Abstract
Aim
To study the possible influence of patient characteristics on abacavir pharmacokinetics.
Methods
A population pharmacokinetic model for abacavir was developed using data from 188 adult patients by the use of a nonlinear mixed effects modelling method performed with NONMEM.
Results
Abacavir pharmacokinetics was well described by a two-compartment open model with linear absorption and elimination. Typical population estimates for the absorption rate constant (Ka), the apparent central distribution volume (Vc/F), the apparent peripheral distribution volume (Vp/F), the apparent intercompartmental clearance (Q/F) and the apparent plasma clearance (CL/F) were 1.8 h−1, 75 l, 23.6 l, 10 l h−1 and 47.5 l h−1, respectively. Apparent plasma clearance was positively related to bodyweight. Individual Bayesian estimates of CL/F were used to calculate abacavir AUC. The latter decreased from 10.7 ± 5.0 to 5.7 ± 1.6 mgh l−1 when bodyweight increased from 36 to 102 kg. This drop in abacavir exposure could lead to suboptimal treatment for the heaviest patients, as antiviral efficacy of abacavir is known to be related to its AUC. A 400 mg abacavir dose would be necessary to achieve adequate exposure to abacavir in patients weighing more than 60 kg.
Conclusions
The apparent plasma clearance of abacavir was positively related to bodyweight. The efficacy of the current recommended abacavir dosage for patients with high bodyweight should be evaluated in further studies.
Keywords: HIV, abacavir, adults, bodyweight, population pharmacokinetics
Introduction
Abacavir is a nucleoside analogue used for the treatment of immunodeficiency virus type 1 (HIV-1) infection. Abacavir is extensively metabolized by the liver and is converted intracellularly into the active compound, carbovir triphosphate [1]. Phase II studies suggested the use of a 300 mg BID dose for an optimal antiviral efficacy of this drug [2, 3]. However, phase II studies are often performed on a limited number of patients who are subject to selection criteria to minimize physiopathological differences between them. Therefore, the estimation of interindividual variability in pharmacokinetic parameters can be poor and the influence of individual characteristics of patients on the disposition of a drug may remain unknown.
Using population pharmacokinetics it has been shown that interindividual variability in the volume of distribution and the clearance of stavudine, zidovudine and didanosine, three other nucleoside analogues, was related to bodyweight [4, 5]. Since only few concentration measurements are needed from each individual, this approach can be performed from routine clinical data.
In France many patients on highly active antiretroviral therapy (HAART) benefit from therapeutic drug monitoring, which has allowed us to perform retrospectively a population pharmacokinetic study in a large population of patients receiving abacavir. The objective of this study was to investigate possible relationships between the pharmacokinetics of abacavir and patient characteristics. Such results could be useful for the optimization of abacavir treatment, since a significant relationship has been demonstrated between its area under the concentration-time curve (AUC) and antiretroviral efficacy [6].
Methods
Patients and treatment
The population included adult patients receiving oral abacavir, as 300 mg tablets, for the treatment of HIV infection and who were monitored for plasma concentrations of antiretroviral drugs on a routine basis. For each patient, the time between administration and sampling times, gender, bodyweight (BW) and age were recorded, as well as information on combined treatment, particularly antiretroviral drugs. Ethics committee approval and patient consent are not necessary in France for the use of therapeutic drug monitoring data.
Drug analysis
Abacavir plasma concentrations were measured using high-performance liquid chromatography with UV detection at 280 nm. Briefly, 3 ml of terbutylmethylether was added to 100 µl of plasma samples. After 20 min of mixing, samples were centrifuged for 20 min at 2200 g and the supernatant was evaporated at 37 °C under a stream of nitrogen. The residue was then reconstituted in 120 µl of the mobile phase (10 mm phosphate buffer, pH = 7.2: acetonitrile; 82 : 18, v/v) and 80 µl was injected onto the chromatographic system. The separation was performed isocratically on a Cluzeau C8 plus satisfaction column at a flow rate of 0.5 ml min−1. The limit of quantification of the method was 0.01 mg l−1, the inter assay precision (expressed as a coefficient of variation) and the bias were less than 15% and 5%, respectively, in the calibration range 0.01–10 mg l−1.
Population pharmacokinetic modelling
Concentration-time data were analysed using the first-order conditional estimation method (FOCE) of the nonlinear mixed effects modelling program NONMEM [7] (version V, level 1.1, double precision). Abacavir data were analysed according to a two-compartment pharmacokinetic model with linear absorption and elimination. Parameters from the final structural model were apparent total and intercompartmental clearance (CL/F and Q/F), apparent central and peripheral distribution volume (Vc/F and Vp/F) and absorption rate constant (Ka), where F is the bioavailability (subroutines ADVAN4 TRANS4).
Several error models were investigated (i.e. proportional, exponential and additive random effects model) to describe interpatient and residual variability.
The influence of each patient covariate on pharmacokinetic parameters was systematically tested via a generalized modelling according to the following equation, using CL as an example,
where TV(CL) is the typical value of clearance for a patient with the median covariate value and θBW is the estimated influential factor for bodyweight. Such covariates included age, bodyweight, dose per unit BW, gender and concomitant antiretroviral therapy.
The effect of a covariate was assessed by the chi-squared test of the difference between the objective functions of the basic model (without covariate) and the model including the covariate. Improvement of the fit was then considered to be significant if the decrease in the objective function value was at least 7 units (P < 0.01, one degree of freedom) compared with the basic pharmacokinetic model.
The accuracy and robustness of the final population model were assessed using a bootstrap method [8], consisting of repeated random sampling with replacement from the original data set. This resampling was repeated 1000 times and the values of the parameters estimated from the bootstrap set were compared with the estimates obtained from the original data set. The entire procedure was performed in an automated fashion using a DOS batch file and Awk scripts [9].
Results
One hundred and eighty-eight patients, ranging in age from 16.1 to 72.8 years, were available for pharmacokinetic evaluation. All received abacavir as the recommended 300 mg BID dose. Their characteristics are listed in Table 1, and the observed combinations with other antiretroviral drugs are summarized in Table 2. No information was available about either the duration of abacavir therapy or the viral load or CD4 count at sampling time. Three hundred and forty-four blood samples were analysed, 30 of them corresponding to plasma concentrations that were below the quantification limit of the analytical method (LOQ). The values of these 30 samples were set to half the LOQ, i.e. 0.005 mg l−1 [10].
Table 1.
Characteristics of the patients (151 men/37 women)
| Characteristics | Mean | SD | Median | Range |
|---|---|---|---|---|
| Age (year) | 39.7 | 11.7 | 40 | 16.1–72.8 |
| Bodyweight (kg) | 65.4 | 12.3 | 65 | 36–102 |
| Dose (mg) | 300 | 0 | 300 | |
| Dose kg−1 | 4.8 | 1.0 | 4.6 | 2.9–8.3 |
| Number of samples | 344 | |||
| Number of samples per patient | 1.9 | 1.5 | 1 | 1–7 |
| Abacavir concentration (mg l−1)* | 0.74 | 0.79 | 0.53 | 0.01–4.36 |
| Delay between administrationand sampling (h) | 5.6 | 4.1 | 3.7 | 0.2–12 |
Below the LOQ concentrations were not taken into account.
Table 2.
Number and type of antiretroviral drug combinations taken by the patiens studied
| Pharmacological class of antiretroviral drugs combined with abacavir | |||||||
|---|---|---|---|---|---|---|---|
| NNRT | |||||||
| NRTI | NRTI | NNRTI | +NNRTI | ||||
| NRTI | NNRTI | PI | +NNRTI | +PI | +PI | +PI | |
| n | 18 | 2 | 1 | 41 | 71 | 26 | 29 |
NRTI: Nucleoside Reverse Transcriptase Inhibitor; NNRTI: Non Nucleoside Reverse Transcriptase Inhibitor; PI: Protease Inhibitor; n: Number of subjects.
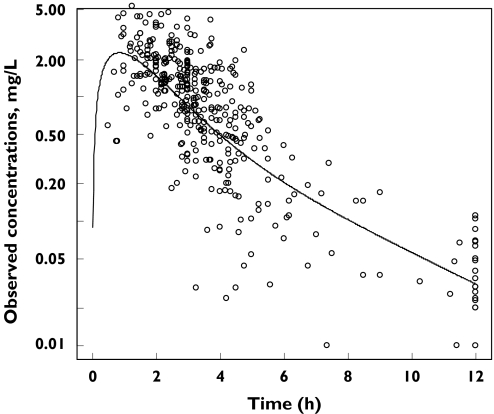
A two-compartment model adequately described the data. The plot of observed concentrations vs. time after dose with the typical pharmacokinetic curve is shown in Figure 1. Inter-patient variability was described by an exponential error model, whereas residual variability was described by an additive error model. All parameters estimates were significant according to the standard errors provided by the NONMEM covariance step (not shown). Interindividual variability in Vp/F and Q/F could not be estimated. The only covariate that decreased the objective function by more than 7 units (i.e. −22 units) was BW, which had a positive influence on CL/F. When BW was included in the model, Q/F had to be fixed to its typical value obtained from the basic model (i.e. 10 l h−1) to produce a successful convergence. The possibility of drug interactions occurring was systematically investigated with respect to CL/F, but there was no evidence for this, even with the potential inducing drugs, nevirapine and efavirenz. The covariate submodel was defined by the equation:
Figure 1.
Observed abacavir concentrations vs. time data and the typical curve
A 17% relative decrease in the interindividual variability of CL/F was observed. Table 3 summarizes the population parameter estimates.
Table 3.
Population pharmacokinetic parameters for abacavir in 188 adult patients and the results of bootstrap validation
| Final model | ||||
|---|---|---|---|---|
| Original dataset | Bootstrap† | |||
| Parameter | Mean | SE | Mean | SE |
| TV (CL/F) (l h−1) | 47.5 | 2.6 | 46.8 | 4.0 |
| CL/F, θBW | 0.80 | 0.23 | 0.77 | 0.27 |
| TV(Vc/F) (l) | 75 | 7.7 | 70.5 | 13.4 |
| TV (Q/F) (l h−1) | 10* | 2.3* | / | / |
| TV(Vp/F) (l) | 24 | 3.5 | 24.3 | 3.5 |
| TV(Ka) (h−1) | 1.8 | 0.4 | 2.0 | 1.1 |
| Residual variability, σ2 (mg l−1) | 0.039 | 0.009 | 0.039 | 0.010 |
| ω2CL/F | 0.18 | 0.03 | 0.18 | 0.03 |
| ω2Vc/F | 0.51 | 0.13 | 0.54 | 0.25 |
| ω2Ka | 1.13 | 0.33 | 1.11 | 0.37 |
| COVCL,V | 0.27 | 0.04 | 0.28 | 0.10 |
Mean of 1000 bootstrap analyses
SE, standard error of the estimate; TV(CL/F), TV(Vc/F) and TV(Ka), typical value of CL/F, Vc/F and Ka, respectively; θCOVARIATE, influential factor for covariate: BW, body weight; ω2, interindividual variability; COVCL,V, covariance between CL/F and Vc/F;
typical value and SE obtained with the basic model
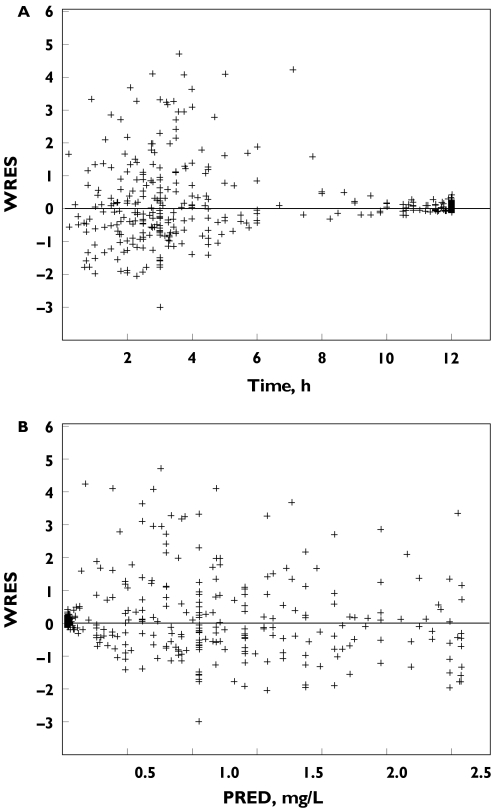
The goodness-of-fit was evaluated graphically by the weighted residuals vs. predicted concentrations and Time after dose plots (Figure 2).
Figure 2.
(A) Goodness of fit evaluated by weighted residuals (WRES) vs. time data; (B) WRES vs. model predicted (PRED) plasma abacavir concentrations
The final model obtained with the original dataset was subjected to a bootstrap analysis. As shown in Table 3, the mean parameter estimates obtained from the bootstrap process, following 1000 runs, were not statistically different from the estimates previously obtained with the original dataset.
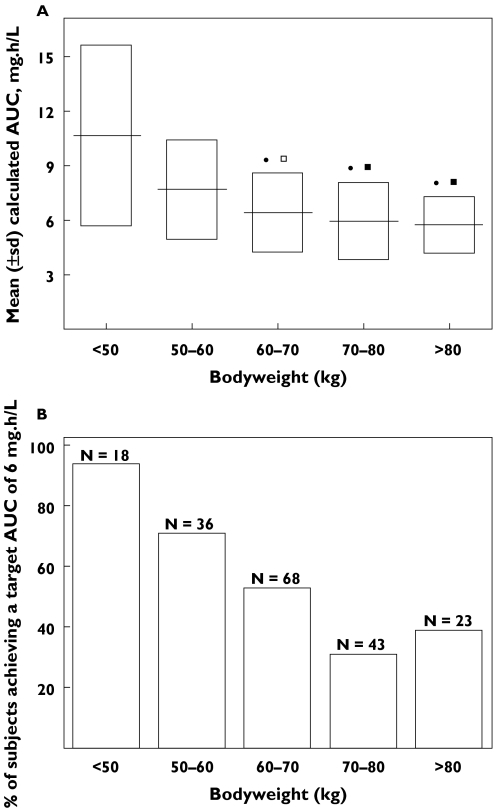
Individual Bayesian clearances were used to calculate AUC values. The mean ± standard deviation AUC decreased from 10.7 ± 5.0 mg h l−1 to 5.7 ± 1.6 mg h l−1 when bodyweight increased from 36 to 102 kg. The mean AUC of patients weighing less than 60 kg was found to be statistically different from that of patients weighing more than 60 kg (Figure 3A).
Figure 3.
(A) Abacavir exposure achieved for a 300 mg BID dose in the 188 adult patients. Mean ± standard deviation (SD) of AUC vs. bodyweight (BW); (B) percentage of patient reaching the 6 mg h l−1 AUC value thought to correspond to the beginning of the plateau phase of abacavir antiviral efficacy vs. bodyweight. anova: •P < 0.001 vs. < 50 kg BW group; □P < 0.01 vs. 50–60 kg BW group; ▪P < 0.1 vs. 50–60 kg BW group; n: number of subjects per BW group
A previously published population pharmacokinetic/pharmacodynamic analysis has shown that the antiviral efficacy of abacavir was related to its AUC by Emax models [6], and that the mean AUC corresponding to the beginning of the plateau phase (5.5 ± 1.4 mg h l−1) was achieved with the 300 mg BID dose. Furthermore, two pharmacokinetic studies have found that the recommended 300 mg abacavir dose led to a mean AUC value of 6 mg h l−1 [11, 12]. Thus, assuming an AUC value of 6 mg h l−1 as a target exposure, the percentage of patients achieving this value in our population markedly decreased as a function of bodyweight (Figure 3B). Thus, 80% of patients weighing less than 60 kg achieved the target AUC value whereas this percentage decreased to 40% for patients weighing more than 60 kg. A 400 mg dose would be necessary to allow 80% of the patients weighing more than 60 kg to achieve a 6 mg h l−1 AUC.
Discussion
Abacavir plasma pharmacokinetics was well described by a two-compartment open model with first order absorption and elimination. However, a one-compartment model was used in a previously published population pharmacokinetic study [6]. This discrepancy could be explained by differences in study design. Blood sampling was performed up to 4 h post dose in the above cited study compared with 12 h in the present work. Two noncompartmental PK studies [11, 13] provided some concentration vs. time curves that could support the use of a one or two-compartment model. A two-compartment model was preferred to describe our data, as it provided a much better fit to the lowest concentrations (not shown). This model provided a mean CL/F estimate of 47.5 l h−1 or 12.2 ml min−1 kg−1 that was in the range of previously reported CL/F values, i.e. 58 l h−1 [6], 46.8 l h−1 [13] and 13.4 ml min−1 kg−1 [11].
Ninety-eight patients received a non nucleoside reverse transcriptase inhibitor (NNRTI) as a concomitant treatment. These drugs are known to be potential inducers of hepatic cytochrome P450 3A4 (CYP 3A4) [14]. Though alcohol dehydrogenase, UDP glucuronyl transferase [15], but not CYP 3A4 are involved in abacavir metabolism, a possible inducing effect of NNRTIs on abacavir CL/F was investigated as it has been previously shown that phase II enzymes can be induced by the same drugs that induce cytocromes 450 [16]. No evidence of induction was found in the present study. A lack of pharmacokinetic interaction was also found with ritonavir and nelfinavir, which are potential glucuronyltransferase inducers [17], and zidovudine, which is extensively metabolized by UDPGT [18]. However, only 36% of abacavir dose is metabolized via glucuronidation [13]. Therefore, a possible inducing or inhibitory effect on UDPGT could result in a modest change in abacavir CL/F, not detected in the present study.
Bodyweight was identified as a potential determinant of abacavir plasma clearance, a relationship that was not found in a previously published population analysis [6]. However, these authors reported a 5.3 points decrease in the objective function, a change that would have been significant if a prior significance level of 5% had been chosen. Nevertheless, this decrease suggested the relationship between BW and CL/F that has been shown in our study as the greater number of patients (188 vs. 41) provided a greater statistical power.
Abacavir is a prodrug that is phosphorylated at the intracellular level to obtain the active moiety, carbovir triphosphate. The fact that intracellular levels of this compound depend on abacavir dosage [1], and the relationship between abacavir AUC and its antiviral efficacy, suggest a possible correlation between the plasma concentration of the prodrug and the intracellular concentration of the active moiety, as it was shown for stavudine [19].
Abacavir plasma AUC is indeed correlated with antiviral efficacy as it is related to both time-averaged decrease in viral load and time-averaged increase in CD4 cell count by an Emax model [7]. For these two parameters, the beginning of the plateau phase was achieved with the standard 300 mg dose. Assuming that the latter dose leads to a mean AUC value of 6 mg h−1 l−1 [6, 11, 13], and that lower AUC values correspond to suboptimal antiviral efficacy, there may be a risk of undertreatment in patients with high bodyweight, since an important decrease in the percentage of patients achieving this target AUC value was observed when bodyweight increased.
As has been shown for protease inhibitors [20], inadequate abacavir plasma concentrations could lead to failure of therapy. Given the current abacavir dosage, heavy bodyweight could be a predictive factor for an unfavourable virological outcome. However, as abacavir is not supposed to be prescribed as a single therapy, sub-optimal abacavir concentrations can be balanced by the co-administered antiretroviral drugs. However, considering only abacavir mono-therapy, achieving an optimal exposure in heavy bodyweight patients could necessitate the administration of a 400 mg dose. This would involve the use of an oral solution, as only 300 mg coated tablets are currently available. Furthermore, abacavir is known to be well tolerated at its recommended 300 mg BID dose [3, 21], but the safety of higher doses has not been studied over a period longer than 24 weeks [3].
In conclusion, this study including 188 adult patients showed that the plasma clearance of abacavir was related to body weight and that exposure to the drug might be insufficient in patients with high bodyweight. The usefulness of dose higher than the recommended 300 mg BID should be evaluated in further studies.
References
- 1.Harris M, Back D, Kewn S, Jutha S, Marina R, Montaner JS. Intracellular carbovir triphosphate levels in patients taking abacavir once a day. AIDS. 2002;16(8):1196–7. doi: 10.1097/00002030-200205240-00021. [DOI] [PubMed] [Google Scholar]
- 2.Saag MS, Sonnerborg A, Torres RA, et al. Antiretroviral effect and safety of abacavir alone and in combination with zidovudine in HIV-infected adults. Abacavir Phase 2 Clin Team AIDS. 1998;12(16):F203–9. doi: 10.1097/00002030-199816000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Staszewski S, Katlama C, Harrer T, et al. A dose-ranging study to evaluate the safety and efficacy of abacavir alone or in combination with zidovudine and lamivudine in antiretroviral treatment-naive subjects. AIDS. 1998;12(16):F197–202. doi: 10.1097/00002030-199816000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Horton CM, Dudley MN, Kaul S, et al. Population pharmacokinetics of stavudine (d4T) in patients with AIDS or advanced AIDS-related complex. Antimicrob Agents Chemother. 1995;39(10):2309–15. doi: 10.1128/aac.39.10.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou XJ, Sheiner LB, D'Aquila RT, et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators Antimicrob Agents Chemother. 1999;43(1):121–8. doi: 10.1128/aac.43.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller S, Radomski KM, Lou Y, Stein DS. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2000;44(8):2052–60. doi: 10.1128/aac.44.8.2052-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beal SL, Sheiner LB. NONMEM user's guide. University of California at San Francisco;: NONMEM Project Group; 1991. [Google Scholar]
- 8.Parke J, Charles BG. Factors affecting oral cyclosporin disposition after heart transplantation: bootstrap validation of a population pharmacokinetic model. Eur J Clin Pharmacol. 2000;56(6–7):481–7. doi: 10.1007/s002280000164. [DOI] [PubMed] [Google Scholar]
- 9.Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Meth Programs Biomed. 1999;59(1):19–29. doi: 10.1016/s0169-2607(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 10.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001. 2002;28(5):481–504. doi: 10.1023/a:1012299115260. Erratum. In: J Pharmacokinet Pharmacodyn 2002; 29 (3): 309. [DOI] [PubMed] [Google Scholar]
- 11.Kumar PN, Sweet DE, McDowell JA, et al. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 1999;43(3):603–8. doi: 10.1128/aac.43.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDowell JA, Lou Y, Symonds WS, Stein DS. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44(8):2061–7. doi: 10.1128/aac.44.8.2061-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDowell JA, Chittick GE, Ravitch JR, Polk RE, Kerkering TM, Stein DS. Pharmacokinetics of [(14) C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob Agents Chemother. 1999;43(12):2855–61. doi: 10.1128/aac.43.12.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36(4):289–304. doi: 10.2165/00003088-199936040-00004. [DOI] [PubMed] [Google Scholar]
- 15.McDowell JA, Chittick GE, Stevens CP, Edwards KD, Stein DS. Pharmacokinetic interaction of abacavir (1592U89) and ethanol in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44(6):1686–90. doi: 10.1128/aac.44.6.1686-1690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinach B, de Sousa G, Dostert P, Ings R, Gugenheim J, Rahmani R. Comparative effect of rifabutin and rifampicin on cytochromes P450 and UDP-glucuronosyl transferases expression in fresh and cryopreserved human hepatocytes. Chem Biol Interact. 1999;121(1):37–48. doi: 10.1016/s0009-2797(99)00089-7. [DOI] [PubMed] [Google Scholar]
- 17.Back D, Gibbons S, Khoo S. Pharmacokinetic drug interaction with nevirapine. JAIDS. 2003;34(Suppl 1):S8–S14. doi: 10.1097/00126334-200309011-00003. [DOI] [PubMed] [Google Scholar]
- 18.Resetar A, Minick D, Spector T. Glucuronidation of 3′-azido-3′-deoxythymidine catalyzed by human liver UDP-glucuronosyltransferase. Significance of nucleoside hydrophobicity and inhibition by xenobiotics. Biochem Pharmacol. 1991;42(3):559–68. doi: 10.1016/0006-2952(91)90319-z. [DOI] [PubMed] [Google Scholar]
- 19.Becher F, Landman R, Mboup S, et al. Monitoring of didanosine and stavudine intracellular triphosphorylated anabolite concentrations in HIV-infected patients. AIDS. 2004;18:181–7. doi: 10.1097/00002030-200401230-00006. [DOI] [PubMed] [Google Scholar]
- 20.Durant J, Clevenbergh P, Garraffo R, et al. Importance of protease inhibitor plasma levels in HIV-infected patients treated with genotypic-guided therapy: pharmacological data from the Viradapt Study. AIDS. 2000;14(10):1333–9. doi: 10.1097/00002030-200007070-00005. [DOI] [PubMed] [Google Scholar]
- 21.Moyle GJ, Datta D, Mandalia S, Morlese J, Asboe D, Gazzard BG. Hyperlactataemia and lactic acidosis during antiretroviral therapy: relevance, reproducibility and possible risk factors. AIDS 2002. 16(10):1341–9. doi: 10.1097/00002030-200207050-00005. Erratum in: AIDS, 2002; 16 (12) 1708. [DOI] [PubMed] [Google Scholar]