Abstract
Aims
It is estimated that two-thirds of cancer patients will at some point during their illness experience breakthrough pain. In this study, the pharmacokinetics of a novel sublingual dosage form of fentanyl developed for breakthrough pain was evaluated.
Methods
Eleven Caucasian patients (seven male and 4 female, aged 34–75 years, median 60 years) with metastatic malignant disease were recruited initially, but three patients withdrew. Prior to the study all patients were on continuous nonfentanyl opiate medication. The study was a double-blind, cross-over trial, consisting of three 1-day treatment periods. A new rapidly dissolving preparation of fentanyl, was administered sublingually in single doses of 100, 200 and 400 µg, respectively, on three separate occasions. Plasma fentanyl concentrations were determined using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). Pharmacokinetic parameters were calculated by noncompartment analysis. Tolerability and the occurrence of adverse events were monitored throughout the study by patient questionnaire.
Results
The data from nine subjects who completed at least two periods were used in the analysis of variance. There were no significant differences between doses (100, 200 and 400 µg) for dose adjusted AUC (F = 0.42, P = 0.6660), dose adjusted Cmax (F = 0.08, P = 0.9206) and Tmax (F = 0.94, P = 0.4107). Thus, these parameters showed dose proportionality. The differences (400–100µg) in dose adjusted AUC from the three-period crossover analysis was −0.016 min·ng/ml (t = 0.71, P = 0.8718). Interindividual variability in systemic exposure to fentanyl was fairly small (25–40%), which may be related to a good in vivo biopharmaceutical performance of the sublingual tablet, and a relatively small fraction of the dose being swallowed. The first detectable plasma concentration of fentanyl was observed between 8 and 11 min after administration. tmax increased from 39.7 ± 17.4 to 48.7 ± 26.3 and 56.7 ± 24.6 min for the 100, 200 and 400 µg doses, respectively. Adverse events were few and did not increase with increasing dose.
Conclusion
With this rapidly dissolving fentanyl formulation, the first detectable plasma concentration of fentanyl was observed at 8–11 min after administration. The pharmacokinetics of the drug showed dose proportionately. This formulation of fentanyl seemed to be well tolerated by the patients.
Keywords: fentanyl, incident pain, pharmacokinetics, sublingual administration
Introduction
It is estimated that more than half of cancer patients still suffer from poorly controlled or breakthrough pain [1]. This is associated with increased morbidity and its treatment has been identified as a primary goal in the improvement of medical care for cancer patients [2]. The development slow-release opioid formulations have been a significant step forward in the treatment of cancer pain. However, the therapeutic effect of these drugs shows high interindividual variability, and it is important to apply and develop different strategies to treat both persistent pain and periods of breakthrough pain [1–5]. Since fentanyl is an opioid drug with rapid onset of action, clinical benefit should be gained by administering it in a rapidly dissolved formulation, such as a sublingual tablet. In addition, fentanyl is a small, lipophilic and potent µ-receptor agonist that is given in low doses (100–1000 µg), and lacks the bitter taste associated with some other opioids [9–11]. In general, the rate and extent of in vivo oral mucosal and intestinal absorption is governed by the physico-chemical properties of the drug as well as physiological factors such as gastric states [6–8]. Major advantages of administering fentanyl by the sublingual route are its rapid onset of action and the avoidance of its extensive and highly variable CYP3A4-mediated gut and/or liver metabolism [7, 12, 13]. The aim of the present patient study was to evaluate the pharmacokinetics and tolerability of a novel rapidly absorbed sublingual fentanyl dosage form following the administration of single doses 100, 200 and 400 µg (fentanyl base) to cancer patients.
Materials and methods
Study design
The study was conducted as a double-blind, randomized, cross-over trial consisting of three treatment periods sublingually administered fentanyl at doses of 100, 200 and 400 µg separated by 3 days.
Blood samples (7 ml) were collected from the basilica vein at 0, 1, 3, 5, 10, 15, 20, 30, 60, 90, 120, 180, 240, 360, 480 and 600 min after dosing in heparinized Vacutainer® tubes, BD Vacutainer Systems, New Jersey, USA. The samples were kept on ice and then centrifuged for 10 min at 1500 g. Plasma was stored at −20 °C until analysis.
Patients, side-effects and tolerability.
Eleven Caucasian patients (seven male and four female, aged 34–75 years, median 60 years) were recruited from the Department of Oncology, Sahlgrenska Academy, Göteborg, Sweden. The study was approved by the Ethics Committee of the Medical Faculty and the Swedish Medical Products Agency, Uppsala, Sweden. Eight patients completed the study and withdrawal was not associated with the administration of fentanyl. All eight patients (weight 53–92 kg, mean 72, height 162–179, mean 172) suffered from a metastatic malignant disease and were being treated with an oral nonfentanyl opioid. One patient had an obstructive pulmonary disease. No patient had signs of increased intracranial pressure. Basal laboratory findings outside the range defined in the protocol were assessed separately and judged according its clinical relevance by the responsible physician. None of the concomitant drugs used were considered to have a clinically important interaction with CYP3A4 [12]. Adverse events were continuously monitored throughout the study period. Full physical examination, routine haematology and clinical chemistry and ECG was performed before the beginning of the study and 2–5 days after administration of the last dose. Side-effects and/or opioid-related symptoms were recorded on a questionnaire on the study day and each event was scored from 1 (no symptom) to 4 (severe). Oxygen saturation (using a finger sensor) was recorded during the study.
Dosage form
The new sublingual tablet is based on interactive mixtures of components to optimize the exposure of fentanyl to the fluids and of the oral cavity mucosa in combination with a mucosal bioadhesion of the dosage form [14].
Fentanyl analysis
The concentration of fentanyl in human plasma was determined by LC-MS/MS at Quintiles, Uppsala, Sweden. Fentanyl and an internal standard (fentanyl-d5) were extracted into heptan containing 3% 2-butanol (v/v) at pH >12. The organic phase was evaporated to dryness and the residue was dissolved in 5 mm formic acid and injected onto a reversed-phase LC column. The compounds were eluted with a mobile phase of acetonitrile:water (18 : 82) containing 5 mm formic acid. This method was validated over the concentration range 0.02–10 ng ml−1 with a lower limit of quantification (LLOQ) for a 1.0 ml sample of 0.02 ng ml−1. The intra-assay precision at concentrations of 0.05, 1.00 and 7.25 ng ml−1 was 5.9%, 1.0% and 1.6%, respectively. The interassay precision at concentrations of 0.05, 1.00 and 7.25 ng ml−1 was 5.0%, 1.2% and 1.5%, respectively. Inaccuracy was <2.8% measured as the percentage difference from nominal value. The recovery was 81.4–95.6%. At each concentration the number of replicates was six.
Data analysis
Pharmacokinetic parameters were calculated using noncompartmental analysis. The first detectable and peak plasma concentrations (Cfirst and Cmax, respectively) and the times when they occurred (tfirstand tmax, respectively) were derived directly from the plasma-concentration data. The area under the plasma concentration-time curve (AUCt) to the last measured concentration (Ct) was calculated by using the linear/logarithmic trapezoidal rule. The area from the Ct to infinite time was obtained by extrapolation, i.e. by dividing the last predicted concentration by the terminal rate constant (Ke) obtained by log-linear regression analysis of the last three to five concentration-time points. The total AUC0–∞was calculated from the equation AUC0–∞= AUCt+ Ct/Ke. The terminal half-life (t½12) was estimated using the terminal rate constant.
The data were analysed in anova using the SAS program proc GLM, with subject, treatment, period and treatment sequence as factors. The pharmacokinetic parameters were compared between each dose after log transformation (SAS System). Variability in the data was expressed as standard deviation (SD) and standard error of the mean (SE mean). The study performed as a two-period crossover study (100 vs. 200 µg) with an extra third period (400 µg) was analysed both as a two-period and a three-period design.
Results
Eleven subjects were recruited. Nine subjects completed two periods and eight subjects completed all three periods according to the protocol. The data from all nine subjects who completed at least two periods were used in the analysis of variance. The results from the two- and three-period crossover analyses were in agreement. The fraction of the dose absorbed and the systemic exposure to fentanyl were linear over the dosage range studied, as reflected by the AUC0–∞, which was 74.3 ± 31.0, 159.1 ± 39.2 and 290.8 ± 92.5 ng ml−1·min for the 100, 200 and 400 µg dose, respectively (Table 1). There were no significant differences between doses (100, 200 and 400 µg) for dose adjusted AUC (74.3 ± 31.0, 79.5 ± 19.6 and 72.7 ± 30.8 min ng−1 ml−1 (F = 0.42,P = 0.6660)), dose adjustedCmax (0.24 ± 0.14, 0.24 ± 0.08 and 0.32 ± 0.15 ng ml−1 (F = 0.08, P = 0.9206)) and Tmax (39.8 ± 17.4, 48.8 ± 26.3 and 53.9 ± 28.8 min (F = 0.94, P = 0.4107)). Estimated differences between doses in dose adjusted AUC were (200–100 µg) = 0.084, (t = 0.88, P = 0.4071, two-period crossover analysis) (200–100 µg) = 0.065 (t = 0.71, P = 0.4878, three-period crossover analysis) and (400–100µg) = −0.016 (t = 0.71, P = 0.8718 three-period crossover analysis).
Table 1.
Non-compartmental pharmacokinetic parameters (mean ± SD) of fentanyl following sublingual administration to eight cancer patients
| 100 µg | 200 µg | 400 µg | |
|---|---|---|---|
| AUC0–∞(ng ml−1 min−1) | 74.3 ± 31.0 | 159.1 ± 39.2 | 290.8 ± 92.5 |
| Cfirst (ng ml−1) | 0.05 ± 0.03 | 0.08 ± 0.08 | 0.17 ± 0.14 |
| tfirst (min) | 10.7 ± 3.2 | 8.0 ± 2.7 | 9.0 ± 4.1 |
| Cmax (ng ml−1) | 0.24 ± 0.14 | 0.41 ± 0.16 | 0.91 ± 0.3 |
| tmax (min) | 39.7 ± 17.4 | 48.7 ± 26.3 | 56.7 ± 24.6 |
| t½12 (h) | 6.1 ± 2.0 | 6.3 ± 1.6 | 5.4 ± 1.7 |
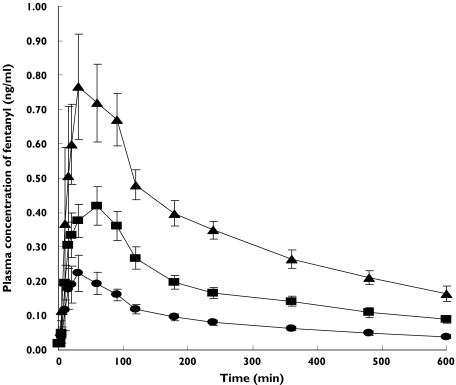
Figure 1.
Mean (± SEM) plasma concentration-time profiles fot fentanyl in eight cancer patients following single sublingual doses of 100 µg (•), 200 µg (▪), or 400 µg (▴), fentanyl on three separate study days
Dose proportionality also occurred with Cmax (Table 1). Fentanyl was rapidly absorbed and its tmax was somewhat slower at the higher doses (Table 1). There was a nonsignificant increase in tmax from 39.7 ± 17.4 to 48.7 ± 26.3–56.7 ± 24.6 min for the 100, 200 and 400 µg doses, respectively (P = 0.19–0.57; t = 0.58–1.36) (Table 1). Cfirst was reasonably dose proportional and tfirst occurred almost at the same time for each dose (P = 0.69–0.92; t = −0.1–0.4) (Table 1). The elimination half-life of fentanyl was 6.1, 6.3 and 5.4 h for the 100, 200 and 400 µg doses, respectively (Table 1).
Sublingual single-dose administration of fentanyl in the current immediate-release formulation was generally well tolerated. Throughout the study period, adverse effects such as pain, diarrhoea, dizziness, vomiting and nausea were observed in seven out of the 11 cancer pain patients, but no serious adverse effect was recorded and none seemed to be related to the administration of fentanyl. Additional symptoms recorded in the patient symptom questionnaires were occasional and were generally not related to the fentanyl. Oxygen treatment was not needed.
Discussion
In the present study we showed that both the plasma AUC and the Cmax of fentanyl given sublingually increased linearly with dosage. The plasma concentrations for the current sublingual formulation were within the established therapeutic range for fentanyl [15]. Our data were in agreement with an earlier pharmacokinetic study where the study drug was given as its oral transmucosal citrate (OTFC; Actiq® Cephalon Inc., Pennsylvania, USA) in a range of single doses (200–1600 µg) to 12 healthy male subjects [16]. The elimination half-life of fentanyl in the present study was also in agreement with values from other studies, in which fentanyl was administered by the pulmonary and oral transmucosal routes [16]. Inter-individual variability in the pharmacokinetic parameters did not increase at higher doses, and there were no secondary plasma concentration peaks that might suggest that only some of the dose was swallowed.
However, interindividual variability (expressed as a coefficient of variation) in the pharmacokinetic parameters (AUC, Cmax and t½12) was 25–40% in the current study, but 60–95% in the study by Streisand et al. in 1998 [16]. This difference suggests a more reliable absorption of fentanyl from the newly developed sublingual tablet, which might be the consequence of less fentanyl being swallowed and a larger portion of the absorption occurring from the mucosal site due to the bioadhesive component.
The time to the first detectable plasma concentration was about the same (8–11 min), throughout the dose range investigated, which indicates that the initial absorption rate of fentanyl from the sublingual tablet is unaffected by the size of the dose. The rapid absorption of fentanyl is expected since its physico-chemical properties govern high passive transcellular membrane diffusion [8, 9, 17]. However, the tendency towards a prolonged tmax of fentanyl at higher sublingual doses may be due to differences in the properties of the highest dose formulation and/or retention of fentanyl in the mucosal tissue in the vicinity of the administration site. Both processes may be explained by the very high liposolubility of fentanyl. It has also been reported that fentanyl may be retained within tissue cell compartments [18, 19]. However, even if the later tmax suggests a somewhat slower absorption rate, bioavailability was unaffected by the dose in our study.
In conclusion, the fraction of the dose absorbed and the systemic exposure of fentanyl administered by the sublingual formulation was linear over the 100–400 µg dose range. Systemic exposure showed fairly small interindividual variability, and the first detactable plasma concentration of fentanyl was at 8–11 min after administration. The reported adverse effects were considered to be unrelated to the administration of fentanyl.
Acknowledgments
This study was supported by Orexo AB, Uppsala, Sweden and B and H Lennernäs received compensation for advicer-work.
References
- 1.McMillan C. Breakthrough pain: assessment and management in cancer patients. Br J Nurs. 2001;10(13):860–6. doi: 10.12968/bjon.2001.10.13.860. [DOI] [PubMed] [Google Scholar]
- 2.Strang P. Cancer pain – a provoker of emotional, social and existential distress. Acta Oncol. 1998;37:641–4. doi: 10.1080/028418698429973. [DOI] [PubMed] [Google Scholar]
- 3.Cherny N. New strategies in opioid therapy for cancer pain. J Oncol Manag. 2000;9:8–15. [PubMed] [Google Scholar]
- 4.McQuay HJ, Jadad AR. Incident pain. Cancer Surv. 1994;21:17–24. [PubMed] [Google Scholar]
- 5.Coluzzi PH, Schwartzberg L, Conroy JD, Charapata S, Gay M, Busch MA, Chavez J, Ashley J, Lebo D, McCracken M, Portenoy RK. Breakthrough cancer pain: a randomized trial comparing oral transmucosal fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR) Pain. 2001;91:123–30. doi: 10.1016/s0304-3959(00)00427-9. [DOI] [PubMed] [Google Scholar]
- 6.Boyle R, Behan PO, Sutton JA. A correlation between severity of migraine and delayed gastric emptying measured by an epigastric impedance method. Br J Clin Pharmacol. 1990;30:405–9. doi: 10.1111/j.1365-2125.1990.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Zhang J, Streisand JB. Oral mucosal delivery: clinical pharmacokinetics and therapeutic applications. Clin Pharmacokin. 2002;41:661–80. doi: 10.2165/00003088-200241090-00003. [DOI] [PubMed] [Google Scholar]
- 8.Winiwarter S, Bonham N, Hallberg A, Lennernäs H, Karlén A. Correlation of human jejunal permeability (in vivo) with experimentally and theoretically derived parameters. A multivariate data analysis approach. J Med Chem. 1999;41:4939–49. doi: 10.1021/jm9810102. [DOI] [PubMed] [Google Scholar]
- 9.Roy SD, Flynn GL. Solubility and related physicochemical properties of narcotic analgesics. Pharm Res. 1988;5:580–6. doi: 10.1023/a:1015994030251. [DOI] [PubMed] [Google Scholar]
- 10.Dollery C, Fentanyl . In: Therapeutic drugs Volume 1. Dollery C, editor. Edinburgh: Churchill Linvingstone; 1991. pp. F26–F29. [Google Scholar]
- 11.Gardner-Nix J. Oral transmucosal fentanyl and sufentanil for incident pain. J Pain Pain Manage. 2001;22:627–30. doi: 10.1016/s0885-3924(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 12.Labroo RB, Paine MF, Thummel KE, Kharasch ED. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy and drug interactions. Drug Metabolism Disposition. 1997;25:1072–80. [PubMed] [Google Scholar]
- 13.Ozdemir V, Kalowa W, Tang BK, Paterson AD, Walker SE, Endrenyi L, Kashuba AD. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10:373–88. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Sundell-Bredenberg S, Nyström C. The possibility of achieving an interactive mixture with high dose homogeneity containing an extremely low proportion of a micronised drug. Eur J Pharm Sci. 2001;12:285–95. doi: 10.1016/s0928-0987(00)00176-7. [DOI] [PubMed] [Google Scholar]
- 15.Dale O, Hjortkjaer R, Kharasch ED. Nasal administration of opioids for pain management in adults. Acta Anaesthesiol Scand. 2002;46:759–70. doi: 10.1034/j.1399-6576.2002.460702.x. [DOI] [PubMed] [Google Scholar]
- 16.Streisand JB, Busch MA, Egan TD, Smith BG, Gay M, Pace NL. Dose proportionality and pharmacokinetics of oral transmucosal fentanyl citrate. Anesthesiol. 1998;88:305–9. doi: 10.1097/00000542-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Roy SD, Flynn GL. Transdermal delivery of narcotic analgesics: comparative permeabilities of narcotic analgesics through human cadaver skin. Pharm Res. 1989;6:825–32. doi: 10.1023/a:1015944018555. [DOI] [PubMed] [Google Scholar]
- 18.Poyhia R, Seppala T. Liposolubility and protein binding of oxycodone in vitro. Pharmacol Toxicol. 1994;74:23–7. doi: 10.1111/j.1600-0773.1994.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 19.Gupta SK, Sathyan G, Phipps B, Klausner M, Southam M. Reproducible fentanyl doses delivered intermittently at different time intervals from an electrotransport system. J Pharm Sci. 1999;88(8):835–41. doi: 10.1021/js980258b. [DOI] [PubMed] [Google Scholar]
- 20.Andrews C, Prys Roberts C. Fentanyl – a review. Clin Anesth. 1983;1:97–122. [Google Scholar]
- 21.Mather LE, Woodhouse A, Ward ME, Farr SJ, Rubsamen RA, Eltherington LG. Pulmonary administration of aerosolised fentanyl: pharmacokinetic analysis of systemic delivery. Br J Clin Pharmacol. 1998;46:37–43. doi: 10.1046/j.1365-2125.1998.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]