Abstract
Aims
The aim of the study was to characterize the population pharmacokinetics of indinavir, define the relationship between the pharmacokinetics of indinavir and ritonavir, and to identify the factors influencing the pharmacokinetics of indinavir alone or when given with ritonavir.
Methods
HIV-1-infected patients being treated with an indinavir-containing regimen were included. During regular visits, 102 blood samples were collected for the determination of plasma indinavir and ritonavir concentrations. Full pharmacokinetic curves were available from 45 patients. Concentrations of indinavir and ritonavir were determined by liquid chromatography coupled with electrospray tandem mass spectrometry. Pharmacokinetic analysis was performed using nonlinear mixed effect modelling (NONMEM).
Results
The disposition of indinavir was best described by a single compartment model with first order absorption and elimination. Values for the clearance, volume of distribution and the absorption rate constant were 46.8 l h−1 (24.2% IIV), 82.3 l (24.6% IIV) and 02.62 h−1, respectively. An absorption lag-time of 0.485 h was detected in patients also taking ritonavir. Furthermore this drug, independent of dose (100–400 mg) or plasma concentration, decreased the clearance of indinavir by 64.6%. In contrast, co-administration of efavirenz or nevirapine increased the clearance of indinavir by 41%, irrespective of the presence or absence of ritonavir. Female patients had a 48% higher apparent bioavailability of indinavir than males.
Conclusions
The pharmacokinetic parameters of indinavir were adequately described by our population model. Female gender and concomitant use of ritonavir and non-nucleoside reverse transcriptase inhibitors strongly influenced the pharmacokinetics of this drug. The results support the concept of ritonavir boosting, maximum inhibition of indinavir metabolized being observed at 100 mg.
Keywords: HIV, indinovir, population pharmacokinetics, ritonavir
Introduction
Indinavir is a potent human immunodeficiency virus (HIV) protease inhibitor [1–3]. The oral bioavailability of the drug when dosed alone is decreased when administered together with food and therefore indinavir should be taken on an empty stomach [4]. However, when co-administered with ritonavir the pharmacokinetic profile of indinavir is improved [5, 6]. This desirable drug–drug interaction is caused by potent inhibition of cytochrome P450 (CYP) 3A4-mediated metabolism in the liver by ritonavir, resulting in a decreased elimination rate of indinavir [7]. In addition, inhibition of drug-transporting cellular efflux proteins such as P-glycoprotein (P-gp) by ritonavir, might further increase the bioavailability of indinavir [8]. However, the inhibitory potential of ritonavir on this transporter has not been established unequivocally [9].
Both toxicity and efficacy have been related to plasma indinavir concentrations [10–12]. Therapeutic drug monitoring of indinavir may be a valuable tool in the treatment with this drug, as has been demonstrated in treatment-naive patients [13].
The aim of this study was to characterize the population pharmacokinetic parameters of indinavir, and, in particular, to model the interaction between ritonavir and indinavir, in a representative HIV-1-infected patient population, in which various dosage regimens were being used. In addition, patient characteristics that might affect the pharmacokinetic parameters of indinavir were investigated.
Methods
Patients
Ambulatory HIV-1-infected patients from the outpatient clinic of the Slotervaart Hospital, Amsterdam, the Netherlands were studied. All patients were taking indinavir as part of their antiretroviral regimen and had at least one plasma indinavir concentration available for analysis. Patients received either indinavir alone, or in combination with ritonavir. Data were collected during regular outpatient clinic visits at random time points. At each visit, which was considered an occasion, a blood sample was obtained for the determination of an indinavir and ritonavir plasma concentration. In addition to the random samples, full pharmacokinetic profiles (8–12 time points per patient) were available from 45 patients, which were obtained as part of several studies performed in our hospital and in the Academic Medical Centre, Amsterdam, the Netherlands. Full details of these studies have been presented elsewhere [5, 14–16]. Protocols were approved by the institutional ethics committees and informed consent was obtained from all patients. The randomly timed blood samples were obtained within the scope of our therapeutic drug monitoring program, which is in accordance with local treatment guidelines.
Blood sampling
Our therapeutic drug monitoring program involves a strict protocol in which plasma concentrations of antiretroviral drugs are routinely and frequently monitored during each visit to the outpatient clinic. As a consequence patients understand the need to record the time of ingestion of the last dose. Additionally, sampling times were recorded electronically at the department of clinical chemistry. The sampling time after dosing was calculated from this information.
Full pharmacokinetic profiles were determined during an administration interval. All concentrations were collected at steady state, at least 2 weeks after initiation of an indinavir-containing regimen.
Drug analysis
Plasma concentrations of indinavir and ritonavir were determined using liquid chromatography coupled with electrospray tandem mass spectrometry. This method was validated over the range 0.01–10 mg l−1 and 0.05–10 mg l−1, respectively, using 100 µl samples of plasma. Recoveries of indinavir and ritonavir were 105.4% and 91.7%, respectively. Within- and between-day precisions were always less than 9.4% for all quality control samples [17].
Population pharmacokinetic analyses
The nonlinear mixed effect modelling software program NONMEM (Version V, level 1.1, GloboMax LLC, Hanover MD, USA) using a Fortran compiler (Compaq Visual Fortran Version 6.5, Compaq Computer Corporation, Houston, TX, USA), was used to perform all analyses. The first-order conditional estimate method (FOCE) procedure with interaction between interindividual, intra-individual and residual variability was used throughout. The adequacy of the developed structural models was evaluated using both statistical and graphical methods. The minimal value of the objective function (OFV) provided by NONMEM was used as goodness-of-fit characteristic to discriminate between hierarchical models using the likelihood ratio test [18]. A P value of 0.05, representing a decrease in OFV of 3.84 points, was considered statistically significant (chi-square distribution, d.f. = 1). Standard errors for all parameters were approximated using the COVARIANCE option of NONMEM. Individual Bayesian estimates of the pharmacokinetic parameters were obtained using the POSTHOC option [18]. The program PDx-Pop (version 1.1, release 4, Globomax LLC, Hanover MD, USA) was used as interface for conducting the population pharmacokinetic analyses with NONMEM and for graphical model diagnostics. In addition, the S-plus (MathSoft, Inc, Seattle, USA) based model-building aid Xpose 3.0 was used for graphical model diagnosis [19].
Basic pharmacokinetic model
First-order absorption models with and without absorption lag-time were tested. Different numbers of transition compartments instead of an absorption lag-time were also tested to describe the absorption process and single and multiple compartment models with linear and nonlinear elimination were used to describe its distribution kinetics.
Since ritonavir is used as a kinetic booster in indinavir-containing regimens, the effect of ritonavir on the pharmacokinetics of indinavir was incorporated in the basic model. This consisted of two phases. In the first phase, a previously developed and validated population pharmacokinetic model for ritonavir, that also included data from patients treated with indinavir/ritonavir, was used to obtain individual Bayesian estimates of the pharmacokinetic parameters for ritonavir [20]. This model used first-order absorption in combination with one-compartment disposition and first-order elimination. Clearance, volume of distribution and absorption rate constant were 10.5 l h−1, 96.6 l and 0.871 h−1, respectively, with interindividual variabilities of 38.3%, 80.0% and 169% (% coefficient of variation), respectively. The interoccasion variability in the apparent bioavailability was 59.1%. Validation of the model indicated precise estimation of the pharmacokinetic parameters [20]. Exposure to ritonavir over a dosing interval (AUC) was calculated by dividing dose by clearance.
In the second phase, the influence of ritonavir on the pharmacokinetics of indinavir was studied. Ritonavir interacts with indinavir during the absorption and the elimination processes, mainly due to inhibition of CYP3A4 in the gut wall and the liver and inhibition of P-gp. Other transporters, such as multidrug resistance proteins (MRPs) and organic anion transporting polypeptides (OATPs) may also be affected.
Of the models tested, the first described the mechanism of the interaction assuming a time-dependent effect of ritonavir on the pharmacokinetics of indinavir. In this model the predicted concentration of ritonavir, based on the Bayesian estimates of clearance, volume of distribution and absorption rate constant, was directly related to the elimination of indinavir (1). In the second model, a direct relationship between the clearance of indinavir and the total exposure to ritonavir, expressed as the predicted AUC, was assumed (2). These relationships were expressed as follows:
in which CL/Fij represents the indinavir clearance of the ith individual on the jth occasion, θ1 is the typical value of clearance, Emax is the maximum inhibitory effect of ritonavir, Cij(t) is the concentration of the ith individual on the jth occasion at time t, C50 is the concentration of ritonavir that is associated with half-maximal inhibition of the clearance of indinavir, AUCij is the AUC of the ith individual on the jth occasion, AUC50 is the AUC of ritonavir that is associated with half-maximal inhibition of the clearance of indinavir, and γ is a constant to be estimated.
The effect of ritonavir on the apparent bioavailability F was also assessed.
Population pharmacokinetic parameters such as clearance, volume of distribution and the absorption rate constant were estimated. Interindividual (IIV) and interoccasion variability (IOV) in the pharmacokinetic parameters were determined using an exponential error model, according to Karlsson & Sheiner [21]. For example, variability in clearance was estimated using the equation
in which CL/Fij represents the clearance of the ith individual on the jth occasion, θ1 is the population value of clearance, ηi is the interindividual random effect with mean 0 and variance ω2 and κij is the interoccasion random effect with mean 0 and variance π2. The parameters ω and π represent % coefficient of variation for interindividual and interoccasion variability, respectively. Residual variability was modelled with a combined additive and proportional error model.
Covariate pharmacokinetic model
To identify possible relationships between the pharmacokinetics of indinavir and patient characteristics, the following covariates were collected at baseline: age, weight, gender, race, alanine aminotransferase (ALAT, in U l−1), aspartate aminotransferase (ASAT, in U l−1), alkaline phosphatase (AP, in U l−1), gamma-glutamyltransferase (GGT, in U l−1), total bilirubin (TBR, in µmol l−1) and serum creatinine (CR, in µmol l−1). Patients were considered to have a chronic hepatitis B or C infection when hepatitis surface antigen (HbsAg) or antihepatitis C antibodies (anti-HCV), respectively, could be detected at baseline. Concomitant use of the CYP3A4-inducing drugs efavirenz and nevirapine was documented. Age and weight were examined as continuous variables and gender, race, hepatitis B infection and hepatitis C infection as dichotomous variables. Values for ALAT, ASAT, AP, GGT, TBR and CR were transformed to dichotomous variables by using 1.5 times the upper limit of normal (ULN) as cut-off value. Some covariates were missing in a small subset of patients. In order to avoid bias, a covariate was included in the model indicating the missing data. For instance, the influence of a dichotomous covariate X on clearance with missing data of X for some individuals was modelled as:
in which TVCL is the typical value of clearance in the population, MIS is 1 for records with missing data and 0 for all other records, θ1 is the typical value of an individual with X = 0 (no missing data) and θ2 is the relative difference in clearance for individuals with X = 1 (no missing data) and θ3 is the relative difference in clearance for individuals with missing data.
The inclusion of a covariate relationship in a pharmacokinetic model was based on a combination of the statistical significance and the clinical importance of the relationship. A stepwise forward inclusion and backward elimination procedure was carried out for the detection of covariate relations. A covariate was considered statistically significant in the forward inclusion process when the inclusion was associated with a decrease in the minimal value of the objective function associated with a P value of ≤0.05 (ΔOFV = 3.84 points, log-likelihood ratio test). In the backward elimination procedure a P value of 0.01 combined with a clinically relevant effect was required. Clinical relevance was considered when the typical value of the pharmacokinetic parameter of interest changed at least 10% in the range of the covariate, as observed in the population to prevent the detection of a clinically unimportant, albeit significant, relationship.
Statistical refinement
The validity of the interindividual variability model was assessed by evaluating the correlations between individual random effects (η) and interoccasion random effects (κ) for all of the pharmacokinetic parameters [22]. When a substantial correlation was present or suspected, covariance between these parameters was included in the model.
Model validation
The bootstrap resampling technique was applied as an internal validation for the final model [23]. Bootstrap replicates were generated by randomly sampling approximately 65% of the original data set with replacement. The final model was fitted to the replicate data set using the bootstrap option in the software package Wings for NONMEM (written by N. Holford, version 406, May 2004, Auckland, New Zealand). Parameter estimates for the replicate data set were obtained in this way [23]. The precision of the model was evaluated by visual inspection of the distribution of model parameters. Furthermore, the median parameter values and 95% prediction intervals of the bootstrap replicates were compared with the estimates of the original data set.
Results
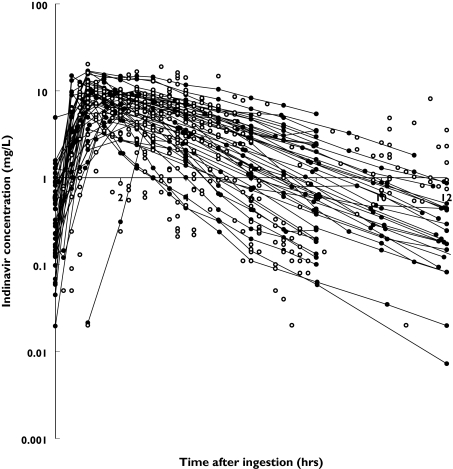
Full pharmacokinetic profiles were available from 45 patients. In addition, indinavir plasma concentrations at a single time point were obtained from 102 patients. In total 853 samples were used for analysis. Most patients received 800 mg indinavir orally either three times daily (112 occasions) or twice daily in combination with 100 mg ritonavir (201 occasions) or 400 mg indinavir and 400 mg ritonavir twice daily (37 occasions). Alternative regimens and patient characteristics of the data set are presented in Table 1. The patient population was predominantly male and Caucasian. Information was not available (depending on the covariable) from 0 to 19.7% of the patients. Excluding the data from the full profiles, two to three samples per patient (range from 1 to 18, follow-up 0–64 months) were used. The concentration-time data of indinavir are shown in Figure 1.
Table 1.
Baseline characteristics of the patients (n = 147) studied on 443 occasions
| Parameter | Number of occasions (n (%)) | Number of patients. (n (%)) | Median | IQR | Number of patients > 1.5 × ULN | Missing (n (%)) |
|---|---|---|---|---|---|---|
| Regimen | ||||||
| 3 × 600 mg IDV | 1 (0.2) | |||||
| 3 × 800 mg IDV | 112 (25.3) | |||||
| 3 × 1000 mg IDV | 26 (5.9) | |||||
| 3 × 1200 mg IDV | 4 (0.9) | |||||
| 2 × 1200 mg IDV | 7 (1.6) | |||||
| 2 × 1400 mg IDV | 5 (1.1) | |||||
| 2 × 200 mg IDV +2 × 100 mg RTV | 1 (0.2) | |||||
| 2 × 600 mg IDV +2 × 100 mg RTV | 18 (4.1) | |||||
| 2 × 800 mg IDV +2 × 100 mg RTV | 201 (45.4) | |||||
| 2 × 1000 mg IDV +2 × 100 mg RTV | 20 (4.5) | |||||
| 2 × 1200 mg IDV +2 × 100 mg RTV | 2 (0.5) | |||||
| 2 × 800 mg IDV +2 × 400 mg RTV | 6 (1.4) | |||||
| 2 × 400 mg IDV +2 × 400 mg RTV | 37 (8.4) | |||||
| 1 × 800 mg IDV +1 × 100 mg RTV | 3 (0.7) | 0 | ||||
| concomitant NNRTI | 35 (7.9) | 0 | ||||
| Gender M/F | 138/9 (93.9/6.1) | 0 | ||||
| Age (years) | 40.3 | 34.9–47.1 | 29 (19.7) | |||
| Weight (kg) | 73.0 | 65.0–80.0 | 0 | |||
| Race | ||||||
| Caucasian | 121 (82.3) | |||||
| Black | 14 (9.5) | |||||
| Asian | 7 (4.8) | |||||
| Latino | 5 (3.4) | |||||
| Clinical chemistry | ||||||
| Baseline ASAT (U l−1) | 31.7 | 24.4–43.5 | 21 | 4 (2.7) | ||
| Baseline ALAT (U l−1) | 34.0 | 22.0–48.1 | 20 | 4 (2.7) | ||
| Baseline GGT (U l−1) | 37.8 | 22.0–62.8 | 27 | 7 (4.8) | ||
| Baseline AP (U l−1) | 78.8 | 65.0–92.6 | 4 | 7 (4.8) | ||
| Baseline TBR (µmol l−1) | 18.0 | 12.3–27.0 | 40 | 5 (3.4) | ||
| Baseline CR (µmol l−1) | 81.0 | 72.0–91.0 | 1 | 4 (2.7) | ||
| Clinical immunology at baseline | ||||||
| CD4 cell count (106 l−1) | 380 | 220–575 | 8 (5.4) | |||
| CD8 cell count (106 l−1) | 1060 | 725–1565 | 12 (8.2) | |||
| Molecular biology at baseline | ||||||
| Plasma log10 HIV-1 RNA (copies/ml) | 2.30 | 2.30–3.67 | 6 (4.1) | |||
| HBV/no HBV | 5/133 (3.4/90.5) | 9 (6.1) | ||||
| HCV/no HCV | 8/130 (5.4/88.4) | 9 (6.1) | ||||
IDV = indinavir, RTV = ritonavir, M = male, F = female, ASAT = aspartate aminotransferase, ALAT = alanine aminotransferase, GGT = gamma-glutamyltransferase, AP = alkaline phosphatase, TBR = total bilirubin, CR = creatinine, HBV = hepatitis B infection, HCV = hepatitis C infection, IQR = interquartile range.
Figure 1.
Concentration-time data for indinavir. Open circles (○) represent concentrations at single time points, dots connected with hairlines (•) represent full pharmacokinetic profiles
The population pharmacokinetics of indinavir were best described by a one-compartment model with first-order absorption and elimination. Both zero-order and first-order models with and without peripheral compartments were tested. However, the use of a zero-order absorption model or inclusion of a peripheral compartment did not increase the goodness-of-fit.
Several models with and without an absorption lag-time, as well as models with several numbers of transition compartments to describe the absorption phase [24] were evaluated. It appeared that concomitant use of ritonavir slowed the absorption of indinavir by introducing an absorption lag-time (0.485 h) which was therefore included in the model for patients taking ritonavir (ΔOFV = 79.5, P < 0.001).
The influence of exposure to ritonavir on the pharmacokinetics of indinavir was modelled in two steps. First, for 105 patients (288 occasions) who used ritonavir concomitantly with indinavir, Bayesian estimates of the ritonavir pharmacokinetic parameters were obtained by use of the earlier developed population pharmacokinetic model for ritonavir [20]. Thereafter, the effect of ritonavir exposure was studied using several models. At first, a direct time-dependent relationship between ritonavir and the clearance of indinavir was tested. However, an increase in the clearance of indinavir during the dose-interval due to the elimination of ritonavir over time could not be demonstrated. Thereafter, a continuous relationship between exposure to ritonavir, expressed as AUC, and the clearance of indinavir was tested. The OFV of this model decreased substantially in comparison with that without the effect of ritonavir. However, AUC50 was estimated to be very small and Emax was 0.638, indicating complete inhibition of indinavir metabolism at very low exposure to ritonavir. Therefore, in the final model the effect of ritonavir was modelled as a dichotomous variable (drug present or not). This model resulted in a decrease in the OFV of 137.4 points (P < 0.001) compared with the model that did not take account of the effect of ritonavir.
No relationship between exposure to ritonavir and the apparent bioavailability of indinavir could be demonstrated (ΔOFV = 2.4, P = 0.121).
A correlation between the individual random effects of the clearance and volume of distribution (ηCL and ηV) of indinavir was observed and the covariance between these parameters was added to the model. The correlation coefficient was 0.729.
The results from the basic pharmacokinetic model are summarized in Table 2. Clearance was estimated to be 49.3 l h−1 with an IIV and IOV of 34.8% and 21.2%, respectively. Corresponding values for the volume of distribution and absorption rate constant were 77.2 l (IIV = 28.5%) and 02.64 h−1, respectively. Ritonavir decreased the clearance of indinavir by 64%.
Table 2.
Parameter estimates for the final pharmacokinetic model for indinavir and the results of bootstrap analysis
| Basic model | Final model | Bootstrap analysis | ||||
|---|---|---|---|---|---|---|
| Est | RSE (%) | Est | RSE (%) | Median | 95% PI | |
| CL/F (l h−1) | 49.3 | 6.19 | 46.8 | 5.75 | 46.6 | 41.5, 55.3 |
| θritonavir* | 0.362 | 7.27 | 0.354 | 6.07 | 0.357 | 0.314, 0.406 |
| θconcomitant NNRTI* | – | – | 1.41 | 4.78 | 1.41 | 1.27, 1.56 |
| V/F (l) | 77.2 | 5.03 | 82.3 | 4.70 | 81.8 | 74.4, 102. |
| ka (h−1) | 2.64 | 16.4 | 2.62 | 16.0 | 2.59 | 1.90, 3.93 |
| Lag-time (h)# | 0.483 | 2.40 | 0.485 | 1.79 | 0.485 | 0.431, 0.545 |
| IIV CL/F (%) | 34.8 | 24.1 | 24.2 | 44.5 | 24.4 | 11.2, 52.3 |
| IIV V/F (%) | 28.5 | 50.6 | 24.6 | 52.3 | 24.0 | 10.6, 77.7 |
| Correlation IIV CL/F-V/F | 0.729 | 44.4 | 0.629 | 84.8 | 0.757 | −1, 1- |
| IOV CL/F (%) | 21.2 | 40.6 | 20.9 | 37.0 | 20.5 | 11.5, 32.6 |
| IOV F (%) | 22.8 | 48.3 | 23.1 | 50.7 | 22.2 | 7.74, 56.5 |
| θfemale* | – | – | 1.48 | 16.7 | 1.46 | 0.882, 2.040 |
| Additive error (mg l−1) | 0.0491 | 17.0 | 0.0491 | 16.7 | 0.0492 | 0.0306, 0.0782 |
| Proportional error (%) | 35.0 | 6.06 | 35.3 | 6.18 | 34.8 | 25.0, 39.4 |
Relative change in pharmacokinetic parameter in the presence of the covariate, resulting in the following equations: CL/F = 46.8 × 0.354RTV × 1.41NNRTI, V/F = 77.2, F = 1 × 1.48SEX.
Only estimated when indinavir and ritonavir were combined. CL/F = clearance, V/F = volume of distribution, F = apparent bioavailability, ka = absorption rate constant, IIV = interindividual variability, IOV = interoccasion variability, Est = parameter estimate, RSE = relative standard error, PI = prediction interval.
The different covariates were introduced separately into the basic model on clearance, volume of distribution and apparent bioavailability, using a univariate procedure. Gender, baseline TBR and concomitant use of efavirenz or nevirapine showed a statistically significant relationship with clearance and apparent bioavailability. None of the covariates was related to the volume of distribution.
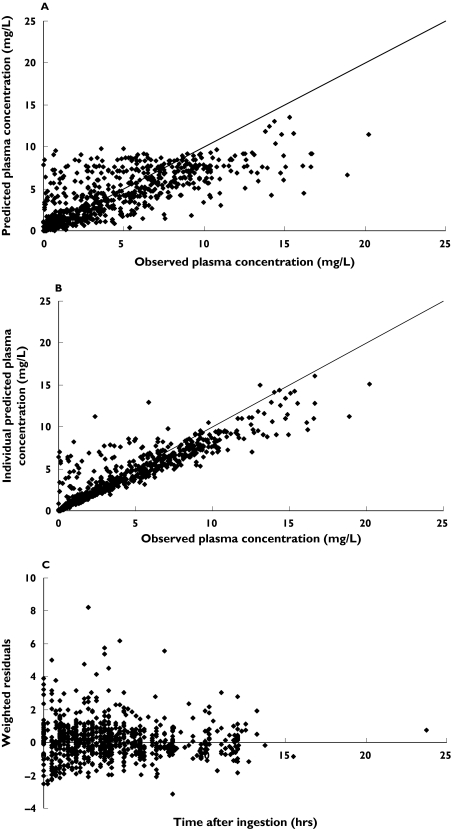
From the backwards elimination, concomitant use of efavirenz or nevirapine (ΔOFV = −34.8, change in clearance = 41%) and gender (ΔOFV = −8.6, change in apparent bioavailability = 48%) had statistically significant and clinically relevant influence on the pharmacokinetics of indinavir. The effect of efavirenz and nevirapine was similar and inclusion of separate effects for each drug did not improve goodness-of-fit. Furthermore, this change in the pharmacokinetics of indinavir was independent of ritonavir. The results of the final model are summarized in Table 2. The model predicted and individual predicted concentrations vs. observed concentrations of indinavir using the final model are presented in Figure 2. The model based predictions were symmetrically distributed around the line of identity, indicating that the model adequately describes the pharmacokinetics of indinavir. Figure 2C shows the plot of the weighted residuals vs. time after ingestion.
Figure 2.
Population (panel A) and individual (panel B) predicted concentrations vs. observed concentrations of indinavir, and weighted residuals vs. time after dosing (panel C) using the final model
The following equation describes the final model for clearance and F:
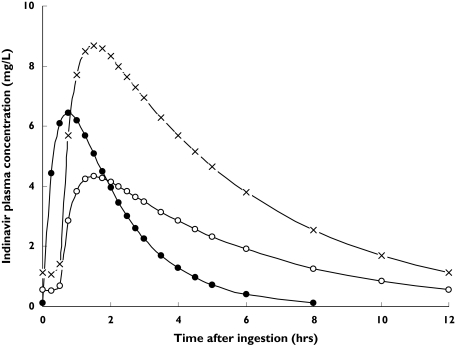
in which RTV is 1 when ritonavir is administered and 0 when it is not, NNRTI is 1 when efavirenz or nevirapine are administered and 0 when they are not, and SEX is 0 for males and 1 for female patients. In Figure 3 typical concentration-time data of the three most commonly used indinavir-containing regimens are shown (three times daily 800 mg indinavir, twice daily 800 mg indinavir with twice daily 100 mg ritonavir, twice daily 400 mg indinavir with twice daily 400 mg ritonavir).
Figure 3.
Concentration-time points for three indinavir-containing regimens. The solid dots represent three times daily 800 mg indinavir, the open circles represent two times daily 400 mg indinavir in combination with 400 mg ritonavir, and the crosses represent two times daily 800 mg indinavir with 100 mg ritonavir
From the original data set 1000 replicate bootstrap data sets were generated and used for the evaluation of the precision of the parameter estimates. In addition to those for the basic and final model, Table 2 lists the results of the bootstrap procedure, presented as medians and 95% prediction intervals. Median values from the bootstrap analysis were close to the parameter estimates from the original data set, and all parameters could be estimated with acceptable precision.
Discussion
A population pharmacokinetic model was developed that characterized the interaction between indinavir and ritonavir with a dichotomous effect of exposure to the latter on the clearance of the former drug. The developed model enabled adequate description of the pharmacokinetics of indinavir. Our estimates of clearance (46.8 l h−1) and volume of distribution (82.3 l) fell within the wide range (25.6–110 l h−1 and 30–195 l, respectively) of values found in previous studies [25–29].
The effect of ritonavir on the pharmacokinetics of indinavir was found not to be time-dependent. Furthermore, it appeared that the boosting effect of ritonavir was independent of dosage (100 mg or higher). Concomitant use of ritonavir caused a decrease in clearance of 64%, resulting in an increase in the elimination half-life from 1.2 h to 3.4 h. In contrast to the findings of Saah et al.[30], our data indicate that 100 mg of ritonavir may be sufficient for maximal inhibition of indinavir metabolism.
The absorption of indinavir was also influenced by ritonavir. The latter may have caused delayed gastric passage [31], since only in patients using indinavir combined with ritonavir could a lag-time be detected. Furthermore, and as observed in other studies [5, 30], the maximum concentration of indinavir occurred later when given with ritonavir, whereas the absorption rate was similar.
No relationship between exposure to ritonavir and the apparent bioavailability of indinavir was demonstrated. At clinical doses indinavir concentrations in the intestinal lumen are substantially higher than its Km (140 µm) for P-gp efflux [32]. As a result, it is likely that intestinal P-gp is saturated by indinavir at these doses. Thus it was not expected that ritonavir would substantially increase the oral bioavailability of indinavir through inhibition of intestinal P-gp, despite the finding that ritonavir is a moderate inhibitor of P-gp [33].
Treatment with efavirenz and nevirapine was associated with a 41% increase in the clearance of indinavir. This induction of metabolism, an effect observed by others [34–36] was also seen when ritonavir was co-administered. No patients took the latter at a dose higher than 100 mg when they were being treated with NNRTIs. Therefore, we were unable to determine whether higher doses of ritonavir may offset the enzyme induction by NNRTIs. In our study we could not demonstrate a difference in the interaction between efavirenz and nevirapine with indinavir, since no additional increase in the goodness-of-fit was gained by the inclusion of individual data for either drug. A marked decrease in the interindividual variability in indinavir clearance was observed when the effects of nevirapine and efavirenz were included in the model.
Gender had a significant effect on the pharmacokinetics of indinavir. Female patients had 48% higher bioavailability compared with male patients, indicating that females develop higher plasma concentrations, an observation also made by Csajka et al.[29]. Investigations using midazolam as a probe of intestinal CYP3A4 and verapamil, a mixed CYP3A and P-gp substrate, showed higher bioavailability of these drugs in women compared with men [37]. The pharmacokinetics of indinavir are probably influenced by both differential expression of drug transporters and CYP enzymes. Hence the factors influencing its transport and/or metabolism can be expected to affect both clearance and bioavailability.
No other tested covariables showed an influence on the pharmacokinetics of indinavir.
Despite significant interoccasion variability, the presence of a relationship between plasma concentration and efficacy and/or toxicity and the substantial interindividual variability in clearance indicates the usefulness of monitoring plasma concentrations to optimize indinavir-containing therapy [13]. Bayesian estimates of individual pharmacokinetic parameters for indinavir based on the developed model can be used to determine maximum and minimum plasma concentrations from a randomly timed blood sample. Appropriate therapeutic drug monitoring can then be performed, thus preventing toxicity and/or virologic failure, especially in females and in patients also taking efavirenz or nevirapine.
In conclusion, a model to describe the pharmacokinetics of indinavir was developed and validated. To this end, a large outpatient population was used, and concentration-time points over the complete dosing interval were incorporated into the model. Cotreatment with ritonavir delayed the absorption of indinavir and substantially affected its elimination. The influence of ritonavir on the clearance of indinavir was independent of dosage and it was found that that 100 mg of ritonavir is sufficient for maximal inhibition of the CYP3A4-mediated metabolism of indinavir. In contrast, concomitant use of NNRTIs increased the clearance of indinavir.
Acknowledgments
Competing Interests: None declared.
References
- 1.Eron JJ, Jr, Murphy RL, Peterson D, Pottage J, Parenti DM, Jemsek J, Swindells S, Sepulveda G, Bellos N, Rashbaum BC, Esinhart J, Schoellkopf N, Grosso R, Stevens M. A comparison of stavudine, didanosine and indinavir with zidovudine, lamivudine and indinavir for the initial treatment of HIV-1 infected individuals: selection of thymidine analog regimen therapy (START II) AIDS. 2000;14:1601–10. doi: 10.1097/00002030-200007280-00016. [DOI] [PubMed] [Google Scholar]
- 2.Lichterfeld M, Nischalke HD, Bergmann F, Wiesel W, Rieke A, Theisen A, Fatkenheuer G, Oette M, Carls H, Fenske S, Nadler M, Knechten H, Wasmuth JC, Rockstroh JK. Long-term efficacy and safety of ritonavir/indinavir at 400/400 mg twice a day in combination with two nucleoside reverse transcriptase inhibitors as first line antiretroviral therapy. HIV Med. 2002;3:37–43. doi: 10.1046/j.1464-2662.2001.00091.x. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Jr, Feinberg JE, Balfour HH, Jr, Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 4.Carver PL, Fleisher D, Zhou SY, Kaul D, Kazanjian P, Li C. Meal composition effects on the oral bioavailability of indinavir in HIV-infected patients. Pharm Res. 1999;16:718–24. doi: 10.1023/a:1018880726035. [DOI] [PubMed] [Google Scholar]
- 5.van Heeswijk RPG, Veldkamp AI, Hoetelmans RMW, Mulder JW, Schreij G, Hsu A, Lange JMA, Beijnen JH, Meenhorst PL. The steady-state plasma pharmacokinetics of indinavir alone and in combination with a low dose of ritonavir in twice daily dosing regimens in HIV-1-infected individuals. AIDS. 1999;13:F95–F99. doi: 10.1097/00002030-199910010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Burger DM, Hugen PW, Aarnoutse RE, Dieleman JP, van der Prins JMPT, ten Veen JH, Mulder JW, Meenhorst PL, Blok WL, van der Meer JT, Reiss P, Lange JM. A retrospective, cohort-based survey of patients using twice-daily indinavir + ritonavir combinations: pharmacokinetics, safety, and efficacy. J Acquir Immune Defic Syndr. 2001;26:218–24. doi: 10.1097/00042560-200103010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hsu A, Granneman GR, Cao G, Carothers L, Japour A, El Shourbagy T, Dennis S, Berg J, Erdman K, Leonard JM, Sun E. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob Agents Chemother. 1998;42:2784–91. doi: 10.1128/aac.42.11.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty MM, Charman WN. The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism? Clin Pharmacokinet. 2002;41:235–53. doi: 10.2165/00003088-200241040-00001. [DOI] [PubMed] [Google Scholar]
- 9.Huisman MT, Smit JW, Wiltshire HR, Hoetelmans RM, Beijnen JH, Schinkel AH. P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol Pharmacol. 2001;59:806–13. doi: 10.1124/mol.59.4.806. [DOI] [PubMed] [Google Scholar]
- 10.Solas C, Basso S, Poizot-Martin I, Ravaux I, Gallais H, Gastaut JA, Durand A, Lacarelle B. High indinavir Cmin is associated with higher toxicity in patients on indinavir-ritonavir 800/100 mg twice-daily regimen. J Acquir Immune Defic Syndr. 2002;29:374–7. doi: 10.1097/00126334-200204010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Dieleman JP, Gyssens IC, van der Ende ME, de Marie S, Burger DM. Urological complaints in relation to indinavir plasma concentrations in HIV-infected patients. AIDS. 1999;13:473–8. doi: 10.1097/00002030-199903110-00005. [DOI] [PubMed] [Google Scholar]
- 12.Burger DM, Hoetelmans RMW, Hugen PW, Mulder JW, Meenhorst PL, Koopmans PP, Brinkman K, Keuter M, Dolmans W, Hekster YA. Low plasma concentrations of indinavir are related to virological treatment failure in HIV-1-infected patients on indinavir-containing triple therapy. Antivir Ther. 1998;3:215–20. [PubMed] [Google Scholar]
- 13.Burger D, Hugen P, Reiss P, Gyssens I, Schneider M, Kroon F, Schreij G, Brinkman K, Richter C, Prins J, Aarnoutse R, Lange J. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive HIV-1-infected individuals. AIDS. 2003;17:1157–65. doi: 10.1097/00002030-200305230-00007. [DOI] [PubMed] [Google Scholar]
- 14.Hoetelmans RMW, van Heeswijk RPG, Profijt M, Mulder JW, Meenhorst PL, Lange JMA, Reiss P, Beijnen JH. Comparison of the plasma pharmacokinetics and renal clearance of didanosine during once and twice daily dosing in HIV-1 infected individuals. AIDS. 1998;12:F211–F216. doi: 10.1097/00002030-199817000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Sankatsing SU, Hoggard PG, Huitema ADR, Sparidans RW, Kewn S, Crommentuyn KML, Lange JMA, Beijnen JH, Back DJ, Prins JM. Effect of mycophenolate mofetil on the pharmacokinetics of antiretroviral drugs and on intracellular nucleoside triphosphate pools. Clin Pharmacokinet. 2004;43:823–32. doi: 10.2165/00003088-200443120-00004. [DOI] [PubMed] [Google Scholar]
- 16.van Heeswijk RPG, Veldkamp AI, Mulder JW, Meenhorst PL, Wit FW, Lange JMA, Danner SA, Foudraine NA, Kwakkelstein MO, Reiss P, Beijnen JH, Hoetelmans RMW. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1-infected individuals. AIDS. 2000;14:F77–F82. doi: 10.1097/00002030-200005260-00001. [DOI] [PubMed] [Google Scholar]
- 17.Crommentuyn KML, Rosing H, Nan-Offeringa LGAH, Hillebrand MJX, Huitema ADR, Beijnen JH. Rapid quantification of HIV protease inhibitors in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2003;38:157–66. doi: 10.1002/jms.425. [DOI] [PubMed] [Google Scholar]
- 18.Beal S, Sheiner L. NONMEM Project. Group San Francisco: University of California at San Francisco; 1998. NONMEM User's Guides. [Google Scholar]
- 19.Jonsson EN, Karlsson MO. Xpose – an S-Plus based population pharmacokinetic-pharmacodynamic model building aid for NONMEM. Comp Meth Prog Biomed. 1998;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 20.Kappelhoff BS, Huitema ADR, Crommentuyn KML, Mulder JW, Meenhorst PL, van Gorp ECM, Mairuhu ATA, Beijnen JH. Development and validation of a population pharmacokinetic model for ritonavir used as a booster or as an antiviral agent in HIV-1-infected patients. Br J Clin Pharmacol. 2005;59(2):174–82. doi: 10.1111/j.1365-2125.2004.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735–50. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson MO, Jonsson EN, Wiltse CG, Wade JR. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm. 1998;26:207–46. doi: 10.1023/a:1020561807903. [DOI] [PubMed] [Google Scholar]
- 23.Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Meth Programs Biomed. 1999;59:19–29. doi: 10.1016/s0169-2607(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 24.Kappelhoff BS, Huitema ADR, Yalvaç Z, Prins JM, Mulder JW, Meenhorst PL, Beijnen JH. Clin Pharmacokinet. 2005. Population pharmacokinetics of efavirenz in an unselected cohort of HIV-1-infected individuals. in press. [DOI] [PubMed] [Google Scholar]
- 25.Boyd MA, Aarnoutse RE, Ruxrungtham K, Stek M, Jr, van Heeswijk RPG, Lange JMA, Cooper DA, Phanuphak P, Burger DM. Pharmacokinetics of indinavir/ritonavir (800/100 mg) in combination with efavirenz (600 mg) in HIV-1-infected subjects. J Acquir Immune Defic Syndr. 2003;34:134–9. doi: 10.1097/00126334-200310010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Letendre SL, Capparelli EV, Ellis RJ, McCutchan JA. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. The HIV Neurobehavioral Research Center Group. Antimicrob Agents Chemother. 2000;44:2173–5. doi: 10.1128/aac.44.8.2173-2175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou XJ, Havlir DV, Richman DD, Acosta EP, Hirsch M, Collier AC, Tebas P, Sommadossi JP. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS. 2000;14:2869–76. doi: 10.1097/00002030-200012220-00008. [DOI] [PubMed] [Google Scholar]
- 28.DiCenzo R, Forrest A, Squires KE, Hammer SM, Fischl MA, Wu H, Cha R, Morse GD. Indinavir, efavirenz, and abacavir pharmacokinetics in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2003;47:1929–35. doi: 10.1128/AAC.47.6.1929-1935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Telenti A, Biollaz J, Buclin T. Population pharmacokinetics of indinavir in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 2004;48:3226–32. doi: 10.1128/AAC.48.9.3226-3232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saah AJ, Winchell GA, Nessly ML, Seniuk MA, Rhodes RR, Deutsch PJ. Pharmacokinetic profile and tolerability of indinavir-ritonavir combinations in healthy volunteers. Antimicrob Agents Chemother. 2001;45:2710–5. doi: 10.1128/AAC.45.10.2710-2715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu JF, Blaschke TF, Flexner C, Rosenkranz SL, Sheiner LB. Model-based analysis of the pharmacokinetic interactions between ritonavir, nelfinavir, and saquinavir after simultaneous and staggered oral administration. Drug Metab Dispos. 2002;30:1455–61. doi: 10.1124/dmd.30.12.1455. [DOI] [PubMed] [Google Scholar]
- 32.Hochman JH, Chiba M, Nishime J, Yamazaki M, Lin JH. Influence of P-glycoprotein on the transport and metabolism of indinavir in Caco-2 cells expressing cytochrome P-450 3A4. J Pharmacol Exp Ther. 2000;292:310–8. [PubMed] [Google Scholar]
- 33.Jorajuria S, Dereuddre-Bosquet N, Becher F, Martin S, Porcheray F, Garrigues A, Mabondzo A, Benech H, Grassi J, Orlowski S, Dormont D, Clayette P. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther. 2004;9:519–28. [PubMed] [Google Scholar]
- 34.Murphy RL, Sommadossi JP, Lamson M, Hall DB, Myers M, Dusek A. Antiviral effect and pharmacokinetic interaction between nevirapine and indinavir in persons infected with human immunodeficiency virus type 1. J Infect Dis. 1999;179:1116–23. doi: 10.1086/314703. [DOI] [PubMed] [Google Scholar]
- 35.Burger DM, Prins JM, van der Ende ME, Aarnoutse RE. The effect of nevirapine on the pharmacokinetics of indinavir/ritonavir 800/100 mg BID. J Acquir Immune Defic Syndr. 2004;35:97–8. doi: 10.1097/00126334-200401010-00015. [DOI] [PubMed] [Google Scholar]
- 36.Aarnoutse RE, Grintjes KJ, Telgt DS, Stek M, Jr, Hugen PW, Reiss P, Koopmans PP, Hekster YA, Burger DM. The influence of efavirenz on the pharmacokinetics of a twice-daily combination of indinavir and low-dose ritonavir in healthy volunteers. Clin Pharmacol Ther. 2002;71:57–67. doi: 10.1067/mcp.2002.121424. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107–21. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 38.Lin JH, Chiba M, Chen IW, Nishime JA, Vastag KJ. Sex-dependent pharmacokinetics of indinavir: in vivo and in vitro evidence. Drug Metab Dispos. 1996;24:1298–306. [PubMed] [Google Scholar]