Abstract
Aims
To investigate the effects of co-administration of cimetidine or omeprazole on the pharmacokinetics of escitalopram.
Methods
Two randomized placebo-controlled crossover studies were carried out. Sixteen healthy subjects were administered placebo, or cimetidine (400 mg twice daily) for 5 days (study 1) or omeprazole (30 mg once daily) for 6 days (study 2). On day 4 (study 1) or day 5 (study 2), a single dose of escitalopram (20 mg) was administered. Blood samples were taken at predetermined times for the measurement of serum concentrations of escitalopram and its demethylated metabolite (S-DCT). Treatment-emergent adverse events were also monitored.
Results
Co-administration with cimetidine caused a moderate increase in the systemic exposure [AUC(0, ∞)] to escitalopram (geometric mean ratio = 1.72, [95% CI 1.34, 2.21]) and a small increase in t½ from 23.7 to 29.0 h (5.24 h [3.75, 6.70]). Co-administration with omeprazole also resulted in a moderate increase in the escitalopram AUC(0, ∞) (1.51 [1.39, 1.64]) and a small increase in t½ from 26.5 to 34.8 h (8.3 h [6.44, 10.2]). There was no significant change in S-DCT AUC(0, ∞) after co-administration of either cimetidine or omeprazole. Co-administration of cimetidine or omeprazole had no effect on the incidence of treatment-emergent adverse events.
Conclusions
In view of the good tolerability of escitalopram, the pharmacokinetic changes observed on co-administration with cimetidine or omeprazole are unlikely to be of clinical concern.
Keywords: CYP2C19, CYP2D6, drug interactions, enantiomers, escitalopram, metabolism
Introduction
The selective serotonin reuptake inhibitor antidepressant escitalopram [1] is metabolized in the liver, mainly to the demethylated (S-DCT) and didemethylated (S-DDCT) metabolites by the cytochrome P450 enzymes CYP2C19, CYP3A4 and CYP2D6 [2, 3]. Both metabolites show only weak pharmacological activity in vitro and no activity in vivo[1, 4]. After multiple dosing, the mean concentrations of S-DCT and S-DDCT in plasma are 28–31% and <5% of the parent compound, respectively.
Cimetidine is a histamine H2-receptor antagonist used for the treatment of peptic ulcers and other hypersecretory conditions. It is a nonspecific inhibitor of the cytochrome P450 system and has been shown to inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 [5] although it does not appear to be a significant substrate for the cytochrome P450 system itself [6].
Omeprazole is a proton pump inhibitor that is widely used for the treatment of peptic ulcers and other hypersecretory conditions. It is metabolized predominantly by CYP2C19 to 5′-hydroxyomeprazole, which is further metabolized by CYP3A to 5′-hydroxyomeprazole sulphone [7]. Omeprazole is also metabolized to a lesser extent by CYP3A to omeprazole sulphone and subsequently to 5′-hydroxyomeprazole sulphone by CYP2C19 [7]. The metabolites formed are essentially inactive. Omeprazole and omeprazole sulphone are competitive inhibitors of CYP2C19 and omeprazole is a weak inhibitor of CYP3A, CYP2C9 and CYP2D6 [8]. As cimetidine and omeprazole are inhibitors of the enzymes that metabolize escitalopram, the present studies were undertaken to investigate their potential effects on the pharmacokinetics of this antidepressant drug.
Methods
Two independent randomized double-blind, two-way cross-over studies of identical design were carried out to evaluate the potential effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram.
Subjects
For each study, 16 healthy subjects (aged 18–45 years; 12 male and four female (14 Caucasian and two black subjects) in the cimetidine study and eight male and eight female (15 Caucasian and one black subject) in the omeprazole study were recruited in the UK.
Study design
The studies were designed and conducted in accordance with the principles of the Declaration of Helsinki. The study protocols were approved by the Independent Review Board at Covance, UK, and all subjects gave written informed consent.
Sixteen subjects were administered cimetidine (400 mg twice daily) or placebo for 5 days (study 1) or omeprazole (30 mg once daily) or placebo for 6 days (study 2). On the morning of day 4 (study 1) or day 5 (study 2), a single dose of 20 mg escitalopram was administered. The wash-out period was 3 weeks between the treatments.
Clinical assessment
Body weight, electrocardiograms (ECG), serum biochemistry, haematology and urinalysis were determined at day −1 and day 14 of each phase of the study. Vital signs and adverse events observed by the investigator or spontaneously reported by the subjects throughout the study periods were recorded.
Blood sampling
Venous blood samples (5 ml) were taken for analysis of serum concentrations of escitalopram and S-DCT at predefined sampling times after escitalopram administration.
Drug and metabolite analysis
Serum concentrations of escitalopram and S-DCT were analyzed at the Department of Drug Analysis (H. Lundbeck A/S, Valby, Denmark) by means of an enantioselective high performance liquid chromatography method with tandem mass spectrometry (HPLC MS/MS) detection [9]. Alkalinized serum was extracted with 1.5% isoamyl alcohol in n-heptane, and back-extracted into dilute hydrochloric acid. Mass-spectrometric detection was performed on a Quattro LC (Micromass, Manchester, UK) using positive electrospray ionization in the multiple reaction-monitoring mode. The lower limit of quantification in serum was 3.08 nmol l−1 for escitalopram and 3.22 nmol l−1 for S-DCT. The accuracy was ±2% across all levels and the precision varied between 4 and 8%.
Genotyping
CYP2D6 and CYP2C19 were determined according to standard methods. The results were only used to aid interpretation of the pharmacokinetic results.
Pharmacokinetic analysis
The pharmacokinetic parameters for escitalopram and S-DCT (AUC(0, ∞), Cmax, tmax and t½) were estimated using standard noncompartmental methods.
Statistical analysis
Comparisons were made by analysis of variance or nonparametric methods. Geometric least squares means ratios and their 95% confidence intervals were calculated.
Results
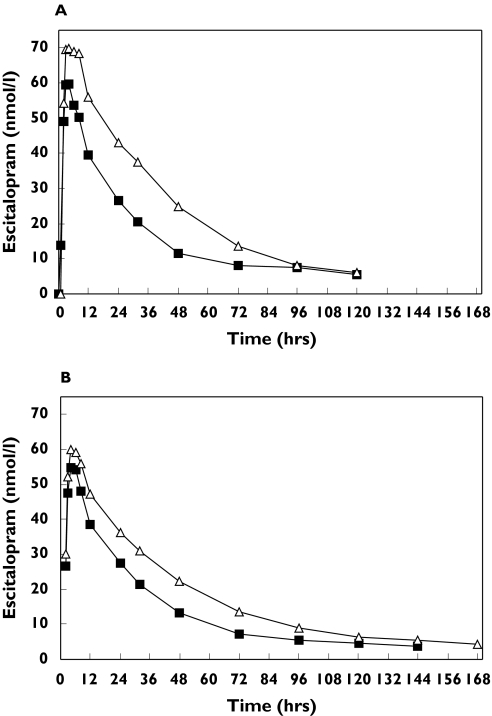
Cimetidine (400 mg twice daily) caused a significant increase of 72% in the AUC(0, ∞) for escitalopram given as a single oral dose (Table 1, Figure 1a). The Cmax for escitalopram was slightly (+22%), but nonsignificantly, greater following cimetidine co-administration.
Table 1.
The effect of multiple dosing with cimetidine or omeprazole on the pharmacokinetics of single dose escitalopram in healthy subjects (n = 16 for each study)
| Parameter | + cimetidine A | + placebo B | Least squares geometric mean ratios (95% CI) (A : B) |
|---|---|---|---|
| AUC(0, ∞) (nmol l−1 h) | 3188 (1612–5935) | 1849 (916–5196) | 1.72 (1.34, 2.21) |
| Cmax (nmol l−1) | 75.5 (44.3–150) | 61.8 (35.6–150) | 1.22 (0.99, 1.51) |
| tmaxa (h) | 3.0 (2.0–8.0) | 3.5 (2.0–6.0) | 0.5 |
| t1/2 (h) | 29.0 (21.1–36.7) | 23.7 (15.3–34.7) | 5.24b (3.75–6.70) |
| + omeprazole C | + placebo D | Least squares means ratios (95% CI) (C : D) | |
|---|---|---|---|
| AUC(0, ∞) (nmol l−1 h) | 2929 (1460–6085) | 1937 (1182–3320) | 1.51 (1.39,1.64) |
| Cmax (nmol l−1) | 65.7 (45.4–111) | 60.4 (46.9–88.8) | 1.09 (1.01,1.17) |
| tmaxa (h) | 4.0 (1.0–8.0) | 4.0 (1.0–6.0) | 0.0 |
| t½ (h) | 34.8 (21.7–47.6) | 26.5 (18.6–36.2) | 8.30b (6.44–10.2) |
AUC(0, ∞) = area under the serum concentration-time curve from time zero to infinity, Cmax = maximum observed serum concentration, tmax = time of maximum observed serum concentration, t½ = serum terminal elimination half-life. Treatment A: 20 mg escitalopram with 400 mg cimetidine (twice daily) (study 1), treatment B: 20 mg escitalopram with placebo (twice daily) (study 1), treatment C: 20 mg escitalopram with 30 mg omeprazole (once daily) (study 2), treatment D: 20 mg escitalopram with placebo (once daily) (study 2).
= arithmetic median and median difference,
= arithmetic least squares mean difference.
Figure 1.
Serum concentrations of escitalopram (nmol l−1) after administration of escitalopram 20 mg alone and (A) with cimetidine (400 mg twice daily on day 4 of 5 days treatment) and (B) with omeprazole (30 mg once daily on day 5 of 6 days treatment). Escitalopram (▪), escitalopram + cimetidine (▵) or escitalopram + omeprazole (▴)
The tmax values were similar, but cimetidine caused a small but significant prolongation of the elimination half-life (t½) of escitalopram (Table 1).
Co-administration of escitalopram with cimetidine did not significantly modify the AUC(0, ∞) of S-DCT. However, Cmax was significantly decreased by 20%. Maximal concentrations of S-DCT were attained only slowly and the tmax was significantly longer following cimetidine (37 vs. 21 h).
Omeprazole (30 mg once daily ) caused a significant increase (+51%) in the AUC(0, ∞) for escitalopram given as a single 20 mg oral dose (Table 1, Figure 1b). Cmax was increased slightly (+9%) but significantly following omeprazole co-administration. Maximal concentrations of escitalopram occurred at similar times (approximately 4 h postdose) following administration of omeprazole or placebo. The elimination half-life (t½) of escitalopram following co-administration with omeprazole was significantly prolonged.
Systemic exposure to the S-DCT metabolite of escitalopram was similar following administration of omeprazole or placebo. However, the Cmax of S-DCT was significantly lower (−21%) following omeprazole co-administration. This was consistent with an observed prolongation of the tmax (33 vs. 22 h).
A single CYP2D6 poor metabolizer was identified in each of the two studies. However, the results from these subjects were within the ranges of those from the other subjects.
Twelve to 15 of the 16 subjects reported at least one treatment-emergent adverse event (TEAE) following each treatment phase. The majority of TEAEs, most commonly nausea, headache, yawning, dizziness, and abdominal pain, were mild in intensity and their incidence was similar when escitalopram was given alone and with cimetidine or omeprazole.
Discussion
The inhibitory effect of cimetidine or omeprazole on the cytochrome P450 system caused a significant increase (+72 or +51%, respectively) in the AUC values for escitalopram. The terminal elimination half-life of escitalopram was also increased by both treatments. Consistent with the decrease in escitalopram clearance, the rate of formation of the primary metabolite S-DCT appeared to be slower and maximal concentrations were attained less rapidly after cimetidine or omeprazole. However, no apparent change in systemic exposure to this metabolite was observed.
In a previous study cimetidine increased the AUC of racemic citalopram, given in multiple doses, by 43%[10]. Although a nonenantioselective analysis method was used, these results are consistent with those reported in the present study.
All drug regimens were equally well tolerated. The majority of TEAEs were reported after the dose of escitalopram, and showed a similar incidence, type and severity in the presence or absence of cimetidine or omeprazole. The most frequently reported TEAEs (headache and nausea) were consistent with those previously associated with citalopram and escitalopram [1].
In conclusion, repeated administration of either cimetidine or omeprazole caused moderate increases in the systemic exposure to escitalopram given as a single dose and a small increase in its terminal elimination half-life in healthy subjects. Because escitalopram is well tolerated, the magnitude of the observed pharmacokinetic changes on escitalopram produced by cimetidine or omeprazole are unlikely to be of clinical concern.
Acknowledgments
Competing interests: None declared.
We thank M. Briley, PhD, for assistance with the manuscript, and Janet Ward, Covance for assistance with the conduct of the study.
References
- 1.Waugh J, Goa KL. Escitalopram: a review of its use in the management of major depressive and anxiety disorders. CNS Drugs. 2003;17:343–62. doi: 10.2165/00023210-200317050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Herrlin K, Yasui-Furukori N, Tybring G, Widén J, Gustafsson LL, Bertilsson L. Metabolism of citalopram enantiomers in CYP2C19/CYP2D6 phenotyped panels of healthy Swedes. Br J Clin Pharmacol. 2003;56:415–21. doi: 10.1046/j.1365-2125.2003.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Moltke LL, Greenblatt DJ, Giancarlo GM, Granda BW, Harmatz JS, Shader RI. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Disp. 2001;29:1102–9. [PubMed] [Google Scholar]
- 4.Baumann P, Larsen F. The pharmacokinetics of citalopram. Rev Contemp Pharmacother. 1995;6:287–95. [Google Scholar]
- 5.Knodell RG, Browne DG, Gwozdz GP, Brian WR, Guengerich FP. Differential inhibition of individual human cytochrome P-450 by cimetidine. Gastroenterology. 1991;101:1680–91. doi: 10.1016/0016-5085(91)90408-d. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues AD. Drugs and the Pharmaceutical Sciences Series. Vol. 116. New York: Marcel Dekker Inc; 2002. Drug–drug interactions. [Google Scholar]
- 7.Rost KL, Roots I. Non-linear kinetics after high-dose omeprazole caused by saturation of genetically variable CYP2C19. Hepatology. 1996;23:1491–7. doi: 10.1002/hep.510230628. [DOI] [PubMed] [Google Scholar]
- 8.Ko J-W, Sukhova N, Thacker D, Chen P, Flockhart DA. Evaluation of omeprazole and lanzoprazole as inhibitors of cytochrome P450 isoforms. Drug Metab Dispos. 1997;25:853–62. [PubMed] [Google Scholar]
- 9.Gutierrez MM, Rosenberg J, Abramowitz W. An evaluation of the potential pharmacokinetic interaction between escitalopram and the cytochrome P450 3A4 inhibitor ritonavir. Clin Ther. 2003;25:1200–9. doi: 10.1016/s0149-2918(03)80076-0. [DOI] [PubMed] [Google Scholar]
- 10.Priskorn M, Larsen F, Segonzac A, Moulin M. Pharmacokinetic interaction study of citalopram and cimetidine in healthy subjects. Eur J Clin Pharmacol. 1997;52:241–2. doi: 10.1007/s002280050282. [DOI] [PubMed] [Google Scholar]