Abstract
List of nonstandard abbreviations mlpc multilumen perfusion catheter TMPD: transmucosal potential difference a.u. arbitrary unit
Aims
To investigate the potential induction by rifampicin of intestinal CYP2C8, CYP2C9, CYP2D6 and CYP3A4 using preparations of human enterocytes.
Methods
Using a multilumen perfusion catheter shed human enterocytes were collected from 6 healthy subjects before and after 10 days of 600 mg day−1 oral rifampicin administration. The protein expression of CYP2C8, CYP2C9, CYP2D6 and CYP3A4 as well as that of CYP3A4 mRNA was determined using Western blotting and RT-PCR, respectively.
Results
CYP3A4 mRNA expression in shed enterocytes increased from 74.6 ± 44.2 to 143.2 ± 68.4 a.u. (P < 0.05, 95% CI: 21.8–115.3). Expression of CYP2C8 and CYP2C9 increased from 5.1 ± 0.9 to 10.4 ± 2.3 pmol mg−1 protein (P < 0.01, 95% CI: 2.8–7.7) and from 4.2 ± 1.4 to 5.7 ± 1.1 pmol mg−1 protein (P < 0.01, 95% CI: 0.6–2.4), respectively. No significant difference in CYP2D6 expression before and during rifampicin intake was observed. Rifampicin administration also resulted in a significant induction of CYP3A4 protein (34.1 ± 10.7 vs. 113.9 ± 31.1 pmol mg−1 protein (P < 0.001, 95% CI: 51.8–107.6)). Ex vivo incubation of enterocyte homogenates with verapamil resulted in a significantly increased production of the metabolites formed via CYP3A4 (D-617: 125.9 ± 118.8 vs. 277.2 ± 145.5 pmol min−1 mg−1 protein (P < 0.05, 95% CI: 30.1–272.5); norverapamil: 113.0 ± 57.9 vs. 398.4 ± 148.2 pmol min−1 mg−1 protein (P < 0.05, 95% CI: 47.2–523.6)).
Conclusion
Our findings indicate that shed enterocytes are a useful tool to study the expression, regulation and function of drug metabolizing enzymes. Induction of intestinal CYP2C8 and CYP2C9 might contribute in part to rifampicin – mediated drug interactions, in addition to their hepatic counterparts and intestinal and hepatic CYP3A4.
Keywords: rifampicin, cytochrome P450 2C, cytochrome P450 3A4, enterocytes, human small intestine
Introduction
Gut wall metabolism and transport are recently recognized as important determinants of presystemic drug elimination [1–3]. The most direct evidence for the importance of gut wall metabolism in humans came from studies during the anhepatic phase of liver transplantations, in which drug and metabolite concentrations were determined in portal venous blood after intraduodenal administration of parent compound [4, 5]. These studies have shown that approximately 50% of the luminally administered CYP3A4 substrates cyclosporin and midazolam were metabolized when they reached the portal vein [4, 5]. Moreover, induction of intestinal drug metabolizing enzymes [6, 7] and transporters (P-glycoprotein) [7] by rifampicin is a now well established mechanism of drug interactions. For example, induction of these intestinal proteins leads to a decreased bioavailability of the P-glycoprotein substrates, digoxin and talinolol [7, 8] and of the CYP3A4 substrates, cyclosporin, midazolam and verapamil [9–13].
Rifampicin also has a significant effect on the elimination of CYP2C9 and CYP2C19 substrates such as tolbutamide, phenytoin, losartan, S-warfarin, antidiabetic drugs (e.g. glibenclamide) and of S-mephenytoin [14–19]. This is in line with several studies with human hepatocytes or in vivo studies using selective probe drugs, showing induction of CYP2C8, CYP2C9 and CYP3A4, but not of CYP2D6 by rifampicin [20–25]. Apart from CYP3A4 [6], it is not yet established whether induction of these enzymes also occurs at the level of the small intestine, thereby possibly contributing to the above mentioned drug interactions.
Using an intestinal multilumen perfusion catheter we have been able to measure directly drug absorption, intestinal drug metabolism and transport [26, 27]. For example, it was shown that gut wall metabolism of the calcium channel blocker verapamil is quantitatively as important as hepatic drug metabolism (mean extraction ratio: 0.49 vs. 0.48) [26]. In addition, methods have been developed to collect large quantities of relatively pure (>84%) populations of viable human enterocytes [28], which can be used for studies on expression and regulation of intestinal proteins. The latter approach may be superior to the use of intestinal biopsies, which contain only a modest amount of enterocytes. Furthermore, protein degradation occurs with surgical samples due to warm and cold ischaemia.
Human shed enterocytes, collected before and during treatment of healthy subjects with rifampicin, were used to determine whether this inducing agent affected the expression and regulation of several intestinal cytochrome P450 enzymes (CYP2C8, CYP2C9, CYP2D6, CYP3A4).
Materials and methods
Subjects
Six healthy male subjects (age: 27.5 ± 6.5 years, weight: 77.3 ± 3.1 kg) were included in this study. Medical histories, physical examination, and routine laboratory tests revealed no abnormalities. All subjects gave written informed consent. The study protocol was approved by the local ethics committee (Ethikkommission der Medizinischen Fakultät, Universität Tübingen, Germany). Subjects did not take any medications before the study, and they refrained from consumption of caffeine, alcohol and grapefruit juice. All of the subjects were nonsmokers.
Study design
A re-usable multiluminal intestinal perfusion catheter (mlpc; Dentsleeve Pty Ltd, Wayville, South Australia, Australia) was used for perfusion of isolated, 20 cm jejunal segments and collection of shed enterocytes. The ones used in the present investigation were obtained from a previous study on intestinal digoxin transport from the quinidine-free jejunal segment [27]. Details of this technique including catheter design, intubation procedure, localization of the catheter via transmucosal potential difference (TMPD) and composition of buffer solutions have been described elsewhere [26–28]. In brief, the 280 cm (tip to luer) long silicon rubber catheter has an external diameter of 4.5 mm and contains 12 channels of different diameters. Isolated jejunal segments can be perfused by separate infusion channels. After an overnight fast and local anaesthesia of the pharynx (Xylocain®-Spray, Astra GmbH, Wedel, Germany), the mlpc was introduced orally and placed into the small intestine. The correct position of the device was verified by measurement of TMPD. The most proximal balloon was positioned at least 20 cm distal from the pylorus and thus distal from the Papilla Vateri. Using a motor-driven syringe (IVAC P7000 Mk II; ALARIS™ Medical Systems, Gießen, Germany) intestinal segments were perfused at 2.0 ml min−1 for 3 h with perfusion buffer as described previously [27]. Perfusate was collected from the isolated intestinal segment at 15 min intervals and was used for protein and RNA preparation, respectively [28]. The samples were stored at −80°C until further use. The shed enterocytes before rifampicin administration were obtained on study day 1 as described above. On study day 15, the procedure was repeated after 10 days of 600 mg day−1 rifampicin p.o. (Rifa; Grünenthal GmbH, Stolberg, Germany) [27]. The CYP3A4 protein expression data (before and after rifampicin administration) used in this study have been published previously [27].
mRNA and protein analysis
Determination of CYP3A4 mRNA expression in shed enterocytes before and during rifampicin administration was performed as described previously [29]. In brief, cDNA synthesis was carried out with the Reverse Transcription reagents kit (Applied Biosystems, Foster City, California, USA) according to the manufacturer's instructions. The detection of CYP3A4 mRNA was carried out with an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, California, USA) using 50 ng total RNA for cDNA synthesis. For normalization, the expression of the housekeeping gene β-actin was determined with a PDAR (Applied Biosystems, USA). For calibration of CYP3A4 mRNA, a CYP3A4 plasmid, kindly provided by Dr F.J. Gonzalez was used, whereas the calibration of β-actin was performed with the cDNA from a human liver.
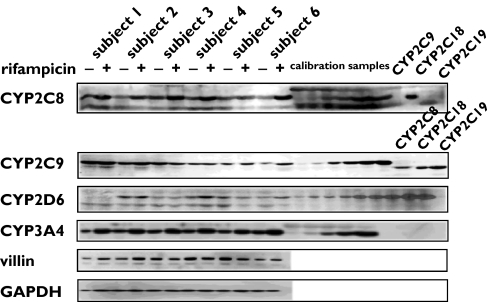
Immunoblotting analyses were performed as described before [28]. The perfusate samples were homogenized using of a 2 ml conical Wheaton glass tube with a motor-driven Teflon pestle (1000 r.p.m. for 2 min) followed by sonicating the homogenates for 30 s at 12 W (Bandelin Sonoplus HD 200, Bandelin Electronics, Berlin, Germany). For detection of CYP2C8, CYP2C9, CYP2D6 and CYP3A4, 40 µg, 30 µg, 50 µg and 7 µg, respectively, of the pooled whole homogenates were separated on 10% SDS-polyacrylamide slab gels according to the method of Laemmli [30]. For immunoblotting analyses a standard protocol was used [31]. Antibodies against CYP2C8, CYP2C9, CYP2C18 and CYP2C19 were obtained from Research Diagnostics (Flanders, NJ, USA). The antibody against CYP2C8 cross-reacts with CYP2C18 recombinat protein (Figure 2). However, because there is no detectable expression of CYP2C18 protein in the intestine [36] the results of the present study represent the actual expression of CYP2C8 in the shed enterocytes. The CYP2D6 antibody was provided by Dr U. Zanger (Dr Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany). Antibodies against CYP3A4, GAPDH and villin were purchased from Gentest (Frankfurt a.M., Germany), Dunn-Labortechnik (Asbach, Germany) and Chemicon (Hofheim, Germany), respectively. Recombinant CYP2C8, CYP2C9, CYP3A4 and CYP2D6 were obtained from Gentest (Frankfurt a.M., Germany). Chemiluminescence (SuperSignal West Dura, Perbio, Bonn, Germany) was measured with a CCD camera (Fuji LAS-1000, Raytest, Straubenhardt, Germany). Densitometric analyses were performed using the densitometric software AIDA 2.1 (Ray test, Straubenhardt, Germany). For quantification of cytochrome P450 expression, serial dilutions (1000, 500, 250, 125, 50, 25 fmol for CYP2C8 and CYP2C9; 1000, 500, 250, 125, 50 fmol for CYP3A4 and 125, 100, 50, 30, 25, 10, 5, 2.5, 1 fmol for CYP2D6) of recombinantly expressed cytochrome P450 enzymes were used. The assays for all cytochrome P450 enzymes were linear over the entire concentration range. The calculation of the cytochrome P450 expression was based on the calibration curves obtained with the recombinant enzymes. Western Blots were performed at least in duplicates.
Figure 2.
Representative Western Blot of CYP2C8, CYP2C9, CYP2D6, CYP3A4, villin and glyceraldehyde 3-phosphate dehydrogenase in six healthy subjects before and during rifampicin administration. (–) lanes: before rifampicin intake (+) lanes: during rifampicin intake (600 mg d−1 for 10 d). Calibration samples of recombinant CYP2C8, CYP2C9, CYP2D6 and CYP3A4 were used for quantification. For controlling the cross activity of the CYP2C8 and CYP2C9 antibodies, the last three lanes of the CYP2C8 and CYP2C9 blots were loaded with recombinant CYP2C9, CYP2C18, CYP2C19 and CYP2C8, CYP2C18, CYP2C19, respectively. Subject 1 was a carrier of the CYP2D6*4/*4 genotype, and therefore no protein was detectable
Cytochrome P450 activity
Formation of the verapamil metabolites D-617 and norverapamil was used to determine the CYP3A4 activity of shed enterocytes. Verapamil metabolites D-702 and D-703 were also measured, since they are primarily formed in liver by CYP2C8 and CYP2C9 [32]. The incubation experiments with verapamil and the analysis of the metabolites by LC/MS were performed as described previously [28, 33]. The lower limit of quantification for the metabolites and verapamil was 1 pmol. These assays were linear over the concentration range 1–500 pmol for all metabolites and verapamil. The intra-assay and interassay coefficents of variation were lower than 10% for all metabolites and lower than 12% for verapamil. Determination of cytochrome P450 activity was carried out in five subjects. Insufficient amounts of shed enterocytes were available in subject 5.
CYP2D6 genotyping
CYP2D6 genotype was determined as described previously [28].
Statistical analysis
Data are presented as mean ± SD. A paired t-test was used to test the effect of rifampicin on enzyme expression and function. The Pearson correlation test was used. Statistical analyses were performed with GraphPad InStat software (GraphPad Software for Science Inc., San Diego, California, USA). A P-value ≤ 0.05 was considered to be statistically significant.
Results
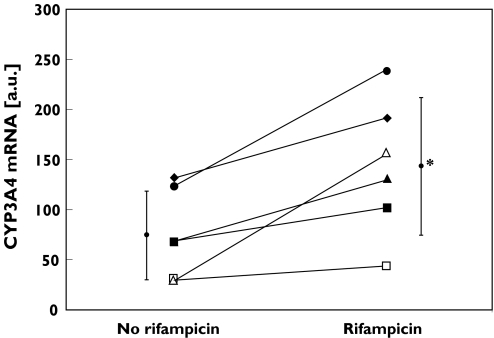
Figure 1 shows the CYP3A4 mRNA expression data before and during rifampicin treatment in each subject. CYP3A4 mRNA increased from 74.6 ± 44.2 to 143.2 ± 68.4 a.u. (2.3-fold increase; P < 0.05, 95% CI: 21.8–115.3). Subject 6 showed the strongest induction.
Figure 1.
Quantification of CYP3A4 mRNA in six healthy subjects before and during rifampicin administration (600 mg d−1 for 10 d) by quantitative RT-PCR. *P < 0.05. Subject 1 (♦), subject 2 (▪), subject 3 (▴), subject 4 (•), subject 5 (□), subject 6 (▵)
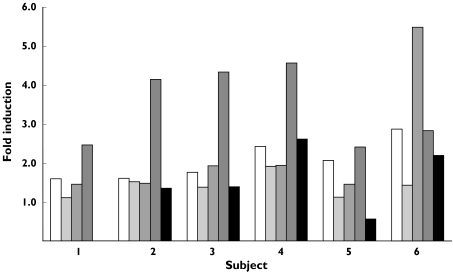
In average, 4.9 ± 1.9 mg of enterocyte protein were collected during each 3 h perfusion experiment. Figure 2 shows a representative Western Blots for all the proteins. Expression of CYP2C8 and CYP2C9 increased from 5.1 ± 0.9 to 10.4 ± 2.3 pmol mg−1 protein (P < 0.01, 95% CI: 2.8–7.7) and from 4.2 ± 1.4 to 5.7 ± 1.1 pmol mg−1 protein (P < 0.01, 95% CI: 0.6–2.4), respectively. No significant difference in CYP2D6 expression was observed (0.16 ± 0.13 vs. 0.26 ± 0.23 pmol mg−1 protein, ns, 95% CI: −0.08–0.27). Subject 1 was homozygous for the CYP2D6*4 allele and therefore CYP2D6 was not detectable. Rifampicin administration also resulted in a significant induction of CYP3A4 expression (34.1 ± 10.7 vs. 113.9 ± 31.1 pmol mg−1 protein, P < 0.001, 95% CI: 51.8–107.6). However, the induction of CYP3A4 protein did not correlate with that of CYP3A4 mRNA. The expression of the enterocyte-specific protein villin and the housekeeping gene product glyceraldehyde 3-phosphate dehydrogenase were not altered by rifampicin. The change in the expression of each CYP is shown in Figure 3, and the extent of induction of the proteins in Figure 4. The only significant correlation was between CYP2C9 and CYP3A4 induction (r = 0.80, P = 0.05, 95% CI: −0.02–0.98).
Figure 3.
CYP2C8, CYP2C9, CYP2D6 and CYP3A4 protein expression in shed enterocytes before and during rifampicin intake. **P < 0.01, ***P < 0.001. Subject 1 (♦), subject 2 (▪), subject 3 (▴), subject 4 (•), subject 5 (□), subject 6 (▵). Data are presented as mean ± SD
Figure 4.
Extent of CYP2C8, CYP2C9, CYP2D6, CYP3A4 mRNA and CYP3A4 protein induction by rifampicin in each subject. CYP2C8 (□), CYP2C9 ( ), CYP3A4 mRNA (
), CYP3A4 mRNA ( ), CYP3A4 (
), CYP3A4 ( ), CYP2D6 (▪)
), CYP2D6 (▪)
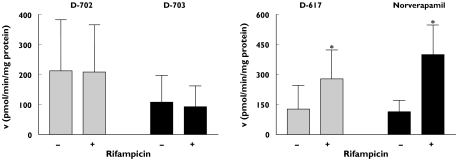
Formation of the verapamil metabolites D-702 and D-703 did not differ before and during rifampicin intake (D-702: 211.7 ± 379.6 vs. 209.0 ± 351.4, 95% CI: −35.4–40.9; D-703: 108.1 ± 198.8 vs. 92.9 ± 155.4 pmol min−1 mg−1 protein, 95% CI: −39.4–69.7) (Figure 5). However, formation of the verapamil metabolites D-617 and norverapamil increased significantly by 2.2- and 3.5-fold, respectively, during rifampicin intake (D-617: 125.9 ± 118.8 vs. 277.2 ± 145.5 pmol min−1 mg−1 protein, P < 0.05, 95% CI: 30.1–272.5; norverapamil: 113.0 ± 57.9 vs. 398.4 ± 148.2 pmol min−1 mg−1 protein, P < 0.05, 95% CI: 47.2–523.6). As expected, formation of the verapamil metabolites D-617 and norverapamil correlated with CYP3A4 expression (D-617: r = 0.70, P < 0.05, 95% CI: 0.19–0.92; norverapamil: r = 0.90, P < 0.001, 95% CI: 0.65–0.98).
Figure 5.
Formation of verapamil metabolites D-702, D-703, D-617 and norverapamil in shed enterocytes before (−) and during (+) rifampicin intake. *P < 0.05
Discussion
In shed enterocytes CYP3A4 mRNA, protein and catalytic activity were induced by rifampicin as described previously using duodenal biopsies [6, 7]. In addition to CYP3A4, CYP2C8, CYP2C9 and CYP2D6 are expressed in human small intestine [34–38]. To the best of our knowledge, these are the first data that rifampicin also induces expression of some (CYP2C8, CYP2C9), but not all (CYP2D6) of these intestinal proteins.
These data are in line with studies using human hepatocytes, which showed induction of CYP2C8, CYP2C9 and CYP3A4, but not of CYP2D6 by rifampicin [21–24]. Moreover, using human duodenal biopsies we have previously found that rifampicin induces the expression of intestinal mRNA for CYP2C8, CYP2C9 and CYP2C19 [39]. Using paired samples of small intestine and liver from the same patients, we showed that enterocyte CYP2C8 and CYP2C9 expression and catalytic activity is considerably lower than in hepatocytes (e.g. Clint D-703: 0.15 vs. 1.17 µl min−1 mg−1 protein; Clint4′-hydroxydiclofenac: 46 vs. 281 µl min−1 mg−1 protein [36]. However, due to the different anatomical situation with enterocytes being the first cell lining after oral administration, it is possible that intestinal cytochrome P450 enzymes other than CYP3A4 also contribute to overall drug elimination. Moreover, induction of intestinal CYP2C9 could contribute to previously observed drug interactions between rifampicin and several CYP2C9 substrates such as tolbutamide, phenytoin, losartan. S-warfarin, and antidiabetic drugs (e.g. glibenclamide) [14–19].
In studies with primary hepatocytes, the induction of CYP2C8 was considerably higher than that of CYP2C9 [22–24]. Our data from shed enterocytes are in line with these findings. Furthermore, the expected stronger induction of CYP3A4 mRNA by rifampicin compared with CYP2C9 using an immortal hepatocyte cell line (Fa2N-4) was recently reported [25]. No influence of rifampicin on the expression of CYP2D6 was observed in the present study. This finding is consistent with a previous in vivo work, in which rifampicin had no effect on the oral clearance of the CYP2D6 substrate sparteine [20].
The pregnane X receptor (PXR) is a key regulator of CYP3A4 induction by rifampicin (for review see [40]). Other genes reported to be regulated by PXR ligands are CYP2C8, CYP2C9, MDR1 and MRP2 [40]. These findings are consistent with the present work on CYP2C8, CYP2C9, and with other studies on P-glycoprotein [7, 8] and MRP2 [41] induction in human small intestine. The correlation between the induction of CYP2C9 and CYP3A4 in shed enterocytes is in accordance with a common regulatory factor being involved.
As previously reported [28], formation of norverapamil and D-617 correlated with enterocyte CYP3A4 expression. Values for the latter and CYP3A4 function (verapamil N-dealkylation and N-demethylation) in the present study are in very good agreement to those from previous studies. For example, the mean CYP3A4 expression in enterocytes obtained from surgical samples was 76 pmol mg−1 protein [42] compared with 34 pmol mg−1 protein in the present study. In one of our previous studies, the CYP3A4 expression in shed enterocytes was 36 pmol mg−1 protein [28]. As a result of an about 3.3-fold increase in enterocyte CYP3A4 content by rifampicin, the formation of norverapamil and D-617 in enterocyte homogenates was induced by treatment with this drug. These data and the 70% contribution of intestinal CYP3A to total intestinal cytochrome P450 content [43] further confirm the importance of intestinal CYP3A4 for presystemic drug metabolism and drug interactions.
D-702 and D-703 are formed from verapamil in the liver by CYP2C8 and CYP2C9 [32]. Interestingly, their formation was not induced by rifampicin in homogenates of shed enterocytes. This is consistent with previous observations that noninducible enzymes contribute to a major extent to the formation of these metabolites in the small intestine [28].
In conclusion, our data indicate that shed enterocytes can be used to study the regulation of intestinal enzyme expression (mRNA and protein) and function, and are therefore a useful tool for investigation of the role of gut wall metabolism and its possible contribution to drug interactions.
Acknowledgments
We are grateful to Mrs Anja Bengel and Mrs Sabine Rekersbrink for excellent technical assistance. This work was supported by the Robert Bosch Foundation (Stuttgart, Germany), a grant from the Bundesministerium für Bildung und Forschung (BMBF grant BEO 0311782) and the Deutsche Forschungsgemeinschaft (DFG Fr1298/2–3)
References
- 1.Suzuki H, Sugiyama Y. Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur J Pharm Sci. 2000;12(1):3–12. doi: 10.1016/s0928-0987(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 2.Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997;27(2–3):161–70. doi: 10.1016/s0169-409x(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Benet LZ. The gut as a barrier to drug absorption. combined role of cytochrome P450, 3A and P–glycoprotein. Clin Pharmacokinet. 2001;40(3):159–68. doi: 10.2165/00003088-200140030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60(1):14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 5.Kolars JC, Awni WM, Merion RM, Watkins PB. First-pass metabolism of cyclosporin by the gut. Lancet. 1991;338(8781):1488–90. doi: 10.1016/0140-6736(91)92302-i. [DOI] [PubMed] [Google Scholar]
- 6.Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampicin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90(5):1871–8. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, et al. The role of intestinal P–glycoprotein in the interaction of digoxin and rifampicin. J Clin Invest. 1999;104(2):147–53. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westphal K, Weinbrenner A, Zschiesche M, Franke G, Knoke M, Oertel R, et al. Induction of P-glycoprotein by rifampicin increases intestinal secretion of talinolol in human beings: a new type of drug/drug interaction. Clin Pharmacol Ther. 2000;68(4):345–55. doi: 10.1067/mcp.2000.109797. [DOI] [PubMed] [Google Scholar]
- 9.Langhoff E, Madsen S. Rapid metabolism of cyclosporin and prednisone in kidney transplant patient receiving tuberculostatic treatment. Lancet. 1983;2(8357):1031. doi: 10.1016/s0140-6736(83)91019-x. [DOI] [PubMed] [Google Scholar]
- 10.Backman JT, Kivisto KT, Olkkola KT, Neuvonen PJ. The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol. 1998;54(1):53–8. doi: 10.1007/s002280050420. [DOI] [PubMed] [Google Scholar]
- 11.Fromm MF, Busse D, Kroemer HK, Eichelbaum M. Differential induction of prehepatic and hepatic metabolism of verapamil by rifampicin. Hepatology. 1996;24(4):796–801. doi: 10.1002/hep.510240407. [DOI] [PubMed] [Google Scholar]
- 12.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42(9):819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 13.Finch CK, Chrisman CR, Baciewicz AM, Self TH. Rifampicin and rifabutin drug interactions: an update. Arch Intern Med. 2002;162(9):985–92. doi: 10.1001/archinte.162.9.985. [DOI] [PubMed] [Google Scholar]
- 14.Williamson KM, Patterson JH, McQueen RH, Adams KF, Jr, Pieper JA. Effects of erythromycin or rifampicin on losartan pharmacokinetics in healthy subjects. Clin Pharmacol Ther. 1998;63(3):316–23. doi: 10.1016/S0009-9236(98)90163-1. [DOI] [PubMed] [Google Scholar]
- 15.Zilly W, Breimer DD, Richter E. Induction of drug metabolism in man after rifampicin treatment measured by increased hexobarbital and tolbutamide clearance. Eur J Clin Pharmacol. 1975;9(2–3):219–27. doi: 10.1007/BF00614021. [DOI] [PubMed] [Google Scholar]
- 16.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivisto KT. Effects of rifampicin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther. 2001;69(6):400–6. doi: 10.1067/mcp.2001.115822. [DOI] [PubMed] [Google Scholar]
- 17.Kay L, Kampmann JP, Svendsen TL, Vergman B, Hansen JE, Skovsted L, et al. Influence of rifampicin and isoniazid on the kinetics of phenytoin. Br J Clin Pharmacol. 1985;20(4):323–6. doi: 10.1111/j.1365-2125.1985.tb05071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimark LD, Gibaldi M, Trager WF, O'Reilly RA, Goulart DA. The mechanism of the warfarin–rifampicin drug interaction in humans. Clin Pharmacol Ther. 1987;42(4):388–94. doi: 10.1038/clpt.1987.168. [DOI] [PubMed] [Google Scholar]
- 19.Feng HJ, Huang SL, Wang W, Zhou HH. The induction effect of rifampicin on activity of mephenytoin 4′-hydroxylase related to M1 mutation of CYP2C19 and gene dose. Br J Clin Pharmacol. 1998;45(1):27–9. doi: 10.1046/j.1365-2125.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichelbaum M, Mineshita S, Ohnhaus EE, Zekorn C. The influence of enzyme induction on polymorphic sparteine oxidation. Br J Clin Pharmacol. 1986;22(1):49–53. doi: 10.1111/j.1365-2125.1986.tb02879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, et al. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos. 2003;31(4):421–31. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- 22.Gerbal-Chaloin S, Pascussi JM, Pichard-Garcia L, Daujat M, Waechter F, Fabre JM, et al. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos. 2001;29(3):242–51. [PubMed] [Google Scholar]
- 23.Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampicin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299(3):849–57. [PubMed] [Google Scholar]
- 24.Raucy JL, Mueller L, Duan K, Allen SW, Strom S, Lasker JM. Expression and induction of CYP2C P450 enzymes in primary cultures of human hepatocytes. J Pharmacol Exp Ther. 2002;302(2):475–82. doi: 10.1124/jpet.102.033837. [DOI] [PubMed] [Google Scholar]
- 25.Mills JB, Rose KA, Sadagopan N, Sahi J, de Morais SM. Induction of drug metabolism enzymes and MDR1 using a novel human hepatocyte cell line. J Pharmacol Exp Ther. 2004;309(1):303–9. doi: 10.1124/jpet.103.061713. [DOI] [PubMed] [Google Scholar]
- 26.von Richter O, Greiner B, Fromm MF, Fraser R, Omari T, Barclay ML, et al. Determination of in vivo absorption, metabolism, and transport of drugs by the human intestinal wall and liver with a novel perfusion technique. Clin Pharmacol Ther. 2001;70(3):217–27. doi: 10.1067/mcp.2001.117937. [DOI] [PubMed] [Google Scholar]
- 27.Drescher S, Glaeser H, Murdter T, Hitzl M, Eichelbaum M, Fromm MF. P-glycoprotein-mediated intestinal and biliary digoxin transport in humans. Clin Pharmacol Ther. 2003;73(3):223–31. doi: 10.1067/mcp.2003.27. [DOI] [PubMed] [Google Scholar]
- 28.Glaeser H, Drescher S, van der Kuip H, Behrens C, Geick A, Burk O, et al. Shed human enterocytes as a tool for the study of expression and function of intestinal drug-metabolizing enzymes and transporters. Clin Pharmacol Ther. 2002;71(3):131–40. doi: 10.1067/mcp.2002.121370. [DOI] [PubMed] [Google Scholar]
- 29.Koch I, Weil R, Wolbold R, Brockmoller J, Hustert E, Burk O, et al. Interindividual variability and tissue-specificity in the expression of cytochrome P450, 3A mRNA. Drug Metab Dispos. 2002;30(10):1108–14. doi: 10.1124/dmd.30.10.1108. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Klempnauer KH, Bonifer C, Sippel AE. Identification and characterization of the protein encoded by the human c-myb proto-oncogene. Embo J. 1986;5(8):1903–11. doi: 10.1002/j.1460-2075.1986.tb04443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busse D, Cosme J, Beaune P, Kroemer HK, Eichelbaum M. Cytochromes of the P450, 2C subfamily are the major enzymes involved in the O–demethylation of verapamil in humans. Naunyn Schmiedebergs Arch Pharmacol. 1995;353(1):116–21. doi: 10.1007/BF00168924. [DOI] [PubMed] [Google Scholar]
- 33.von Richter O, Eichelbaum M, Schonberger F, Hofmann U. Rapid and highly sensitive method for the determination of verapamil, [2H7]verapamil and metabolites in biological fluids by liquid chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;738(1):137–47. doi: 10.1016/s0378-4347(99)00508-3. [DOI] [PubMed] [Google Scholar]
- 34.de Waziers I, Cugnenc PH, Yang CS, Leroux JP, Beaune PH. Cytochrome P 450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990;253(1):387–94. [PubMed] [Google Scholar]
- 35.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80(4):1029–36. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapple F, von Richter O, Fromm MF, Richter T, Thon KP, Wisser H, et al. Differential expression and function of CYP2C isoforms in human intestine and liver. Pharmacogenetics. 2003;13(9):565–75. doi: 10.1097/00008571-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Ding X, Kaminsky LS. Human extrahepatic cytochromes P450. function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149–73. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- 38.Krishna DR, Klotz U. Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet. 1994;26(2):144–60. doi: 10.2165/00003088-199426020-00007. [DOI] [PubMed] [Google Scholar]
- 39.Lapple F. Charakterisierung der Expression und Induzierbarkeit Von Arzneimittelmetabolisierenden Enzymen der CYP2C-Subfamilie in Humanen Geweben. Stuttgart: University of Stuttgart; 2000. [Google Scholar]
- 40.Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- 41.Fromm MF, Kauffmann HM, Fritz P, Burk O, Kroemer HK, Warzok RW, et al. The effect of rifampicin treatment on intestinal expression of human MRP transporters. Am J Pathol. 2000;157(5):1575–80. doi: 10.1016/S0002-9440(10)64794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Richter O, Burk O, Fromm MF, Thon KP, Eichelbaum M, Kivisto KT. Cytochrome P450 3A4 and P-glycoprotein expression in human small intestinal enterocytes and hepatocytes: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther. 2004;75(3):172–83. doi: 10.1016/j.clpt.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Wacher VJ, Silverman JA, Zhang Y, Benet LZ. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J Pharm Sci. 1998;87(11):1322–30. doi: 10.1021/js980082d. [DOI] [PubMed] [Google Scholar]