Abstract
Background
Controversy remains about the interaction between mycophenolate mofetil (MMF) and the calcineurin inhibitors cyclosporin (CsA) and tacrolimus (TACR). The need to double the dose of MMF in case of CsA co-administration to achieve the same mycophenolic acid (MPA) levels as in TACR co-administration, has been attributed to either inhibition by CsA of the enterohepatic cycle, or an inhibition of glucuronidation to mycophenolate glucuronide (MPAG) by TACR. We explored these interactions clinically in 64 kidney transplant patients.
Methods
Plasma MPA/MPAG curves were determined during the first year post transplantation. Using nonlinear mixed effect modelling, MPA/MPAG data were fitted to a four-compartment model, in which a rate constant describing transfer from the fourth to the first compartment (k41), and therefore enterohepatic recycling, could be introduced.
Results
MPA and MPAG plasma concentrations were adequately described by a four-compartment model, which was significantly improved by introduction of k41 in case of TACR co-administration (minimum value of the objective function decreased by 181 points, P < 0.0001). Using this model, no statistically significant difference was observed in clearance of MPA between TACR and CsA co-administration (11.9 and 14.1 l h−1, respectively). Total clearance of MPAG was lower in case of CsA co-administration (1.45 and 0.92 l h−1, respectively), while there was no difference in renal clearance of MPAG (1.09 and 0.92 l h−1, respectively).
Conclusions
Our study supplies supportive clinical evidence that inhibition of the enterohepatic cycle in case of CsA co-administration explains some of the differences observed in PK of MMF when co-administered with either TACR or CsA. This finding may have clinical consequences for the immunosuppressive management of kidney transplant patients.
Keywords: pharmacokinetics, mycophenolic acid, metabolism, modelling, enterohepatic cycle, kidney transplantation
Introduction
Kidney transplant recipients usually receive multiple immunosuppressive drugs. Today, the majority of these patients receives the glucocorticosteroid prednisolone, the inosine monophosphate dehydrogenase (IMPDH) inhibitor mycophenolate mofetil (MMF), and one of the calcineurin inhibitors cyclosporin micro emulsion (CsA) or tacrolimus (TACR). These combinations have been shown to be successful in the prevention of acute rejection episodes [1–3].
During absorption, the prodrug MMF is hydrolysed completely into the active metabolite mycopenolic acid (MPA), which undergoes further metabolism [4]. Quantitatively, the most important metabolite is the pharmacologically inactive mycophenolate glucuronide (MPAG), which is excreted either into urine or into the bile [5]. Biliary excreted MPAG undergoes substantial enterohepatic cycling, and is back-transformed to the pharmacologically active MPA during re-absorption [5].
Earlier studies have shown that for patients receiving CsA, roughly twice the dose of MMF is needed to achieve the same systemic exposure to MPA when compared with patients receiving TACR [6, 7]. This has been attributed to either an inhibition by CsA of the enterohepatic cycle [8], or an inhibition of glucuronidation by TACR [6]. Where the latter has been shown only in in vitro studies [9], the former has also been shown in vivo in rodents [10].
We further explored these interactions clinically using both MPA and MPAG plasma concentration data derived from a study with 64 kidney transplant patients who were on controlled systemic exposure to either CsA or TACR during the first year after transplantation. MPA/MPAG concentration–time curves were taken at regular time points during this year. Using nonlinear mixed effect modelling (NONMEM) we developed an integrated metabolite pharmacokinetic model for both MPA and its glucuronide, in which an enterohepatic cycle was incorporated.
Patients and methods
Sixty-four patients, transplanted at the Leiden University Medical Center (Leiden, the Netherlands) between 2000 and 2002, entered the study that primarily investigated the clinical, biochemical and pathological effects of triple therapy with prednisolone, MMF and either CsA (n = 33) or TACR (n = 31). Details of this study have been described elsewhere [11]. Patient characteristics are listed in Table 1. No statistical difference (Student's t-test, P > 0.05) was shown between both groups for the patients’ age, weight, length and renal function. Comedication known to influence PK of MMF, CsA or TACR was avoided. After kidney transplantation, CsA and TACR doses were adjusted to reach a predefined AUC (area under the blood concentration–time curves) as described earlier [12, 13]. AUCs were determined around the morning dose at week 2, 4, 6, 8, 10, 17, 21, 26, 39 and 52. During the first 6 weeks after Tx, the target-AUCs0−12 h were 5400 and 210 h µg−1 l−1 for CsA and TACR, respectively. Thereafter, the target-AUCs0−12 h were 3250 and 125 h µg−1 l−1, respectively. Mean accuracy and precision for reaching the predefined AUCs0−12 h were 7 and 18% for CsA, and 11 and 22%, respectively, for TACR (data from 12 weeks−1 year post Tx). Calcineurin inhibitors were given either at a twice daily or a once daily dose regimen. However, because total daily systemic exposure was kept the same for each dose regimen, we analysed each calcineurin inhibitor group as one group, thereby also preventing the study groups from becoming too small. MMF doses were 1000 mg bid and 500 mg bid for the CsA and TACR group, respectively.
Table 1.
Demographic and baseline characteristics
| Characteristic | Cyclosporine (n = 33) | Tacrolimus (n = 31) |
|---|---|---|
| Recipient age (year) ± SD | 48.3 ± 12.5 | 44.9 ± 12.5 |
| Recipient gender n male (%) | 26 (78.8) | 23 (76.4) |
| Recipient weight (kg) ± SD | 77.0 ± 16.9 | 75.3 ± 11.9 |
| Recipient height (m) ± SD | 1.74 ± 0.12 | 1.74 ± 0.09 |
| Primary disease (n, %) | ||
| Primary glomerular disease | 12 (36.4) | 12 (38.7) |
| Diabetic nephropathy | 2 (6.1) | 1 (3.2) |
| Hypertension | 5 (15.2) | 4 (12.9) |
| Hereditary disease | 4 (12.1) | 6 (19.4) |
| Congenital dysplasia/reflux | 3 (9.1) | 1 (3.2) |
| Aetiology uncertain, other | 7 (21.1) | 7 (22.6) |
| Donor age (year) ± SD | 44.0 ± 14.6 | 46.8 ± 13.3 |
| Donor gender n male (%) | 13 (39.4) | 19 (61.3) |
| Procedure (n, %) | ||
| Cadaveric, heart beating | 16 (48.6) | 13 (41.9) |
| Cadaveric, non heart beating | 7 (21.1) | 4 (12.9) |
| Living related | 6 (18.2) | 9 (29.0) |
| Living unrelated | 4 (12.1) | 5 (16.2) |
| Delayed graft function (n, %) | 9 (27.3) | 6 (19.3) |
| GFR (ml min−1) ± SD* | 62.5 ± 13.2 | 65.1 ± 4.0 |
= Nankivell formula.
A total of seven patients (n = 6 for CsA, n = 1 for TACR) was treated for suspected acute rejection (methylprednisolone iv 1000 mg for 3 days), most of them during the first 3 months post Tx. Data collected during acute rejection episodes, were not discarded a priori. Two patients switched from CsA to TACR 8 and 12 weeks post Tx, respectively. These patients were regarded TACR users, while the data of the first weeks post Tx were discarded. All patients gave oral and written informed consent before study entry. The study was approved by the Medical Ethics Committee of the Leiden University Medical Center.
At week 2, 6, 12, 26, 39 and 52, blood samples were taken around the morning dose at 0, 1, 2, 3, 4 and 6 h after drug administration. At week 4, 8, 10, 17 and 21, blood samples were taken at 0, 2 and 3 h after drug administration. Blood samples were taken for both CsA/TACR and MPA/MPAG determination. Whole blood concentrations of CsA and TACR were determined by fluorescence polarization immunoassay (FPIA) (Axsym; Abbott Diagnostics, Abbott Park, IL, USA) and microparticle enzyme immunoassay (MEIA; Abbott Laboratories, Abbott Park, IL, USA), respectively. MPA and MPAG plasma concentrations were determined using a validated high performance liquid chromatography (HPLC) method including on-line solid phase extraction and ultraviolet detection at 305 nm. The method was linear for MPA and MPAG from 0.2 to 60 and 10–400 mg l−1, respectively. Lower limits of quantification were 0.2 and 10 mg l−1, respectively. Accuracy and precision were 97 and 7% and 98 and 3% for MPA at 0.54 and 10.8 mg l−1. Accuracy and precision were 104 and 7% for MPAG at 160 mg l−1. A detailed description of the method of analysis of MPA and MPAG in plasma is available from the authors on request.
Two data sets were analysed; all available data from 2 weeks post Tx (2748 MPA and 2648 MPAG concentration measurements) and a subset where only measurements obtained starting 12 weeks post Tx were included (1563 MPA and 1514 MPAG concentration measurements). Analysis of the full data set indicated that up to 12 weeks, pharmacokinetic parameters tended to change possibly due to residual effects of the transplantation, changing exposure to CsA or TACR, and variations in comedication. Therefore the restricted data set (12 weeks and later) was used for the final parameter estimation, while the full data set was used for illustrative purposes only.
Pharmacokinetics of MPA and MPAG was investigated using Non-linear Mixed Effect Modelling (NONMEM Version V, GloboMax LLC, Hanover, MD), using the first order method and constant coefficient of variation interindividual error models and a constant coefficient of variation residual error model. Estimation of intraindividual variability was not attempted and all occasions were analysed as if originating from different subjects. More accurate estimation methods were attempted (FOCE and FOCE with interaction) but these did not lead to model convergence due to numerical instability. As the main thrust of this study was in describing combined PK of MPA and MPAG and the influence of CsA co-administration, modelling interoccasion variability was not regarded essential. To develop a metabolite PK model, MMF doses and MPA and MPAG plasma concentrations were transformed into molar equivalents. Pharmacokinetic parameters therefore apply to molar equivalents. For reasons of recognition by clinicians and pharmacologists, however, in the figures, these molar equivalents have been recalculated to MPA and MPAG plasma concentrations.
A wide range of models was investigated and compared using the minimum value of the objective function (MVOF) and visual inspection of all individual plasma concentration curves. Likelihood ratio tests to compare models were performed by comparing differences in MVOF between models to chi-square distributions with degrees of freedom equal to the difference in the number of parameters, where P < 0.01 was required for significance.
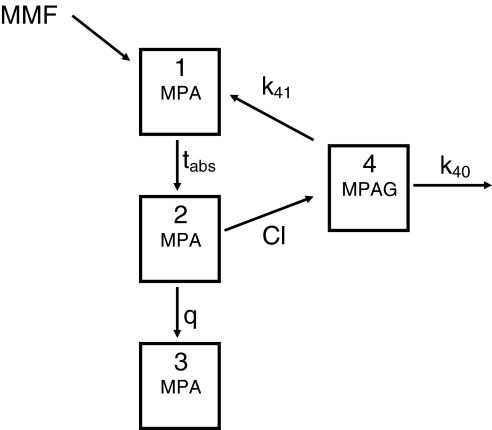
Visual inspection of the curves revealed that MPA was best described by a two-compartment model, while MPAG could be described by a one-compartment model. Probably due to the relatively sparse nature of the data, multiple peaks commonly associated with enterohepatic recycling were not observed. This means that the data do not contain information on discrete biliary excretion episodes and therefore, enterohepatic recycling was modelled as a continuous process. As a result, combined MPA/MPAG PK after oral administration of MMF was described by a four-compartment model. The MMF dose was entered into the first compartment. The second and fourth compartment represented MPA and MPAG plasma concentrations, respectively, while the third compartment accounted for the peripheral distribution of MPA. The enterohepatic recycling step was modelled by introducing a rate constant describing transfer from the fourth (MPAG) to the first (MMF dosing) compartment assuming complete transformation of MPAG into MPA.
We primarily investigated whether PK of both MPA and MPAG after MMF administration could be described simultaneously by a four-compartment model, and whether introduction of an enterohepatic cycle in case of TACR and not in case of CsA co-administration improved description of the data. Within the overall population analysis, separate population parameter estimates were obtained for TACR and CsA co-administration. The population average parameters describing CsA were parameterized as the difference between the population average TACR parameter and the population average CsA parameter. This means that population estimates for CsA co-administration can be calculated (by simple addition) but standard errors are not available for CsA parameters. It would also have been possible to estimate absolute population average values for both co-administration situations. However, estimation of the difference instead allows testing of the significance of the difference between the two co-administrations for each parameter separately, using confidence intervals based on the approximate standard error. For this purpose, the approximate standard errors (SEM) for the difference estimates were used to arrive at approximate 95% confidence limits (difference ± 2*SEM). Figure 1 gives a schematic representation of the final PK model used. The detailed NONMEM syntax of this model is available from the authors on request.
Figure 1.
Four-compartment model for the pharmacokinetics of mycophenolate mofetil (MMF); MPA = mycophenolic acid, MPAG = mycophenolate glucuronide, tabs = absorption half-life, q = intercompartmental clearance, Cl = clearance of MPA into MPAG, k40 = elimination rate constant of MPAG, k41 = rate transfer constant describing biliary excretion of MPAG
Influence of covariates on pharmacokinetic variability was investigated using Pearson's correlation coefficients between empirical Bayes estimates of the pharmacokinetic parameters and patient characteristics (age, height, weight, haemoglobin, serum albumin, creatinine clearance estimated using Cockcroft and Gault, and creatinine clearance estimated using Nankivell).
Results
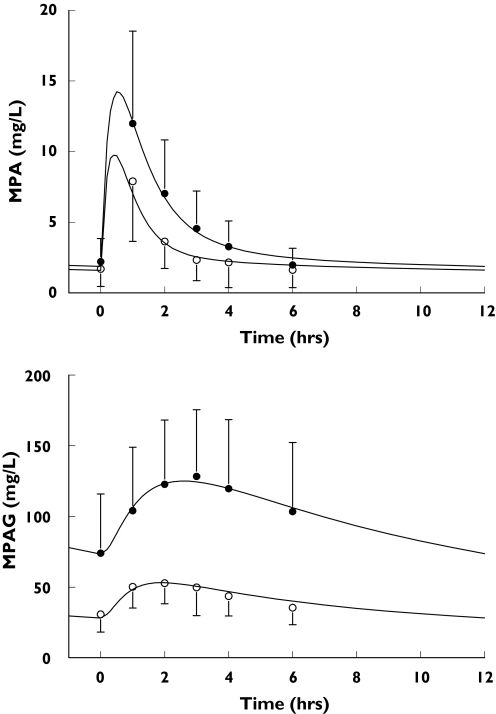
Both MPA and MPAG plasma concentrations after MMF administration starting from 12 weeks after Tx were adequately described by a four-compartment model. Absence of systematic model deviation can be seen in Figure 2, illustrating that the mean predicted curves are in close agreement with the mean of the observations. Standard goodness of fit plot (e.g. observed vs. predicted concentrations) are not shown because the high number of data points results in uninformative graphs.
Figure 2.
Average (±SD) graph of observed data (black markers: CsA, white markers: TACR) with average predicted curves from weeks 12, 26 and 52 combined; top graph: MPA, bottom graph: MPAG
Introduction of k41 into the model in case of TACR co-administration, significantly improved the description of the data (MVOF decreased by 181 points, P < 0.0001).
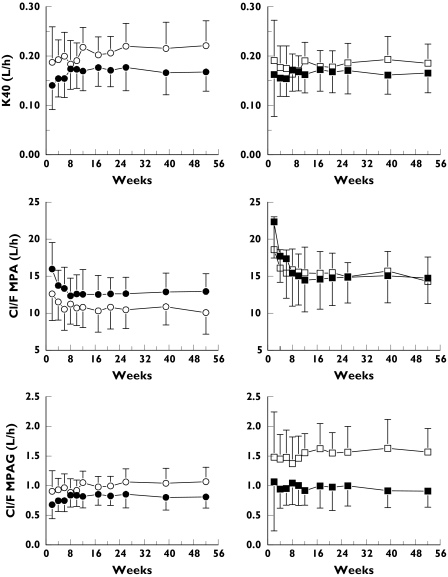
Table 2 summarizes all PK parameters of the final model for both the CsA and TACR group. Figure 2 shows values of predicted and observed MPA and MPAG data, illustrating altered description of the data after incorporating enterohepatic recycling, while Figure 3 shows the value of relevant PK parameters (mean and SD of empirical Bayes estimates) during 1 year post transplantation for the two groups with and without incorporating enterohepatic recycling (Cl MPA, Cl MPAG, k40) using the full data set. As can be seen, PK is relatively stable from 12 weeks on, in both study groups.
Table 2.
Final NONMEM parameters for the full model using data from 12 weeks and later
| Parameter | Mean (SE) TACR | Mean CsA | Difference (95% CI) | CV (%) |
|---|---|---|---|---|
| Cl/F (l h−1) | 11.9 (1.75) | 14.1 | 2.2 (−1 to 6) | 30 |
| T1/2a (h) | 0.567 (0.137) | 0.690 | 0.123 (−0.18 to 0.42) | 57 |
| Q/F (l h−1) | 11.2 (4.09) | 20.1 | 8.9 (0 to 18) | 51 |
| Vc/F (l) | 10.3 (16.8) | 11.7 | 1.4 (−3 to 6) | 74 |
| Vp/F (l) | 183 (75.0) | 465 | 282 (90 to 480) | 330 |
| V4/F (MPAG) (l) | 8.91 (2.27) | 5.60 | −3.31 (−7.9 to 1.2) | 20 |
| K40 (MPAG) (h−1) | 0.122 (0.0343) | 0.165 | 0.043 (−0.03 to 0.12) | 28 |
| K41 (MPAG) (h−1) | 0.0410 (0.0209) | NA | NA | 78 |
| Error MPA (%) | 35 | |||
| Error MPAG (%) | 14 |
Figure 3.
Average (±SD) empirical Bayes estimates of clearance of MPA, clearance of MPAG and k40 during 1 year post transplantation calculated using the four-compartment model without (left side of the figure) and with the use of k41 in case of TACR co-administration (right side of the figure); white markers represent TACR and black markers CsA comedication
Using the model incorporating enterohepatic recycling, no difference was observed in clearance of MPA between CsA and TACR co-administration (difference: 2.2 l h−1; 95% CI: −1 to 6 l h−1). This clearance represents almost entirely the glucuronidation of MPA. In case of CsA co-administration, a somewhat higher clearance from the central to the peripheral compartment, and a higher peripheral volume was observed. No significant differences were observed for either absorption half-life or central volume.
No statistically significant difference was observed in the volume of distribution of MPAG, although the average value was about 40% higher in case of TACR co-administration. Consequently, total clearance of MPAG calculated as (k40 + k41)*V4/F using empirical Bayes estimates was 40% lower in case of CsA co-administration. This difference, however, was caused by the difference in volume of distribution of MPAG, as the sum of the rate constants for TACR co-administration (k41 + k40) equalled the rate constant for CsA co-administration (k40).
The strongest relationships between patient characteristics and pharmacokinetic parameters were shown for creatinine clearance and k40, with correlation coefficients of 0.51 for the Nankivell estimate (Clnk) and 0.45 for the Cockroft and Gault (Clcr) estimate (P < 0.0001, n = 340, data from 12 weeks and later). All other correlations were less than 0.39.
Discussion
Earlier pharmacokinetic studies have demonstrated an interaction between calcineurin inhibitors and MMF. However, controversy remains regarding the exact mechanism through which this interaction takes place [14]. Zucker et al. reported significantly increased MPA trough plasma concentrations and MPA AUCs in patients treated with TACR, compared with patients treated with MMF and CsA [6], leading to the hypothesis that increased MPA levels are the result of inhibition of glucuronidation of MPA by TACR, which is supported by an in vitro study showing inhibition of uridine-diphosphate-glucuronyl-transferase (UGT) formation of MPAG by TACR but not CsA [9]. Smak Gregoor et al. however, reported significantly decreased MPA plasma trough concentrations in kidney transplant recipients in case of CsA co-administration when compared with no calcineurin inhibitor as comedication, leading to the hypothesis that CsA inhibits the enterohepatic recirculation of MPAG [15]. This hypothesis was later confirmed by in vivo studies in rodents showing no effect of TACR on MPA concentrations when compared with placebo, in contrast to the known effect of CsA on MPA concentrations [10]. This interaction may occur at the level of the biliary excretion of MPAG, where MPAG can be expected to be a substrate for the active transport by ATP-binding cassette transporters such as multidrug resistance-associated protein 2 (MRP-2) [8], which was recently confirmed by an in vivo study in rodents [16].
Pharmacokinetic (PK) modelling may capture pharmacological information about a drug, especially in complex systems such as the metabolism and biodistribution of a drug such as MMF. It has become clear, however, that PK modelling based solely on MPA concentrations is difficult after oral administration of MMF [17, 18]. In the present study, we modelled both MPA and MPAG plasma concentrations simultaneously, and it seems that this enhanced data set is more useful for the description of MMF PK. Moreover, our data were derived from transplant patients who were stable and on well controlled systemic exposure to the calcineurin inhibitors [12, 13], thereby eliminating some potential factors influencing MMF PK.
The present study clearly shows two major findings. First, MPA and MPAG PK in stable renal transplant patients, or at least those data between 0 and 6 h after drug administration, can be described adequately by a compartmental metabolite PK model. Such a model may be of use for therapeutic drug monitoring purposes in case both MPA and MPAG concentrations are determined on a routine basis. Moreover, the model may be of help describing PK in other patient populations and describing PK of other formulations with MPA as active compound. Second, this study strongly suggests that inhibition of the enterohepatic cycle in case of CsA co-administration explains some of the differences observed in PK of MMF when co-administered with either TACR or CsA. This conclusion is based mainly on the significant improvement of description of the data by introduction of an enterohepatic cycle into the model in case of TACR co-administration. The finding may explain the less frequent gastrointestinal side-effects caused by MMF in case of CsA co-administration, since biliary excretion of MPAG is believed to play a role in this adverse event [16].
The present study, however, did assume an extreme situation, namely complete inhibition of biliary excretion in case of CsA co-administration, where it is known from animal studies [16] that inhibition is not complete. The reason for not estimating k41 in the presence of CsA, however, has to do with identifiability. There is a trade-off between all parameters, where an increase in one will lead to a decrease in another with (almost) the same curve as result. The presence of k41 effectively increases the amount available for take-up in the gut. Incorporation of k41 in the CsA group would probably lead to nonzero estimates for this parameter compensated by and increase in distribution volume. Posing an extreme situation (presence/absence of k41) enables to see if all other parameters are essentially similar while there is a substantial difference in (dose-normalized) concentrations.
According to the model, no difference exists in glucuronidation between TACR and CsA. However, this finding should not be extrapolated easily to the conclusion that there is no effect on glucuronidation by TACR, especially since we did not investigate specifically whether inhibition of glucuronidation by TACR significantly improves description of the data. To address these issues more appropriately, a third patient group consisting of patients receiving only MMF should be studied. However, such a patient group was not available.
In the present study we only determined MPA and MPAG plasma concentrations during 6 h after MMF administration, thereby possibly missing part of the second plasma peak MPA sometimes shows, and which has been attributed to the enterohepatic cycle. However, as in our final data set all patients had received MMF for at least 3 months, substantial contribution of the enterohepatic cycle had also resulted in general accumulation of both MPA and MPAG and thereby an increase in basal levels. In the present study, we also did not investigate possible other metabolites of MMF such as the pharmacologically active acyl-glucuronide of MPA (Acyl-MPAG) [4, 19]. However, these metabolites, although pharmacologically active, are quantitatively negligible in the biodistribution and metabolism of MMF. Nevertheless, in the future, it would be interesting to expand the present model with such metabolites.
The present study shows that MPA and MPAG PK in stable renal transplant patients can be described adequately by a compartmental metabolite PK model. Moreover, it shows the ability of modelling techniques to help unravel complex pharmacological systems, supplying supportive clinical evidence that inhibition of the enterohepatic cycle in case of CsA co-administration explains some of the differences observed in PK of MMF when co-administered with either TACR or CsA. This may have clinical consequences for the immunosuppressive management of kidney transplant patients.
Acknowledgments
Competing interests: None declared.
References
- 1.Sollinger HW. Mycophenolate mofetil for the prevention of acute rejections in primary cadaveric renal allograft recipients. US Renal transplant Mycophenolate Study Group. Transplantation. 1995;60(3):225–32. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Marsh JW, McCauley J, Johnston J, Randhawa P, Irish W, Gritsch HA, Naragh R, Hakala TR, Fung JJ, Starzl TE. A prospective, randomized trial of tacrolimus/prednisone versus tacrolimus/prednisone/mycophenolate mofetil in renal transplant recipients. Transplantation. 1999;67(3):411–5. doi: 10.1097/00007890-199902150-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keown PA, Landsberg D, Halloran PF, Shoker A, Rush D, Jeffery J, Russell D, Stiller C, Muirhead N, Cole E, Paul L, Zaltzman J, Loertscher R, Daloze P, Dandavino R, Boucher A, Handa P, Lawen J, Belitsky P, Parfrey P. A randomized, prospective multicenter pharmacoepidemiological study of cyclosporine microemulsion in stable renal graft recipients. Report of the Canadian Neoral Renal Transplantation Study Group. Transplantation. 1996;62(12):1744–52. doi: 10.1097/00007890-199612270-00009. [DOI] [PubMed] [Google Scholar]
- 4.Shipkova M, Armstrong VW, Wieland E, Niedmann PD, Schuetz E, Brenner-Weiss G, Voihsel M, Braun F, Oellerich M. Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mophetil. Br J Clin Pharmacol. 1999;126:1075–82. doi: 10.1038/sj.bjp.0702399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullingham RES, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 2003;34(6):429–55. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- 6.Zucker K, Rosen A, Tsaroucha A, de Faria L, Roth D, Ciancio G, Esquenazi V, Burke G, Tzakis A, Miller J. Augmentation of mycophenolate mofetil pharmacokinetics in renal transplant patients receiving Prograf and Cellcept in combination therapy. Transplant Proc. 1997;29(1–2):334–6. doi: 10.1016/s0041-1345(96)00292-8. [DOI] [PubMed] [Google Scholar]
- 7.Filler G, Zimmering M, Mai I. Pharmacokinetics of mycophenolate mofetil are influenced by concomitant immunosuppression. Pediatric Nephrol. 2000;14(2):100–4. doi: 10.1007/s004670050021. [DOI] [PubMed] [Google Scholar]
- 8.van Gelder T, Klupp J, Sawamoto T, Christians U, Morris RE. ATP-binding cassette transporters and calcineurin inhibitors: potent clinical implications. Transplant Proc. 2001;22:2420–1. doi: 10.1016/s0041-1345(01)02059-0. [DOI] [PubMed] [Google Scholar]
- 9.Zucker K, Tsaroucha A, Olson L, Esquenazi V, Tzakis A, Miller J. Evidence that tacrolimus augments the bioavailability of mycophenolate mofetil through the inhibition of mycophenolic acid glucuronidation. Ther Drug Monit. 1999;21(1):35–43. doi: 10.1097/00007691-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 10.van Gelder T, Klupp J, Barten MJ, Christians U, Morris RE. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit. 2001;23(2):119–28. doi: 10.1097/00007691-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Scholten EM, Rowshani AT, Surachno J, van Kan E, Cremers SCLM, Paul L, Bemelman FJ, Florquin S, ten Berge IJ, de Fijter JW. Calcineurin-inhibitor neprhotoxicity and efficacy study: the CANNES trial. Am J Transplantation. 2003;3(Suppl 5):554. [Google Scholar]
- 12.Cremers SCLM, Scholten EM, Schoemaker RC, Lentjes EGWM, Vermeij P, Paul LC, de Fijter JW. A compartmental pharmacokinetic model of cyclosporin and its predictive performance after Bayesian estimation in kidney and simultaneous pancreas-kidney transplant recipients. Nephrol Dial Transplant. 2003;18:1201–8. doi: 10.1093/ndt/gfg065. [DOI] [PubMed] [Google Scholar]
- 13.Scholten EM, Cremers SCLM, Rowshani AT, Cohen AF, van Kan E, Den Hartigh J, Paul LC, de Fijter JW. AUC-guided treatment with tacrolimus in renal transplant recipients submitted. 2004. in press.
- 14.Christiansens U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41(11):813–51. doi: 10.2165/00003088-200241110-00003. [DOI] [PubMed] [Google Scholar]
- 15.Smak Gregoor PJH, van Gelder T, Hesse CJ, van der Mast BJ, van Besouw NM, Weimar W. Mycophenolic acid plasma concentrations in kidney allograft recipients with or without cyclosporin: a cross-sectional study. Nephrol Dial Transplant. 1999;14:706–8. doi: 10.1093/ndt/14.3.706. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Saitoh H, Kobayashi M, Tadano K, Takahashi Y, Hirano T. Cyclosporin A, but not tacrolimus, inhibits the biliary excretion of mycophenolic acid glucuronide possibly mediated by Mrp2 in rats. J Pharmacol Exp Ther. 2004;309:1029–1035. doi: 10.1124/jpet.103.063073. [DOI] [PubMed] [Google Scholar]
- 17.Shum B, Duffull SB, Taylor PJ, Tett SE. Population pharmacokinetic analysis of mycophenolic acid in renal transplant recipients following oral administration of mycophenolate mofetil. Br J Clin Pharmacol. 2003;56:188–97. doi: 10.1046/j.1365-2125.2003.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Guellec C, Bourgoin H, Buechler M, Le Meur Y, Lebranchu Y, Marquet P, Paintaud G. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in stable renal transplant patients. Clin Pharmacokinet. 2004;43(4):253–66. doi: 10.2165/00003088-200443040-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kuypers DR, Vanrenterghem Y, Squifflet JP, Mourad M, Abramowicz D, Oellerich M, Armstrong V, Shipkova M, Daems J. Twelve-month evaluation of the clinical pharmacokinetics of total and free mycophenolic acid and its glucuronide metabolites in renal allograft recipientson low dose tacrolimus in combination with mycophenolate mofetil. Ther Drug Monit. 2003;25(5):609–22. doi: 10.1097/00007691-200310000-00011. [DOI] [PubMed] [Google Scholar]