 19 | 73 ± 18 | 74 ± 20 |
| (mean ± SD, range) | (45–117) | (60–99) | (45–100) | (52–110) | (50–117) |
| Positive urinalyses (n) | |||||
| Opiates | 14 | 3 | 6 | 5 | 0 |
| Benzodiazepines | 23 | 7 | 8 | 6 | 2 |
| Sympathomimetic amines | 5 | 2 | 1 | 2 | 0 |
Procedures and measures
The pharmacokinetics and pharmacodynamics of methadone were assessed over a single 24-h interdosing interval at steady-state for each subject under open-label conditions using previously described methods [19, 20]. At the beginning of each session, a urine sample was obtained for the detection of additional drug use and an intravenous cannula (18–22 G) (Becton Dickinson, Sandy, UT, USA) was inserted into a suitable forearm vein. To permit quantification of plasma (R)- and (S)-methadone concentrations, blood samples (5 ml) were obtained prior to dosing and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 9, 11 and 23 h following dosing. There were two minor variations in this schedule across the four studies: (i) Study 2, a sample was collected at 12 h instead of 11 h following dosing; and (ii) Study 4, an additional 14th sample was collected at 8 h following dosing. Plasma (R)- and (S)-methadone concentrations were quantified by high-performance liquid chromatography using previously described methods [4, 21]. Precision and inaccuracies were <10% for all quality control samples (high 300 ng ml−1, medium 100 ng ml−1, and low 30 ng ml−1) for all analytes. The concentration range of the standard curve was 15–1000 ng ml−1 for each enantiomer.
Pharmacodynamic responses were recorded at baseline prior to the administration of methadone and subsequently just after each blood sampling time, but no more frequently than hourly. Subjective self-report measures included:
Methadone Symptoms Checklist (MSC) [22]: used to record 16 withdrawal symptoms as present or absent to yield an overall measure of withdrawal severity (0–16).
Profile of Mood States (POMS) [23]: consists of 65 adjectives that are rated on a scale from 0 (not at all) to 4 (extremely) according to how subjects feel. These items produce scores for subscales measuring six distinct affective states (score ranges in parentheses): Tension (0–36), Anger (0–48), Depression (0–60), Vigour (0–32), Fatigue (0–28), and Confusion (0–28). High scores indicate negative affective states for all scales except Vigour; a positive mood measure. The Total Mood Disturbance scale provides a global assessment of affective state and is calculated by adding the subscales scores with Vigour weighted negatively. Total Mood Disturbance scores can range from −32 to 200 such that high scores indicate more negative mood states.
Physiological measures included:
Pupil diameter: measured using a video (Studies 1–3) or digital (Study 4) camera and ruler placed directly beneath the subject's eye under constant lighting conditions.
Respiratory rate: measured by direct observation of the subject, without their awareness, after at least 10 min of rest.
Heart rate: measured manually at the wrist.
Systolic and diastolic blood pressure: measured using a sphygmomanometer and stethoscope.
Area under the curve (AUC) was calculated (using the linear trapezoidal rule) for plasma concentrations of (R)-, (S)-, and (rac)-methadone, and all pharmacodynamic responses. The AUC for plasma concentrations (AUCCP) and pharmacodynamic responses was divided by the duration of the study (23 h) to yield average steady-state plasma concentrations (Cav) and pharmacodynamic responses for each subject across the interdosing interval. As an index of the fluctuation in (S)-: (R)-plasma methadone concentration ratios within subjects during the interdosing interval, the ratio of maximum to minimum ratios for each subject was calculated. Two indices of relative exposure to (S)- and (R)-methadone were also calculated for each subject:
AUCCP ratio: the ratio of AUCCP values for (S)-:(R)-methadone concentrations was used as a measure of total relative exposure to each enantiomer across the entire dosing interval.
Trough ratio: since plasma samples are most often and most readily collected at the time of presentation for dosing, the plasma concentration ratio of (S)-:(R)-methadone prior to dosing (trough) was considered clinically relevant.
Statistical analyses
Since each of the four studies used the same patient selection criteria, data were combined and analysed as a single sample. Variation between studies on the demographic and treatment-related variables collected was explored using analysis of variance for continuous variables (i.e. age, dose) and χ2 statistics for categorical variables (i.e. gender, urinalysis results), and no significant effects for study were observed. Linear regression was used to investigate relationships between average pharmacodynamic responses and (S)-methadone Cav controlling for (R)-methadone Cav and rac-methadone dose. The latter two predictors were entered into the equations first, followed by (S)-methadone Cav, in order to determine whether (S)-methadone explained significant additional variability in pharmacodynamic responses independent of (R)-methadone and total rac-methadone exposure. Pearson product moment correlations were used to investigate relationships between pharmacodynamic responses and indices of relative (S)- vs. (R)-methadone exposure. These analyses were also characterized separately for subjects with daily rac-methadone doses of at least 100 mg because previous studies suggest that (S)-methadone is likely to produce clinically significant effects at rac-methadone equivalent doses of 100 mg or more [11, 12, 17]. Partial correlations were used to determine whether relationships between relative (S)- and (R)-methadone exposures were influenced by the presence of additional drugs (listed in Table 1) in subjects’ urine samples. For linear regression analyses, the assumptions of normality and homoscedascity were verified by inspection of scatter plots for the residuals. An α level of 0.05 was used for all analyses. Data are presented as mean ± SD (range) unless stated otherwise.
Results
Means for Cav (uncorrected for dose) during the interdosing interval for (R)-, (S)-, and rac-methadone were 175 ± 100 (27–493), 185 ± 117 (26–591) and 361 ± 213 (52–1067) ng ml−1, respectively. Plasma concentrations for (R)-, (S)-, and rac-methadone ranged from 19 to 742, 21 to 1026, and 44 to 1768 ng ml−1, respectively.
Linear regression analyses indicated that (S)-methadone Cav accounted for significant additional variance in pharmacodynamic responses beyond that accounted for by (R)-methadone Cav and rac-methadone dose (increase in R2, P-value; absolute R2) for Tension (0.08, 0.04; 0.10), Fatigue (0.09, 0.02; 0.12), and Confusion (0.10, 0.02; 0.12) and the MSC withdrawal scale (0.09, 0.03; 0.12); marginally nonsignificant results were also obtained for Total Mood Disturbance (0.06, 0.07; 0.08) and heart rate (0.07, 0.06; 0.10). Regression coefficients for each of these response variables (Table 2) indicated that (S)-methadone Cav was positively associated with the intensity of negative mood states, MSC withdrawal scores, and heart rate; an opposite pattern of relationships was evident for (R)-methadone Cav. Rac-methadone dose showed a similar pattern of regression coefficients to (S)-methadone Cav but none were statistically significant. All regression coefficients for the pharmacodynamic measures not shown in Table 2 were non-significant.
Table 2.
Linear regression slope coefficients for (S)-methadone Cav, (R)-methadone Cav, and rac-methadone dose as predictors of average pharmacodynamic responses over a 24-h interdosing interval (n = 55)
 (SE) (SE) |
t | P | Partial r | ||
|---|---|---|---|---|---|
| Total Mood Disturbance | |||||
| (S)-methadone Cav | 0.18 (0.10) | 0.68 | 1.82 | 0.07 | 0.25 |
| (R)-methadone Cav | −0.30 (0.14) | −0.99 | 2.09 | 0.04 | −0.28 |
| Rac-methadone dose | 0.24 (0.15) | 0.38 | 1.55 | 0.13 | 0.21 |
| Tension | |||||
| (S)-methadone Cav | 0.04 (0.02) | 0.77 | 2.07 | 0.04 | 0.28 |
| (R)-methadone Cav | −0.06 (0.03) | −1.08 | 2.31 | 0.03 | −0.31 |
| Rac-methadone dose | 0.05 (0.03) | 0.42 | 1.73 | 0.09 | 0.24 |
| Fatigue | |||||
| (S)-methadone Cav | 0.05 (0.02) | 0.85 | 2.32 | 0.02 | 0.31 |
| (R)-methadone Cav | −0.08 (0.03) | −1.23 | 2.65 | 0.01 | −0.35 |
| Rac-methadone dose | 0.06 (0.03) | 0.46 | 1.91 | 0.06 | 0.26 |
| Confusion | |||||
| (S)-methadone Cav | 0.04 (0.01) | 0.88 | 2.40 | 0.02 | 0.32 |
| (R)-methadone Cav | −0.05 (0.02) | −1.10 | 2.38 | 0.02 | −0.32 |
| Rac-methadone dose | 0.04 (0.02) | 0.42 | 1.75 | 0.09 | 0.24 |
| Withdrawal | |||||
| (S)-methadone Cav | 0.02 (0.01) | 0.82 | 2.22 | 0.03 | 0.30 |
| (R)-methadone Cav | −0.03 (0.02) | −1.01 | 2.17 | 0.04 | −0.29 |
| Rac-methadone dose | 0.03 (0.02) | 0.43 | 1.79 | 0.08 | 0.24 |
| Heart rate | |||||
| (S)-methadone Cav | 0.05 (0.03) | 0.73 | 1.98 | 0.05 | 0.27 |
| (R)-methadone Cav | −0.08 (0.04) | −0.93 | 1.98 | 0.05 | −0.27 |
| Rac-methadone dose | 0.07 (0.04) | 0.41 | 1.70 | 0.10 | 0.23 |
Cav, Average steady-state plasma concentration;  , estimated regression slope coefficient; SE, standard error of
, estimated regression slope coefficient; SE, standard error of 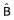 ;
; 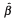 , estimated standardized regression slope coefficient. t and P-values relate to the test of the null hypothesis that the true slope (B) is equal to zero. Partial r is the correlation between the independent and dependent variable when the linear effects of other independent variables in the model have been held fixed.
, estimated standardized regression slope coefficient. t and P-values relate to the test of the null hypothesis that the true slope (B) is equal to zero. Partial r is the correlation between the independent and dependent variable when the linear effects of other independent variables in the model have been held fixed.
Relative plasma concentrations for (S)- vs. (R)-methadone concentrations showed considerable intra- and interindividual variation during the methadone interdosing interval (see examples in Figure 1). Mean fluctuation in the (S)-:(R)-methadone ratio within subjects during the interdosing interval (ratio of maximum to minimum values) was 1.50 ± 0.25 (1.15–2.31). Pharmacodynamic responses were not related to the (S)-:(R)-methadone AUCCP ratio (mean 1.05 ± 0.21, 0.65–1.51) for the subject group as a whole. However, within the ≥100-mg dose range (n = 17), the (S)-:(R)-methadone AUCCP ratio showed significant positive associations (r, P) with the intensity of negative mood states including Total Mood Disturbance (0.61, 0.01), Tension (0.69, 0.002), Fatigue (0.65, 0.005), Confusion (0.64, 0.006) and Depression (0.49, 0.047), and also with heart rate (0.59, 0.01) (scatterplots for Total Mood Disturbance and Tension are shown in Figure 2). Trough plasma (S)-:(R)-methadone concentration ratios (mean 0.95 ± 0.25, 0.44–1.56) correlated strongly with AUCCP ratios (r = 0.89, P < 0.001) and showed a similar pattern of relationships with pharmacodynamic responses, although statistically significant relationships (r, P) were observed only for Tension (0.53, 0.03) and Fatigue (0.52, 0.03) within the ≥100-mg dose range. Controlling for the presence of additional drugs in urine did not alter the pattern of relationships described above.
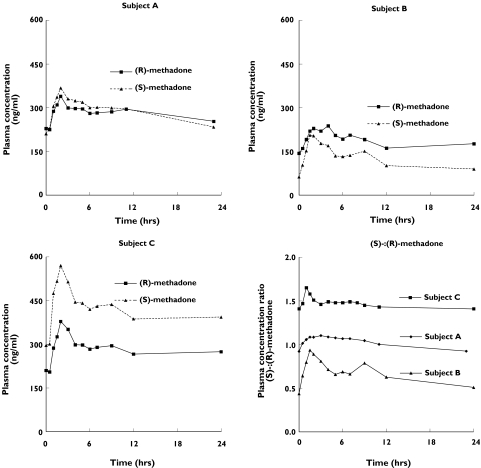
Figure 1.
Plasma concentration–time profiles for (R)- and (S)-methadone during a 24-h interdosing interval in three methadone maintenance patients. Inter- and intrasubject variation in the relative plasma concentrations of (R)- and (S)-methadone are exemplified by three different subjects showing similar concentrations of each enantiomer (subject A) and relatively greater concentrations for (R)- (subject B) or (S)- (subject C) methadone. Concentrations have been normalized to a 70-mg rac-methadone dose. Plasma concentration ratios for (S)-:(R)-methadone are shown for each subject in the bottom right-hand panel
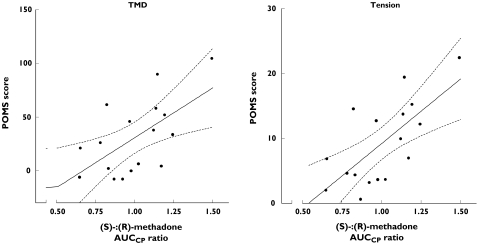
Figure 2.
Relationship between the plasma AUCCP ratio for (S)-:(R)-methadone and Total Mood Disturbance (TMD) (r2 = 0.37, P = 0.01) and tension (r2 = 0.48, P = 0.002) scores from the Profile of Mood States (POMS) during a 24-h interdosing interval in 17 patients maintained on ≥100 mg rac-methadone. Lines shown are the line of best fit (unbroken) and 95% confidence interval (broken) calculated using linear regression. Data represent averages for each variable calculated by dividing the area under the curve by the period of measurement (23 h)
Discussion
During a 24-h interdosing interval in 55 rac-methadone maintenance patients (S)-methadone Cav accounted for significant variability in pharmacodynamic responses unaccounted for by (R)-methadone Cav and rac-methadone dose. The pattern of relationships evident for (S)-methadone Cav was opposite to that of (R)-methadone Cav, such that the former showed positive associations with the intensity of negative mood states (such as Tension, Fatigue, and Confusion) and opioid withdrawal. Rac-methadone dose did not account for significant variability in pharmacodynamic response independent of Cav for each enantiomer. Relative exposure to (S)- vs. (R)-methadone over the full interdosing interval, as measured by the (S)-:(R)-methadone AUCCP ratio, showed significant variation across individuals (range 0.65–1.51). The (S)-:(R)-methadone AUCCP ratio was not associated with pharmacodynamic responses for the subject group as a whole but was positively associated with the intensity of negative mood states and heart rate amongst higher dose patients (100 mg or more). The ratio of (S)-:(R)-methadone at trough (i.e. the time of presentation for dosing) showed a similar pattern of results, with significant relationships evident for Tension and Fatigue, and was a strong predictor of AUCCP ratios (r = 0.89).
The results of this study are consistent with earlier findings that (S)-methadone, particularly at higher doses (50–1000 mg), may produce an adverse profile of subjective and symptomatic effects distinct from those of (R)-methadone [11, 12, 17]. To this extent, it would be expected that the use of (R)-methadone alone instead of rac-methadone for maintenance treatment would yield improved treatment outcomes. Although previous comparisons of clinical efficacy and acceptability for rac- and (R)-methadone have found no significant differences [13–16, 24], interpretation of these findings is complicated by several factors. These studies generally failed to account for variation in methadone dose or enantiomeric ratio, featured few measures of subjective effects (e.g. mood states), used small samples sizes (n ≤ 30) [14–16, 24], and in some cases compared each formulation following a single subcutaneous injection [24] or focused only on ‘substantial’ symptom complaints [13]. It is also noteworthy that a significant proportion of patients (41% overall) required an increase in dose following the transfer from (R)-methadone to an equivalent rac-methadone dose in each of three studies for which such data were presented (10/16, 10/22 and 6/26) [14–16]. Important and potentially subtle differences in response for (R)- and rac-methadone may thus have been overlooked in these previous investigations.
Differences in pharmacodynamic responses for (R)- and (S)-methadone may also include effects mediated by non-opioid mechanisms [25], which could be difficult to detect using instruments designed for measurement of opioid-mediated effects. For example, both methadone enantiomers have moderate binding affinity for the NMDA receptor [26] and inhibit the neuronal re-uptake of serotonin and noradrenaline [9]. Compared with (R)-methadone, (S)-methadone appears significantly less potent as an opioid agonist and inhibitor of serotonin and noradrenaline re-uptake [9]. However, both enantiomers noncompetitively inhibit the binding of NMDA receptor ligands with a potency comparable to that of the established NMDA antagonist ketamine [26], use of which has been associated with numerous adverse subjective, physiological and psychomimetic effects [27–32]. The NMDA antagonist characteristics of (S)-methadone, which are associated with significant effects in animals (e.g. antinociception, attenuation of morphine tolerance and NMDA-induced hyperalgesia) [33, 34], may contribute to adverse subjective effects in humans[17]. NMDA antagonism is also hypothesized to influence positively the analgesic efficacy [35] and level of opioid tolerance [36] associated with use of methadone for pain management. However, there remains no direct evidence for NMDA antagonist effects in humans because of the difficulty of response measurement. Other mechanisms that may hypothetically account for variation in pharmacodynamic response to racemic methadone due to variability in relative exposure to (S)- vs. (R)-methadone include competition between each enantiomer for binding sites on traditional opioid receptors [11] and the existence of receptor subtypes and splice variants [37] possessing different stereoselective properties (e.g. less stereoselectivity for either enantiomer) [38]. It is also notable that methadone inhibits the activity of CYP2D6 [39, 40], CNS endogenous substrates for which may be important in regulating mood [41].
Irrespective of the mechanisms involved, the possibility that (S)-methadone produces adverse subjective and physiological responses has potential clinical implications regarding the use of rac-methadone for maintenance treatment in some patients. The magnitudes of these subtle effects, whilst less pronounced than the dominant opioid actions of (R)-methadone, may be important given significant variability in the plasma concentration ratio of (S)- to (R)-methadone between individuals. Therefore, in patients for whom an unfavourable profile of subjective responses to high-dose rac-methadone is accompanied by significantly greater exposure to (S)- vs. (R)-methadone, transfer to (R)-methadone alone or another alternative maintenance pharmacotherapy may be advantageous. Variability between individuals in relative exposure to (S)- vs. (R)-methadone and the possibility that each enantiomer produces distinct pharmacodynamic responses also highlights the importance of using stereoselective assays when monitoring plasma methadone concentrations in maintenance patients [42]. Further studies featuring administration of (R)- and (S)-methadone alone and in combination under controlled conditions, suitable for the application of advanced pharmacodynamic–pharmacokinetic modelling techniques, are needed to characterize further the importance of (S)-methadone and intra- and interindividual variability in the methadone enantiomeric ratio in relation to treatment outcomes.
Acknowledgments
The authors acknowledge the assistance of patients and staff associated with the Drug and Alcohol Services Council of South Australia and Next Step Clinical Drug Services in Western Australia. We also thank Andrew Menelaou, David Foster, Ingvild Quinn (Studies 1–3), and Belinda Wright (Study 4) for their analysis of plasma samples and Peta Prindiville (Study 4) for assistance with data collection. This research was funded in part by the Victorian Department of Health, the National Health and Medical Research Council of Australia (Grant No. 990586), and the Faculty of Medicine & Dentistry, University of Western Australia. D.N. and T.B.M. were supported by Australian Postgraduate Award scholarships (Studies 2 and 3). K.R.D. was supported by a National Drug Strategy Postgraduate Research Scholarship (Study 1).
References
- 1.Kreek MJ, Hachey DL, Klein PD. Stereoselective disposition of methadone in man. Life Sci. 1979;24:925–32. doi: 10.1016/0024-3205(79)90343-6. [DOI] [PubMed] [Google Scholar]
- 2.Boulton DW, Arnaud P, DeVane CL. Pharmacokinetics and pharmacodynamics of methadone enantiomers after a single oral dose of racemate. Clin Pharmacol Therapeutics. 2001;70:48–57. doi: 10.1067/mcp.2001.116793. [DOI] [PubMed] [Google Scholar]
- 3.Kristensen K, Blemmer T, Angelo HR, et al. Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther Drug Monit. 1996;18:221–7. doi: 10.1097/00007691-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Foster DJ, Somogyi AA, Dyer KR, White JM, Bochner F. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2000;50:427–40. doi: 10.1046/j.1365-2125.2000.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster DJ, Somogyi AA, White JM, Bochner F. Population pharmacokinetics of (R)-, (S)- and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2004;57:742–55. doi: 10.1111/j.1365-2125.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiltunen AJ, Beck O, Hjemdahl P, et al. Rated well-being in relation to plasma concentrations of l- and d-methadone in satisfied and dissatisfied patients on methadone maintenance treatment. Psychopharmacology. 1999;143:385–93. doi: 10.1007/s002130050963. [DOI] [PubMed] [Google Scholar]
- 7.Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–14. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen K, Christensen CB, Christrup LL. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45–50. doi: 10.1016/0024-3205(94)00426-s. [DOI] [PubMed] [Google Scholar]
- 9.Codd EE, Shank RP, Schupsky JJ, Raffa RB. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Therapeutics. 1995;274:1263–70. [PubMed] [Google Scholar]
- 10.Isbell H, Eisenman AJ. The addiction liability of some drugs of the methadone series. J Pharmacol Exp Therapeutics. 1948;93:305–13. [PubMed] [Google Scholar]
- 11.Olsen GD, Wendel HA, Livermore JD, Leger RM, Lynn RK, Gerber N. Clinical effects and pharmacokinetics of racemic methadone and its optical isomers. Clin Pharmacol Therapeutics. 1977;21:147–57. doi: 10.1002/cpt1977212147. [DOI] [PubMed] [Google Scholar]
- 12.Scott CC, Robbins EB, Chen KK. Pharmacological comparison of the optical isomers of methadone. J Pharmacol Exp Therapeutics. 1948;93:282–6. [PubMed] [Google Scholar]
- 13.Judson BA, Horns WH, Goldstein A. Side effects of levomethadone and racemic methadone in a maintenance program. Clin Pharmacol Therapeutics. 1976;20:445–9. doi: 10.1002/cpt1976204445. [DOI] [PubMed] [Google Scholar]
- 14.Scherbaum N, Finkbeiner T, Leifert K, Gastpar M. The efficacy of 1-methadone and racemic methadone in substitution treatment for opiate addicts—a double-blind comparison. Pharmacopsychiatry. 1996;29:212–15. doi: 10.1055/s-2007-979573. [DOI] [PubMed] [Google Scholar]
- 15.Eap CB, Finkbeiner T, Gastpar M, Scherbaum N, Powell K, Baumann P. Replacement of (R)-methadone by a double dose of (R,S)-methadone in addicts: interindividual variability of the (R)/(S) ratios and evidence of adaptive changes in methadone pharmacokinetics. Eur J Clin Pharmacol. 1996;50:385–9. doi: 10.1007/s002280050128. [DOI] [PubMed] [Google Scholar]
- 16.de Vos JW, Ufkes JG, Kaplan CD, et al. L-methadone and D,1-methadone in methadone maintenance treatment: a comparison of therapeutic effectiveness and plasma concentrations. Eur Addict Res. 1998;4:134–41. doi: 10.1159/000018936. [DOI] [PubMed] [Google Scholar]
- 17.Fraser HF, Isbell H. Human pharmacology and addictiveness of certain dextroisomers of synthetic analgesics. Bull Narcotics. 1962;14:25–35. [Google Scholar]
- 18.Mitchell TB, White JM, Somogyi AA, Bochner F. Comparative pharmacodynamics and pharmacokinetics of methadone and slow-release oral morphine for maintenance treatment of opioid dependence. Drug Alcohol Depend. 2003;72:85–94. doi: 10.1016/s0376-8716(03)00190-x. [DOI] [PubMed] [Google Scholar]
- 19.Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration–effect relationships. Clin Pharmacol Therapeutics. 1999;65:685–94. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 20.Dyer KR, White JM, Foster DJ, Bochner F, Menelaou A, Somogyi AA. The relationship between mood state and plasma methadone concentration in maintenance patients. J Clin Psychopharmacol. 2001;21:78–84. doi: 10.1097/00004714-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Doverty M, Somogyi AA, White JM, et al. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001;93:155–63. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 22.Dyer KR, White JM. Patterns of symptom complaints in methadone maintenance patients. Addiction. 1997;92:1445–55. [PubMed] [Google Scholar]
- 23.McNair D, Lorr M, Droppleman L. EITS Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 24.Denton JE, Straus OH, Waddell WE, Beecher HK. A comparison of side actions and analgesic effects of morphine, amidone, (4–4-diphenyl-6-dimethylamino-heptanone-3) and its isomers in man. Federation Proc. 1948;7:214–15. [PubMed] [Google Scholar]
- 25.Leander JD, McCleary PE. Opioid and nonopioid behavioral effects of methadone isomers. J Pharmacol Exp Therapeutics. 1982;220:592–6. [PubMed] [Google Scholar]
- 26.Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neuroscience Lett. 1997;223:5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- 27.Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH. The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry. 1998;31:104–9. doi: 10.1055/s-2007-979354. [DOI] [PubMed] [Google Scholar]
- 28.Adler CM, Malhotra AK, Elman I, et al. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–9. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- 29.Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction. 2000;95:575–90. doi: 10.1046/j.1360-0443.2000.9545759.x. [DOI] [PubMed] [Google Scholar]
- 30.Curran HV, Monaghan L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction. 2001;96:749–60. doi: 10.1046/j.1360-0443.2001.96574910.x. [DOI] [PubMed] [Google Scholar]
- 31.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra AK, Pinals DA, Weingartner H, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–7. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 33.Davis AM, Inturrisi CE. d-Methadone blocks morphine tolerance and N-methyl-D-aspartate-induced hyperalgesia. J Pharmacol Exp Therapeutics. 1999;289:1048–53. [PubMed] [Google Scholar]
- 34.Shimoyama N, Shimoyama M, Elliott KJ, Inturrisi CE. d-Methadone is antinociceptive in the rat formalin test. J Pharmacol Exp Therapeutics. 1997;283:648–52. [PubMed] [Google Scholar]
- 35.Ebert B, Thorkildsen C, Andersen S, Christrup LL, Hjeds H. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem Pharmacol. 1998;56:553–9. doi: 10.1016/s0006-2952(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 36.Gagnon B, Bruera E. Differences in the ratios of morphine to methadone in patients with neuropathic pain versus non-neuropathic pain. J Pain Symptom Manage. 1999;18:120–5. doi: 10.1016/s0885-3924(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 37.Pasternak GW. Insights into mu opioid pharmacology: the role of mu opioid receptor subtypes. Life Sci. 2001;68:2213–19. doi: 10.1016/s0024-3205(01)01008-6. [DOI] [PubMed] [Google Scholar]
- 38.Veatch RM, Adler TK, Way EL. The importance of steric configuration in certain morphine-mimetic actions of synthetic analgetics. J Pharmacol Exp Therapeutics. 1964;145:11–19. [PubMed] [Google Scholar]
- 39.Shiran MR, Chowdry J, Rostami-Hodjegan A, et al. A discordance between cytochrome P450 2D6 genotype and phenotype in patients undergoing methadone maintenance treatment. Br J Clin Pharmacol. 2003;56:220–4. doi: 10.1046/j.1365-2125.2003.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu D, Otton SV, Sproule BA, et al. Inhibition of human cytochrome P450 2D6 (CYP2D6) by methadone. Br J Clin Pharmacol. 1993;35:30–4. doi: 10.1111/j.1365-2125.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003;13:307–19. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- 42.Rentsch KM. The importance of stereoselective determination of drugs in the clinical laboratory. J Biochem Biophys Meth. 2002;54:1–9. doi: 10.1016/s0165-022x(02)00124-0. [DOI] [PubMed] [Google Scholar]