Abstract
Aims
The aim of this study was to compare lung deposition of budesonide administered from two dry powder inhalers, Giona® Easyhaler® 200 µg/dose and Pulmicort® Turbuhaler® 200 µg/dose by utilizing a pharmacokinetic method.
Methods
This was an open, randomized, crossover study in 33 healthy subjects. The study consisted of four treatment periods separated by at least 4 wash-out days. Equivalence in lung deposition was assessed after a single inhaled 1000 µg (5 × 200 µg) dose of budesonide from Giona® Easyhaler® and from Pulmicort® Turbuhaler®. Concomitant oral charcoal administration (40 g) was used to prevent gastrointestinal (GI) absorption of budesonide. The efficacy of the charcoal was studied after oral administration of a budesonide 2 mg capsule. The subjects were trained to inhale the study drugs with controlled flow rates, which resulted in an equal pressure drop (4 kPa) across both inhalers. Venous blood samples for the determination of budesonide concentrations in plasma were drawn before and at predetermined time points up to 8 h after drug administration. Budesonide concentrations in plasma were determined using liquid chromatography-tandem mass spectrometry. Several pharmacokinetic parameters were estimated, the area under the budesonide concentration in plasma vs time curve from dosing to infinity (AUC(0, ∞)) being the primary response variable. Equivalence in lung deposition was concluded if the 90% confidence interval (CI) for the Easyhaler® : Turbuhaler® ratio of AUC(0, ∞) fell within the limits of 0.8–1.25.
Results
The mean AUC(0,∞) value after Easyhaler® treatment was 3.48 (standard deviation (SD) 0.93) ng ml−1 h and after Turbuhaler® treatment 3.46 (1.13) ng ml−1 h. The Easyhaler® : Turbuhaler® AUC(0, ∞) ratio was 1.02 and the 90% CI was from 0.96 to 1.09. The mean Cmax values (SD) for budesonide in plasma after Easyhaler® and Turbuhaler® treatments were 1.22 (0.41) ng ml−1 and 1.29 (0.44) ng ml−1, respectively. There was no statistically significant difference (P = 0.39) between the median tmax for Easyhaler® (30 min) and Turbuhaler® treatment (23 min). Charcoal impaired the GI absorption of budesonide by 96%. The occurrence of adverse events was similar during both treatments.
Conclusions
The results show that the lung deposition of budesonide from Giona® Easyhaler® 200 µg/dose and Pulmicort® Turbuhaler® 200 µg/dose dry powder inhalers is equivalent. The charcoal block used to prevent GI absorption of swallowed budesonide was found to be effective.
Keywords: budesonide, dry powder inhaler, lung deposition, pharmacokinetics
Introduction
Inhaled budesonide is extremely effective for controlling inflammation in the asthmatic airway. Relatively low doses of inhaled budesonide (400–800 µg) suppress the disease process and maintain adequate control of inflammation. The anti-asthmatic activity of inhaled budesonide has been proposed to be due to its local intrapulmonary action [1].
Equivalence testing of inhaled corticosteroids is a troublesome issue [2]. By measuring clinical outcomes the pulmonary doses delivered by two inhalers are difficult to compare. The onset of action of corticosteroids is slow, and the dose–response curve relatively flat, with substantial variance in clinical effects [3]. Pharmacokinetic methods may be used to evaluate pulmonary bioavailability of inhaled drugs without the numerous confounding factors related to outpatient clinical trials.
Budesonide is not metabolized in the lungs but is rapidly and extensively absorbed after inhalation [4, 5]. Since the absorption of budesonide from the lungs is 100%, the systemic availability can be assumed to be proportional to the fraction reaching the lungs [6]. Only part of the inhaled drug reaches the lungs, because the major fraction is deposited in the oropharynx [5, 7]. If the mouth is not rinsed, this is swallowed and absorbed from the gastrointestinal (GI) tract. For budesonide, hepatic first pass metabolism is substantial (approximately 90%). Nevertheless, the nonmetabolized drug portion contributes to the total systemic availability of the drug. Therefore the absorption of the drug via the GI tract needs to be prevented when the pulmonary bioavailability of an inhaled drug is evaluated. This can be done by oral charcoal administration concomitant with inhalation [5, 8, 9]. If drug absorption from the GI tract is prevented, the appearance of drug in the systemic circulation can be attributed solely to the absorption of the drug from the lungs after inhalation.
In this study, equivalence of budesonide lung deposition for Giona® Easyhaler® 200 µg/dose, and Pulmicort® Turbuhaler® 200 µg/dose dry powder inhalers was assessed by comparing the pulmonary absorption of budesonide from both devices. Absorption of the swallowed fraction of the dose was prevented by giving charcoal.
Methods
Subjects
Thirty-three healthy Caucasian male (28) and female (5) subjects were included in the study. They were nonsmokers, had normal blood pressure and had not used corticosteroids or regular medication for at least 4 weeks prior to the study. Oral contraceptives were allowed. Normal health was determined by previous medical history and physical examination and laboratory assessments. The ability to generate an adequate inspiratory flow rate through both inhalers was required. The study was approved by the Ethics Committee of Orion Pharma and by the Joint Ethics Committee for Human Research at the University of Kuopio and the Kuopio University Hospital. The study was conducted according to the principles of the Declaration of Helsinki of the World Medical Assembly and in compliance with the applicable regulatory requirements. Oral and written information was given to the subjects and signed informed consents were obtained before the study. The subjects were free to withdraw from the study at any time without providing a reason.
Treatments
The study drugs were Giona® Easyhaler® 200 µg/dose (Orion Pharma, Finland), Pulmicort® Turbuhaler® 200 µg/dose (AstraZeneca, Sweden), and budesonide 2 mg capsule (Orion Pharma, Finland).
The treatments were (A) budesonide 1000 µg as a single dose inhaled from Giona® Easyhaler® coinciding with charcoal administration, (B) budesonide 1000 µg as a single dose inhaled from Pulmicort® Turbuhaler® coinciding with charcoal administration, (C) budesonide 2 mg capsule as a single oral dose and (D) budesonide 2 mg capsule as a single oral dose coinciding with charcoal administration. Charcoal was given as a charcoal-water suspension (50 g in 250 ml of tap water, Carbomix® granular, Leiras-Schering, Finland), in a dose of 10 g immediately before dosing and 30 g during the 1.5 h after dosing. Each subject was randomized to one of the following sequences: ABBD, ABBC, BAAD or BAAC. The replicate design for treatments A and B was used to decrease the variance of the treatment effect and to obtain estimates of intrasubject variabilities for both treatments. Oral budesonide was administered to each subject either with or without concomitant charcoal (treatment C or D).
Methodology
This was open, randomized, crossover study, which consisted of a prestudy examination, four treatment periods, and a poststudy examination. There was a wash-out period of at least 4 days between treatments.
Budesonide was administered under the supervision of the study personnel as a single dose after an overnight fast. The inhaled treatments (A and B) were administered by five consecutive inhalations, with an interval of 1 min. A nose-clip was used to prevent nose breathing. Before administration, the subjects were trained to inhale the drug according to the manufacturers' instructions except that they were taught to inhale at flow rates resulting in an equal pressure drop (4 kPa) across both inhalers. The target inspiratory flow rates were 45 l min−1 for Easyhaler® and 60 l min−1 for Turbuhaler®. Inspiratory parameters (peak inspiratory flow rate (PIFR), inspiratory volume, and inspiratory volume after 1 s) were measured by using a spirometer (Vitalograph Compact II, Vitalograph Ltd, UK) with a specially designed interface. To avoid contamination of blood samples the administration of inhaled treatments was performed away from the blood sampling station. The oral capsule was administered with 150 ml of tap water.
Charcoal (40 g) was given using a procedure modified from Thorsson et al. [5]. The mouth was thoroughly rinsed with 2 × 25 ml of charcoal-water suspension and with 25 ml of tap water. After rinsing, the charcoal suspension and the water were swallowed. The procedure was performed immediately before and after drug administration, and repeated after 45 min and 1.5 h.
An overnight fast of at least 10 h preceded all study treatments and caffeine-containing products were forbidden for 10 h before and during treatment periods. Subjects fasted and maintained an upright position for 4 h after dosing. A standard lunch was then served and caffeine free coffee or tea was given 6 h after dosing.
Clinical and laboratory safety assessments were included in the pre- and poststudy examinations. Adverse events were recorded during the study.
Blood sampling and drug analysis
Venous blood samples (7 ml) for analysis of budesonide in plasma were taken before and 15, 30, 45 min and 1, 2, 3, 4, 5, 6, and 8 h after drug administration. With inhaled treatments, time zero was defined as the time when the first inhalation started. The plasma was separated by centrifugation and stored at −20 °C until analyzed. Budesonide was extracted into ethyl acetate : hexane (50 : 50) after adding internal standard. Analysis was by liquid chromatography-tandem mass spectrometry using selected reaction monitoring. The method was validated with respect to linearity, accuracy, specificity and precision. The method was linear over the concentration range 0.05–2.0 ng ml−1. Recoveries for budesonide were between 83 and 99%. The between-run precisions determined during the study with quality control samples at concentrations of 0.1, 0.5 and 1.5 ng ml−1 were 9.77%, 10.3% and 8.45%, respectively (n = 52, 55 and 53). The within-run precisions determined before the study with spiked plasma samples at concentrations of 0.1, 0.5 and 1.5 ng ml−1 were for budesonide 9.58%, 3.60% and 6.11%, respectively (n = 11−12). The limit of determination was 0.05 ng ml−1.
Data analysis
The estimated sample size was based on previously published results [5, 10] and on the following power calculations. The coefficient of variation (CV) of the AUC after an inhaled dose was estimated to be 35%. The equivalence margin for the test : reference ratio of AUC was defined as 0.8 to 1.25 (CPMP Guideline CPMP/EWP/QWP/1401/98 [11]). The significance level in the two-sided test was set at 5% and the sample size was computed to achieve a power of 90%. This yielded a sample size of approximately 26 subjects. To allow for a discontinuation rate of 20%, 33 subjects were studied.
The pharmacokinetic parameters for budesonide were calculated using WINNONLIN 3.0 computer programme (Pharsight Co., Cary, NC, USA). They were the area under the budesonide concentration in plasma vs time curve from dosing to infinity (AUC(0, ∞)) and to 8 h (AUC(0, 8 h)), peak concentration (Cmax), time to reach peak concentration (tmax), and mean residence time (MRT). The primary variable was AUC(0, ∞).
The statistical method used for the equivalence analysis of lung deposition was the 90% confidence interval (CI) for the Easyhaler® : Turbuhaler® ratio of AUC(0, ∞). This was estimated by using an appropriate linear mixed model after conversion to the natural logarithm of the corresponding variable. For AUC(0, 8 h), testing for equivalence was also performed. No equivalence criterion was used forCmax although a similar modelling strategy with the corresponding 90% CIs for Easyhaler® : Turbuhaler® ratio was applied. tmax was compared by using the Wilcoxon signed rank test. A two-sided P value of less than 5% was considered statistically significant in applicable analyses.
Results
Thirty out of the 33 subjects completed the study. The mean age of the subjects was 23.1 (2.0) years, weight 73.5 (9.6) kg and height 178.7 (8.6) cm. One subject withdrew informed consent before the study, and one discontinued before treatment due to a respiratory tract disorder. Another subject discontinued the study after the third treatment period due to an adverse event (stomatitis). Sixteen subjects used other drugs during the study. The most common one was ibuprofen (for the treatment of headache). Four subjects used contraceptive pills. No subjects were excluded because of inappropriate concomitant medication.
Mean inspiratory parameters during inhalation treatment are shown in Table 1. Deviations from the target PIFRs of 45 l min−1 and 60 l min−1 for Easyhaler® and Turbuhaler®, respectively, were minor.
Table 1.
The mean (SD) inspiratory parameters during administration of inhaled study treatments
| Parameter | Giona® Easyhaler® | Pulmicort® Turbuhaler® |
|---|---|---|
| Peak inspiratory flow rate (l min−1) | 46 (3) | 61 (4) |
| Inspiratory volume (l) | 2.8 (0.6) | 3.1 (0.6) |
| Inspiratory volume in 1 s (l) | 0.75 (0.06) | 1.00 (0.06) |
The target peak inspiratory flow during inhalations using Easyhaler® and Turbuhaler® were 45 and 60 l min−1, respectively.
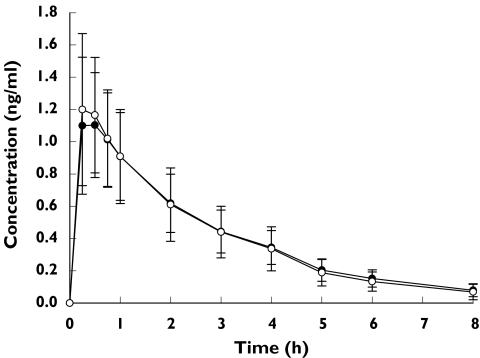
The mean budesonide concentration in plasma vs time curves after Easyhaler® and Turbuhaler® administration with charcoal are illustrated in Figure 1.
Figure 1.
Budesonide concentrations in plasma (ng ml−1) after a single inhaled dose of 1000 µg budesonide given together with charcoal (mean, SD). Giona® Easyhaler® (n = 43) (•), Pulmicort® Tarbuhaler® (n = 44) (○)
The mean AUC(0, ∞) value after Easyhaler® treatment was 3.48 ng ml−1 h, and 3.46 ng ml−1 h after Turbuhaler® treatment, both in combination with charcoal (Table 2). The Easyhaler® : Turbuhaler® ratio for the mean AUC(0, ∞) was 1.02, and the corresponding 90% CI was well within the equivalence range of 0.8–1.25. The 90% CI for Easyhaler® : Turbuhaler® ratio for AUC(0, 8 h) was slightly narrower (0.95–1.08). The Easyhaler® : Turbuhaler® ratio of Cmax values was 0.94 and the 90% CI for the Cmax ratio was 0.86–1.03. In addition, there was no statistically significant difference in the median tmax between Easyhaler® and Turbuhaler® treatment (P = 0.39).
Table 2.
The pharmacokinetic parameters after inhalation of 1000 µg of budesonide with concomitant charcoal administration
| Parameter | Giona® Easyhaler® (n = 43) | Pulmicort® Turbuhaler® (n = 44) | 90% CI for the Easyhaler : Turbuhaler ratio |
|---|---|---|---|
| AUC(0,∞) (ng ml−1 h) | 3.48 (0.93) | 3.46 (1.13) | (0.96, 1.09) |
| AUC(0,8 h) (ng ml−1 h) | 3.22 (0.86) | 3.22 (1.06) | (0.95, 1.08) |
| Cmax (ng ml−1) | 1.22 (0.41) | 1.29 (0.44) | (0.86, 1.03) |
| tmax (min) | 30 (11) | 23 (10) | – |
| MRT(0,8 h) (h) | 2.37 (0.27) | 2.25 (0.26) | – |
| MRT(0,∞) (h) | 3.05 (0.48) | 2.85 (0.38) | – |
Values are means (SD) except tmax values, which are medians (SD). AUC = area under the time-concentration curve; Cmax = peak concentration; tmax = time to reach peak concentration; MRT = mean residence time; CI = Confidence interval.
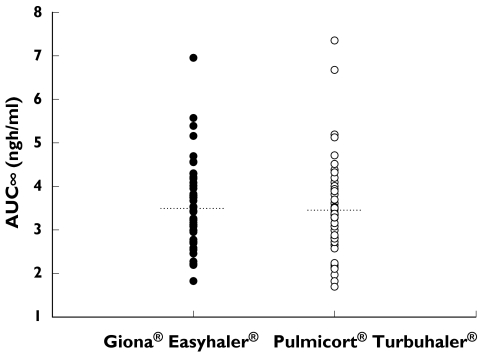
The individual AUC(0, ∞) values are shown in Figure 2. The between subject variances were 0.050 for Easyhaler® and 0.076 for Turbuhaler®, and the within subject variances were 0.024 and 0.015, respectively. The model-based estimates for intra-individual CVs of AUC(0, ∞) were 15.6% for Easyhaler® and 12.4% for Turbuhaler®, giving a ratio of 1.26.
Figure 2.
Individual AUC(0,∞) values (ng ml−1 h) after administration of budesonide in a Giona® Easyhaler® (n = 43) and a Pulmicort® Turbuhaler® (n = 44) treatments. The dotted lines indicate the mean values
The mean AUC(0, 8 h) (SD) after budesonide 2 mg capsule without charcoal was 1.23 (0.83) ng ml−1 h (n = 16). When the capsule was administered with charcoal, the AUC(0, 8 h) could be calculated for only six out of 14 subjects because the budesonide concentrations for eight subjects were below the limit of determination (0.05 ng ml−1) at every time point. By assuming that concentrations below the limit of determination were zero, the mean AUC(0, 8 h) value after administration with charcoal was 0.05 ng ml−1 h, which is 4% of the value after capsule administration without charcoal. The mean Cmax value (SD) after oral administration of 2 mg budesonide capsule was 0.4 (0.2) ng ml−1 which decreased by 90% (0.04 (0.1) ng ml−1) during concomitant charcoal administration. The median tmax of orally administered budesonide was 1 h.
Twenty-four subjects out of 33 reported adverse events (AEs) at least once during the study and the total number of events was 51. The AEs occurred mostly during the inhalation treatment periods (69%). The occurrence of AEs was similar during Easyhaler (n = 18) and Turbuhaler (n = 17) treatments. The severity of AEs was graded mild or moderate in all except one case of stomatitis, which was graded severe. The subject with stomatitis discontinued the study after three treatment periods, and causality with budesonide treatment was thought to be probable. The most frequently reported AEs were headache (19 cases) and upper respiratory tract infection (11 cases). There were no clinically significant differences between the pre- and poststudy laboratory values. Two subjects suffered from anaemia after the study, but this was treated successfully by oral ferrous sulphate supplement.
Discussion
The pulmonary deposition of budesonide for two dry powder inhalers was compared in this open, randomized, crossover pharmacokinetic study. The results show that Giona® Easyhaler® 200 µg/dose and Pulmicort® Turbuhaler® 200 µg/dose dry powder inhalers deliver an equivalent amount of drug to the lungs. The plasma budesonide concentration curves for Easyhaler® and Turbuhaler® treatments were similar and overlapping. The pharmacokinetic parameters also indicated equivalency as well. The 90% CI for Easyhaler® : Turbuhaler® AUC(0,∞) ratio was narrow and well within the prespecified acceptance range (from 0.8 to 1.25, CPMP Guideline CPMP/EWP/QWP/1401/98 [11]). In addition, the values for AUC)(0,8 h), Cmax, and tmax were similar between the two treatments. In the case of Turbuhaler® treatment, similar pharmacokinetic parameters have been published earlier by Thorsson et al. [5].
The pharmacokinetic parameters for the budesonide 2 mg capsule given with and without charcoal showed that the amount of charcoal and the dosing schedule used were sufficient to prevent GI absorption of budesonide by about 96%. Since the bioavailability of orally administrated budesonide is about 10% [4], only 0.4% of the swallowed budesonide was bioavailable when charcoal was given. In a previous study by Thorsson & Edsbäcker [10], charcoal was shown not to affect the distribution or elimination of budesonide. Therefore, it can be concluded that almost all the drug present in plasma originated from pulmonary absorption.
In this study the inhalation technique for both devices was taught and well practised beforehand. The aim was to minimize variability in subject-related factors, of which inhalation technique was considered to be the most critical. The manufacturers' instructions were used except that the subjects inhaled at controlled flow rates. The target inhalation flow rate was set to equal a pressure drop of 4 kPa across both devices during inhalation. This approach was adopted from in vitro testing of dry powder inhalers suggested by Clark & Holligworth [12] and described in the European Pharmacopoeia [13]. An inhalation flow rate of 60 l min−1 through the Turbuhaler® is consistent with flow rates used in previous studies and is the optimal inhalation rate when using this device [14]. Asthmatic patients can also typically generate flow rates of 45 l min−1 for Easyhaler® and 60 l min−1 for Turbuhaler® with a comfortable inspiratory effort [15, 16]. The inspiratory parameters during drug administration confirm that the subjects adopted the techniques well. The mean PIFRs differed by only 1 l min−1 from the target and the variations were small (SD 3 l min−1 for Easyhaler® and 4 l min−1 for Turbuhaler®). Approximately 30% of the inspiratory volume was inhaled during the first second of inhalation.
In addition to the inhalation technique the other study conditions were standardized as much as possible. The between subject variance for AUC(0,8 h) was somewhat smaller for Easyhaler® than for Turbuhaler® (0.047 vs 0.080). This result most probably indicates that dry powder inhalers as such show a small variability in lung dose when administered in a standardized manner. Taking into consideration the numerous sources of variation of inhaled products, the intra-individual CVs of AUC(0,∞) were small. The CVs for lung deposition for budesonide administered by a pressurized metered dose inhaler and by Turbuhaler® have been previously shown to be 77% and 33%, respectively [5].
The observation of equivalent pulmonary delivery of budesonide for the products studied correlates well with the results of an in vivo scintigraphic study by Hirst et al. [17], which showed similar lung depositions of budesonide for both Easyhaler® (18.5%) and Turbuhaler® (21.8%) in asthmatic patients. In addition, the peripheral zone : central zone deposition ratio was similar (0.8). Systemic effects of budesonide delivered from Easyhaler® and Turbuhaler® have been assessed in a separate study by Hämäläinen et al. [18]. Treatment for 1 week at doses of 800 and 1600 µg day−1 had comparable systemic effects irrespective of the type of inhaler. In addition, previous clinical studies have shown that the inhalers are equally effective and safe in the treatment of asthma in adults and in children [19–21].
The result of the present study suggests that pharmacokinetic studies, possibly supplemented with pharmacodynamic endpoints (e.g. effects on HPA axis function for inhaled corticosteroids), offer an alternative approach to clinical studies to show equivalent efficacy and safety of an inhaled generic product compared with an innovator. The use of the method can be extended to product development as Borgström & Lipniunas [22] have reported. In the case of the Giona® Easyhaler®, the results of this pharmacokinetic study correlate well with the results of clinical studies. However, the validation of the pharmacokinetic method as a surrogate for clinical testing requires additional studies.
In conclusion, Giona® Easyhaler® and Pulmicort® Turbuhaler® dry powder inhalers deliver equivalent amounts of budesonide to the lungs. The charcoal block used to prevent the GI absorption of swallowed budesonide was shown to be effective.
Acknowledgments
We are grateful to Ms Marianna Elo, Ms Lea Porthan, Mr Tommi Heikura, Ms Maija Hälikkä, Ms Terttu Kupila, Ms Suvi Forsberg and Mr Tommi Koskela for technical assistance. Orion Pharma, Finland funded the study.
References
- 1.Toogood JH, Frankish CW, Jennings BH, Baskerville JC, Borga O, Lefcoe NM, et al. A study of the mechanism of the antiasthmatic action of inhaled budesonide. J Allergy Clin Immunol. 1990;85:872–80. doi: 10.1016/0091-6749(90)90071-b. [DOI] [PubMed] [Google Scholar]
- 2.Snell NJC. Equivalence testing – the special case of inhaled medications. Int J Pharm Med. 1998;12:245–6. [Google Scholar]
- 3.Lipworth BJ. Airway and systemic effects of inhaled corticosteroids in asthma: dose–response relationship. Pulmonary Pharmacol. 1996;9:19–27. doi: 10.1006/pulp.1996.0002. [DOI] [PubMed] [Google Scholar]
- 4.Ryrfeldt Å, Andersson P, Edsbäcker S, Tönnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis. 1982;63(Suppl. 122):86–95. [PubMed] [Google Scholar]
- 5.Thorsson L, Edsbäcker S, Conradson T-B. Lung deposition of budesonide from Turbuhaler® is twice that from a pressurised metered-dose inhaler P-MDI. Eur Respir J. 1994;7:1839–44. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 6.Thorsson L. Influence of inhaler systems on systemic availability, with focus on inhaled corticosteroids. J Aerosol Med. 1995;8(Suppl. 3):S29–37. doi: 10.1089/jam.1995.8.suppl_3.s-29. [DOI] [PubMed] [Google Scholar]
- 7.Pauwels R. Therapeutic index for inhaled steroids. Eur Respir Rev. 1997;7:366–8. [Google Scholar]
- 8.Newman SP. A comparison of lung deposition patterns between different asthma inhalers. J Aerosol Med. 1995;8(Suppl. 3):S21–7. doi: 10.1089/jam.1995.8.suppl_3.s-21. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen S, Steffenson G, Ohlsson SV. The influence of orally deposited budesonide on the systemic availability of budesonide after inhalation from a Turbuhaler®. Br J Clin Pharmacol. 1993;36:211–14. doi: 10.1111/j.1365-2125.1993.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorsson L, Edsbäcker S. Lung deposition of budesonide from a pressurised metered dose inhaler attached to a spacer. Eur Respir J. 1998;12:1340–5. doi: 10.1183/09031936.98.12061340. [DOI] [PubMed] [Google Scholar]
- 11.CPMP Guideline CPMP/EWP/QWP// Investigation of bioavailability and bioequivalence. 2001.
- 12.Clark AR, Hollingworth AM. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers: implications for in vitro testing. J Aerosol Med. 1993;6:99–110. doi: 10.1089/jam.1993.6.99. [DOI] [PubMed] [Google Scholar]
- 13.3. Suppl. Strasbourg: Council of Europe; 1999. European Pharmacopoeia. [Google Scholar]
- 14.Borgström L. On the use of dry powder inhalers in situations perceived as constrained. J Aerosol Med. 2001;14:281–7. doi: 10.1089/089426801316970231. [DOI] [PubMed] [Google Scholar]
- 15.Brown PH, Geening AP, Crompton GK. Peak inspiratory flow rates in acute asthma. Are they adequate for efficient use of a Turbuhaler®? Thorax. 1992;47:239. [Google Scholar]
- 16.Malmström K, Sorva R, Silvasti M. Application and efficacy of the multi-dose powder inhaler, Easyhaler®, in children with asthma. Pediatr Allergy Immunol. 1999;10:66–70. doi: 10.1034/j.1399-3038.1999.101002.x. [DOI] [PubMed] [Google Scholar]
- 17.Hirst PH, Bacon RE, Pitcairn GR, Silvasti M, Newman SP. A comparison of the lung deposition of budesonide from Easyhaler®, Turbuhaler® and pMDI plus spacer in asthmatic patients. Respir Med. 2001;95:720–7. doi: 10.1053/rmed.2001.1107. [DOI] [PubMed] [Google Scholar]
- 18.Hämäläinen KM, Granander M, Toivanen P, Malinen A. Assessment of the systemic effects of budesonide inhaled from Easyhaler® and from Turbuhaler® in healthy male volunteers. Respir Med. 2001;95:863–9. doi: 10.1053/rmed.2001.1157. [DOI] [PubMed] [Google Scholar]
- 19.Schweisfurth H, Malinen A, Koskela T, Toivanen P, Ranki-Pesonen M German Study Group. Comparison of two budesonide powder inhalers, Easyhaler® and Turbuhaler®, in steroid-naive asthmatic patients. Respir Med. 2002;96:599–606. doi: 10.1053/rmed.2002.1311. on behalf of the. [DOI] [PubMed] [Google Scholar]
- 20.Tukiainen H, Rytilä P, Hämäläinen KM, Silvasti M, Keski-Karhu J. Safety, tolerability and acceptability of two dry powder inhalers in the administration of budesonide in steroid-treated asthmatic patients. Respir Med. 2002;96:221–9. doi: 10.1053/rmed.2001.1261. on behalf of the Finnish Study Group. [DOI] [PubMed] [Google Scholar]
- 21.Vanto T, Hämäläinen KM, Vahteristo M, Wille S, Hyldebrand N. Comparison of two budesonide dry powder inhalers in the treatment of asthma in children. J Aerosol Med. 2004;17:15–24. doi: 10.1089/089426804322994424. on behalf of the Study Group. [DOI] [PubMed] [Google Scholar]
- 22.Borgström L, Lipniunas P. Budesonide/formoterol in Turbuhaler© is not affected by storage in hot and humid conditions. A clinical pharmacokinetic comparison with fluticasone/salmeterol in Diskus™ [abstract] Eur Respir J. 2003;22(Suppl. 45):237s. [Google Scholar]