Abstract
Background
Therapeutic failure with antiretroviral therapy (ART) is a substantial issue where viral rebound, viral resistance and drug-related toxicity remain serious concerns. Drug exposure-response relationships have been described for the protease inhibitors, pharmacokinetic variability is substantial for this class of drugs and drug interactions can also alter ART exposure. Given this background we established a therapeutic drug monitoring (TDM) service to monitor atazanavir (ATV) plasma concentrations early after the therapy was made available to treatment-experienced people infected with HIV who were managed in a clinical setting.
Methods
This was a prospective observational study which evaluated plasma samples from 110 highly treatment-experienced people with HIV using TDM and applied pharmacokinetic analysis over a five month period.
Results
ATV trough concentrations exhibited substantial intersubject variability (<25–2108 µg l−1). A substantial number of subjects (50%,13/26) who received ATV400 mg daily had low exposure to ATV. Serum bilirubin concentrations correlated significantly with higher ATV trough concentrations (ρ = 0.803; P < 0.001) and 55% (29/53) of subjects who received ATV300/100 mg RTV daily had plasma concentrations above a proposed target concentration associated with elevated bilirubin concentrations. This study confirmed low ATV exposure in eight subjects with HIV receiving ATV 400 mg daily. Reasons for low ATV exposure in this cohort include administration of interacting drugs, including a possible interaction with ritonavir, fluticasone and ATV, impaired ATV absorption secondary to suspected achlorhydria and potential interactions with colchicine and nandrolone. Viral load remained undetectable in most of these subjects with low ATV exposure.
Conclusions
TDM and targeted pharmacokinetic studies should be viewed as fundamental tools in the development and clinical application of ART, to improve pharmacotherapy for people with HIV.
Keywords: HIV, atazanavir, pharmacokinetics, therapeutic drug monitoring, toxicity
Introduction
Forty per cent of therapy-naïve people with HIV experience virological failure during the first two years of antiretroviral therapy (ART), with low plasma concentrations of antiretroviral drugs predictive of more rapid immunological failure and failure to achieve virological success in the first year of therapy [1]. Furthermore, a substantial number of people receiving ART discontinue therapy in the first 45 weeks of treatment, the majority stop therapy because of drug related toxicity [2]. The therapeutic strategy of giving the same antiretroviral dose to all people has been challenged and therapeutic drug monitoring (TDM) has been proposed as an alternative strategy to individualize therapy for people with HIV [3].
Atazanavir (ATV) is an azapeptide protease inhibitor. Unlike other protease inhibitors ATV has not been associated with abnormal lipid profiles [4]. Furthermore, ATV has a unique pharmacokinetic profile among the protease inhibitors and is suitable for once-daily oral administration [4]. A clinical study in 56 treatment-naïve people with HIV assessing the efficacy of ATV has shown that exposure measured by area under the concentration–time curve (AUC) was a predictor of viral suppression and people with higher AUCs were more likely to have an increased serum bilirubin concentration [5]. This study evaluated therapeutic and safety response relationships of ATV at three doses (200, 400, and 500 mg daily) given as monotherapy for 2 weeks before commencing stavudine and didanosine [5]. Logistic regression identified low AUC as a predictor of failure to achieve 1.5 log reduction in HIV RNA [5]. Furthermore, the probability of an increase in serum bilirubin concentration (>43 µmol l−1) was greater in subjects with higher ATV AUC values. Similar drug exposure-response relationships have been demonstrated for other protease inhibitors [6, 7].
The management of HIV infection with antiretroviral therapy is complex. Interpatient variability in the absorption, distribution and elimination of antiretroviral agents is substantial [3]. The effect of food on drug absorption is variable, and antiretroviral drug–drug interactions and interactions with other drugs or complementary medicines can also alter ART exposure [1]. Furthermore, pharmacokinetic ‘boosting’ of ART with low doses of ritonavir (typically 100 mg once or twice daily), one of the most potent drug metabolizing inhibitors adds more complexity to ART therapy [8]. The rising complexity of ART regimens has increased the risk of adverse outcomes caused by prescribing errors [9] and research has shown that polypharmacy in general practice has been associated with adverse drug reactions [10]. Major polypharmacy (concurrent use of five or more drugs) is not uncommon in people with HIV and there may be substantial drug interactions that have not yet been identified. Furthermore, in the clinic setting there are many clinical and social variables, logistical complexities, and cost considerations that have the potential to alter therapeutic effectiveness and safety [11, 12]. Therefore, given this background we established a TDM service at St. Vincent's Hospital, Sydney, Australia to monitor ATV pharmacotherapy, which was made available through a special access programme to treatment-experienced people with HIV who were treated in a clinic setting. The aim of this study is to report a series of interesting clinical observations from the early use of ATV therapy by primary care practitioners and highlight the utility of TDM and the importance of surveillance when new antiretroviral therapies are introduced in the clinic.
Methods
This is a prospective observational study which evaluated TDM data from a cohort of 110 people with HIV who received ATV over a 5-month period.
A therapeutic drug monitoring service for ATV was established at St. Vincent's Hospital, Sydney, in December 2003 and 160 requests for ATV drug monitoring from 110 treatment-experienced people with HIV (who received ART for at least 1 year) were received over a 5-month period. The mean age of the study participants receiving 300 mg ATV plus 100 mg RTV daily (ATV300/r) or 400 mg ATV daily (ATV400) was 46 ± 9 and 46 ± 11 years, respectively. There were only two females in the study cohort. Forty-two requests (26%) were rejected because of insufficient information to interpret the result (e.g. time of last dose, sample time or dose missing). Clinical data from people who received ATV300/r (n = 92) or ATV400 (n = 26) were reviewed after gaining approval from the St. Vincent's Hospital Human Research Ethics Committee. Plasma samples for TDM were collected at various times over the dosing interval for ATV300/r: 0–8 h (n = 5), 8–16 h (n = 20) and 16–30 h (n = 67) and ATV400: 8–16 h (n = 2) and 16–30 h (n = 24). Although a 24 h trough collection was preferred, when samples were collected at other times the estimated trough concentration was calculated using standard pharmacokinetic formulae [13] and half-lives reported for ATV300/r [14] and ATV400 given as a single daily doses [15]. In some people samples were collected more than 24 h after the previous ATV dose which reflects the logistical complexity of sampling in the clinic setting.
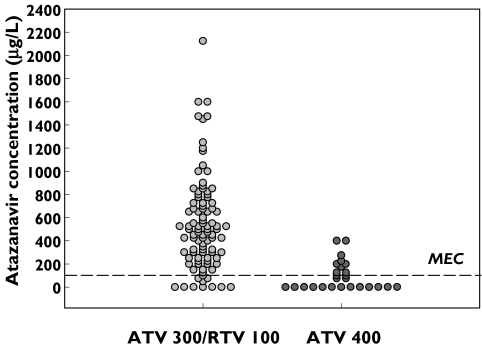
A number of people (11 of 20; Figure 1) with trough plasma ATV concentrations below the limit of detection (25 µg l−1) of the assay and who agreed to participate, were selected for pharmacokinetic evaluation. Each person was interviewed to assess medication adherence and medical records were examined to identify potential interacting drugs. Steady-state pharmacokinetic analysis was performed on 11 people (eight received 400 mg ATV daily and three ATV300/r daily) who had plasma samples collected 0, 3, 6, 9 and 24 h after an observed ATV dose was taken with a standard meal (14 g fat; 1900 kJ). After the 24 h sample was collected the next ATV dose was given with 100 ml of an acidic beverage (Coca-Cola Classic, pH 2.5) and a single 3 h blood sample was collected to observe the effect on ATV concentrations. Subjects were reluctanct to participate in a second pharmacokinetic study to assess the effect of the acidic beverage so a single 3 h collection was selected to represent an approximate maximum plasma concentration that occurred 2–3 h after the dose [15]. One person who received RTV, fluticasone and ATV and who had clinical signs of Cushing Syndrome was studied for a possible dual drug interaction. This person used inhaled corticosteroids for asthma prophylaxis (fluticasone, 250 µg bid) with ‘boosted’ ATV for 7 months before TDM was done. ATV pharmacokinetics were studied in the presence of RTV and fluticasone after an observed dose of ATV300/r was taken with a standard meal. Plasma samples were collected at 0, 3, 6, 9 and 24 h after the ATV300/r dose. The subsequent dose of ATV300/r was taken with 100 ml of an acidic beverage (Coca-Cola Classic, pH 2.5) and a 3 h blood sample was collected to examine the effect on the ATV concentration. A second ATV pharmacokinetic study was conducted 28 days after fluticasone was ceased.
Figure 1.
Distribution of observed trough ATV plasma concentrations in 92 people with HIV receiving ATV300/RTV100 mg daily and 26 people receiving ATV 400 mg daily (MEC is the minimum effective concentration required to suppress viral replication [5])
ATV plasma concentrations were quantified by high-performance liquid chromatography (HPLC) adapted from a previously reported method [16]. Plasma (0.5 ml) and standards were extracted with 5.0 ml 1-chlorobutane. After mixing and centrifugation the organic solvent was evaporated to dryness and the residue resuspended in mobile phase (0.5 ml acetonitrile: 10 m m potassium phosphate buffer pH 7.0; 50 : 50). The mobile phase was washed twice with 3 ml 99% hexane and 50 µl was injected onto the HPLC column. Separation was performed on a phenyl hexyl column (250 × 4.6 mm; 5 µm) with ultraviolet detection at 205 nm. The ATV calibration standards ranged from 50 to 10 000 µg l−1 (r = 0.999) and the lower limit of ATV quantification was 50 µg l−1. Precision was better than 6% RSD and accuracy was within 4% of the expected values for the assay. Cross validation of the ATV assay was done by analysis of selected samples, calibrators and quality control aliquots by an independent laboratory (Tandem Laboratories, Ewing, NJ, USA). Comparison of all results showed a difference of <17% and samples submitted for validation that were reported as <25 µg l−1 (LOD) for this study were recorded as <5 µg l−1 by the reference laboratory. All measurements of HIV-1 RNA and CD4 cell counts were performed at one of two laboratories and were collected as standard of care for most patients. The detection limit of the HIV-1 RNA assays was 50 copies ml−1.
Population pharmacokinetic analysis was performed on data from 77 people including 90 ATV plasma concentrations that were collected at various times over the dosing interval. Additional measurements were taken from four subjects to obtain a full concentration–time profile, with five blood samples drawn over 24 h. These data were combined for the population pharmacokinetic analysis of ATV300/r daily. Population pharmacokinetic analysis was also performed on data from 13 patients (excluding those patients with low ATV exposure) who received ATV400 and had ATV plasma concentrations collected at various times over a dosing interval. Concentration–time rich data sets were obtained from four additional subjects and combined with the single data points collected from the other 13 subjects to perform the population pharmacokinetic analysis in subjects who received ATV400 daily. All samples were obtained during steady-state conditions and patients received ATV daily doses in combination with other antiretroviral agents. Population pharmacokinetic analysis was performed using Kinetica V 4.2 (InnaPhase Corp. PA, USA) [17]. A two-compartment pharmacokinetic model with first-order absorption was selected for the population analysis and initial pharmacokinetic estimates were selected from previously published reports [4, 5]. The adequacy of the pharmacokinetic model in describing the data was evaluated by using AIC, visual examination of the predictions for individual concentration–time profiles, the distribution pattern of the scatter plot of observed vs. predicted concentrations of the drug in plasma, statistical comparison of observed vs. predicted concentrations of the drugs in plasma by Student paired t-test, and visual inspection of the weighted residual and individual pharmacokinetic parameter distribution probability curves.
Pharmacokinetic analyses were also performed on data sets obtained from patients with low exposure to ATV and who provided multiple blood samples over the dosing interval using Kinetica V 4.2. Statistical tests (e.g. correlation analysis using Spearman Rank Correlation for data that was not normally distributed) were performed with Sigma Stat (version 2.03; SPSS Inc., Chicago, IL).
Results
ATV exhibited substantial interpatient variability with observed median Cmin concentrations of 30 µg l−1 (range <25–390 µg l−1) for ATV400 and 476 µg l−1 (range <25–2108 µg l−1) for ATV300/r (Figure 1). The median ATV Cmin was significantly higher in the ATV300/r regimen (P < 0.001; Mann–Whitney Rank Sum Test) when compared with the ATV400 effect target desired from the regimen. Eighteen per cent of results were below the protein-binding adjusted IC90 of ATV for Wild Type virus (100 µg l−1; personal communication Bristol-Myers Squibb). Additionally, 49% of ATV results from subjects who received ATV300/r were greater than the suggested concentration associated with elevated serum bilirubin concentrations [5]. Estimates of the ATV pharmacokinetic parameters were generated from sparse data sets using population modelling as indicated by optimal goodness-of-fit criteria (e.g. observed vs. calculated plasma concentrations correlated significantly for ATV300/r; ρ = 0.998, P < 0.0001 and ATV 400; ρ = 0.989, P < 0.0001). Population pharmacokinetic analysis in people receiving ATV400 found the individual Bayesian model-based estimates of half-life varied from 1.9 to 4.1 h and that 13% of people receiving ATV300/r had a half-life <4 h (median half-life was 5.1 h; range 2.3–11.2 h in the ATV300/r cohort).
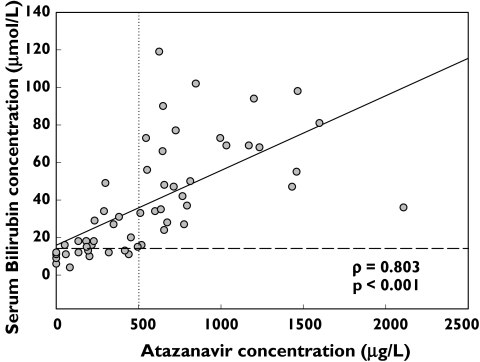
Figure 2 shows the relationship between serum bilirubin concentrations and the steady-state observed Cmin concentration of ATV. Serum bilirubin concentrations correlated significantly with higher ATV Cmin concentrations (ρ = 0.803; P < 0.001, Figure 2). Only 9 of 26 ATV Cmin concentrations (35%) that were below the ATV concentration thought to correlate with raised serum bilirubin (500 µg l−1[5]), produced abnormal serum bilirubin results (median 15.0; range 4–49 µmol l−1, Figure 2); however, all ATV Cmin plasma concentrations >500 µg l−1 were associated with abnormal serum bilirubin results (median 55.5; range 24–119 µmol l−1, Figure 2).
Figure 2.
Spearman correlation between serum bilirubin and observed ATV trough plasma concentrations for people receiving 300 ATV/RTV 100 mg daily. Horizontal dashed line represents normal serum billirubin target of 18 µmol l−1. Vertical dotted line (500 µg l−1) is the suggested target above which the probability of serum bilirubin elevation >43 µmol l−1 is likely [5]. Solid line is the regression line
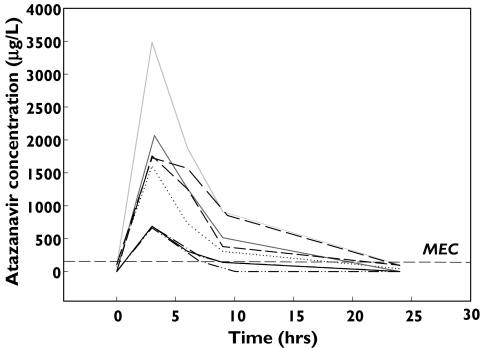
A number of ATV trough plasma concentrations below the limit of detection (25 µg l−1) of the assay (Figure 1) were recorded. Seven people who received ATV300/r and 13 people who received ATV400 had trough plasma concentrations reported as ‘not detected’. Eight of 13 people receiving ATV400 were available to be interviewed by their primary care practitioners who were confident that nonadherence was unlikely in this group of people. A detailed review of each person's medical history (including self-administered complementary medicines) revealed coadministration of medicines suspected to interact with ATV in two patients (efavirenz and esomeprazole). Multiple timed samples over a dosing interval were obtained from eight people who received ATV400 and this confirmed low ATV exposure (Figure 3 and Table 1) compared with population mean pharmacokinetic data from people with HIV reported elsewhere [18]; the mean area under the curve (AUC0−24) was 13 027 µg l−1 h (range 3,499–23 354; n = 8) in this cohort of people with low ATV exposure, compared with the population mean AUC0−24 reported in people receiving standard dosing regimens (22 262 µg l−1 h; SD 20 159, Table 1). Furthermore, the mean model-based estimate of ATV half-life tended to be lower in this group (3.8 h; range 1.7–5.1 h) compared with people infected with HIV who received ATV400 daily (mean half-life 6.5 ± 2.6 h [15]. Three additional patients who received ATV400 showed a 23–300% increase in Cmax after administration of the ATV dose with an acidic beverage (Coca-Cola) suggesting increased absorption of ATV under acidic conditions (Table 1). Colchicine and nandrolone were coadministered with ATV in two other patients and a possible interaction with these agents is suspected and worthy of further investigation. In one patient there was no clear reason for the observed low ATV exposure. Surprisingly, viral load remained undetectable (<50 copies ml−1) in these patients with low ATV exposure. Seven of eight subjects who had received ATV 400 mg daily for at least 2 months (range 2–6 months) had low ATV exposure and undetectable viral loads. These subjects received dual or triple nucleoside therapy with ATV 400 mg daily.
Figure 3.
Observed ATV concentration–time profiles in 8 people with HIV receiving 400 mg daily who had undetectable trough plasma concentrations (MEC is the minimum effective concentration required to suppress viral replication [5])
Table 1.
Pharmacokinetic parameters at steady-state for eight people with HIV who had low ATV exposure after an ATV 400 mg daily dose compared with data in HIV patients who received the same dose [18]
| AUC0−24µg l−1 h | Tmax (h) | Cmaxµg l−1 | Cmaxµg −1 plus Coke | C0µg l−1 | C24µg l−1 | Half-life (h) | |
|---|---|---|---|---|---|---|---|
| HIV patients* | 22 262 (20 159) | 2.0 | 3152 (2231) | NA† | – | 273 (298) | 6.5 (2.6) |
| Patients | |||||||
| 1 | 9 574 | 3.0 | 1605 | 1331 | 77 | 42 | 4.6 |
| 2 | 23 354 | 3.0 | 3485 | ND | 74 | 104 | 4.5 |
| 3 | 11 599 | 3.2 | 2065 | 576 | <25 | <25 | 1.7 |
| 4 | 12 970 | 3.0 | 1756 | 2154 | <25 | 94 | 5.1 |
| 5 | 17 167 | 3.0 | 1728 | 2793 | 106 | 89 | 4.4 |
| 6 | 3 499 | 3.0 | 689 | 1934 | <25 | <25 | 2.2 |
| 7 | 4 229 | 3.0 | 685 | ND‡ | <25 | <25 | 2.6 |
| 8 | 2 877 | 3.0 | 664 | 3347 | <25 | <25 | ND§ |
HIV Patients data expressed as Mean (SD);
NA = not applicable;
ND = not done, ATV dose not given with Coca-Cola in this patient;
ND = not done, insufficient samples on terminal phase to calculate half-life; AUC0−24 (area under the curve from 0 to 24 h), Tmax (time to maximum ATV plasma concentration), Cmax (maximum ATV plasma concentration), C0 (ATV plasma concentration just before observed dose was given), C24 (ATV plasma concentration 24 h after the observed dose was given), half-life (elimination half-life).
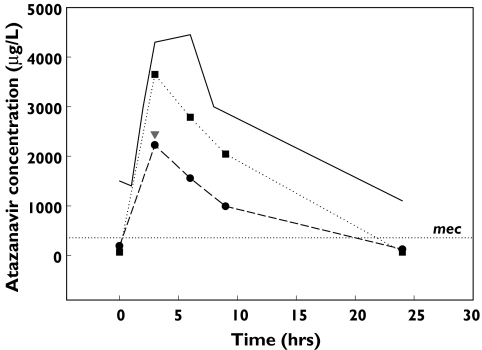
Only three of seven subjects who received ATV300/r and who had ATV plasma concentrations below the limit of detection of the assay, were interviewed to confirm their adherence to the prescribed regimen and had their medical records reviewed to assess potential drug interactions. Pharmacokinetic studies in three people confirmed low exposure to ATV. Coadministration of esomeprazole resulting in raised gastric pH and poor ATV solubility was believed to be responsible for low ATV exposure in one person. Review of a second case identified a subject with Cushing Syndrome and a possible interaction between fluticasone, RTV and/or ATV was suspected. This subject was a 44-year-old Caucasian male who had been HIV positive since 1986 and had a long history of ART. The patient also had longstanding hypertension and asthma and commenced fluticasone (Flixotide) accuhaler 500 µg bid and eformoterol (Oxis) 12 µg bid 2 years earlier. His asthma had been well controlled. ART was altered to include lopinavir/ritonavir and the subject received concomitant therapy with fluticasone for 11 months before being switched to ATV300/r. Seven months later the patient was reviewed by a general practitioner who diagnosed steroid related side-effects including mild Cushingoid appearance, bruising, acne, hirsutism, mild hypertension and low serum cortisol concentrations. The physician concluded that the patient had iatrogenic Cushing Syndrome, probably due to inhibition of fluticasone metabolism by RTV and/or ATV. Multiple blood samples were collected over the dosing interval to confirm low ATV exposure. Fluticasone was ceased and the subject was started on montelukast (Singulair, Merck Sharp and Dohme (Aust) Pty Ltd, Granville, NSW, Australia; 10 mg daily) before a second pharmacokinetic study was performed after a two week washout period. The AUC for ATV increased from 21 447 µg h−1 l during coadministration of fluticasone to 38 384 µg h−1 l when fluticasone was ceased in this person (Figure 4). There was no effect from coadministration of an acidic beverage in this patient and while the data suggest that an interaction between fluticasone, RTV and ATV is possible the mechanism is unknown and requires further investigation. The second of these three patients with low ATV exposure who received ATV300/r also received this combination of fluticasone, RTV and ATV. The three patients who had received ATV300/r for at least 2 months (2, 5 and 6 months for the three subjects) had undetectable viral loads (<50 copies ml−1) over this period despite low exposure to ATV.
Figure 4.
Concentration–time profile in a person with HIV receiving boosted ATV demonstrating a dual interaction between RTV, fluticasone and ATV. 400/100 + fluticasone (•), HIV pop 300/100 ( ), 400/100 + fluticasone + cola drink (▾), 400/100 − fluticasone (▪)
), 400/100 + fluticasone + cola drink (▾), 400/100 − fluticasone (▪)
Discussion
We report the results of a prospective observational study using TDM as a surveillance tool to monitor ATV pharmacotherapy in treatment-experienced people managed in a clinic setting. A number of observations uncovered during TDM were evaluated using assessment of subjects by interview, review of medical records and subsequent pharmacokinetic analysis. ATV was found to exhibit substantial pharmacokinetic variability when given as an ATV400 or ATV300/r daily dose which is consistent with previously reported data [15, 19]. Thirteen per cent of people in this cohort who received ATV300/r had a half-life <4 h while the mean ATV half-life reported for people infected with HIV receiving the same dose was 6.5 ± 2.6 h [15]. People with shorter half-lives of ATV may need regular monitoring and dose individualization to prevent low exposure to ART. Based on these observations and those of others the current therapeutic practice in ART of giving the same dose to all subjects (a ‘one-size- fits-all’ dosing strategy) without regard to differences in systemic exposure caused by pharmacokinetic variability may contribute to variability in response [20]. The characterization of the pharmacokinetics of a drug in the patient population of interest with particular consideration of influential factors and other patient related variables provides understanding to allow optimization of drug dose.
This study confirmed a relationship between elevated serum bilirubin concentration and higher ATV concentrations, and the data appear to support a derived target of 500 µg l−1 above which serum bilirubin concentrations are likely to double (Figure 2;[5]). However this preliminary safety target concentration requires further validation in a prospective cohort of people with HIV taking ART.
ART drug–drug interactions are complex and at times unpredictable [21, 22]. This study has described a possible drug interaction between RTV, fluticasone and ATV. Additionally, observations from this study suggest that interaction studies involving colchicine and nandrolone are worthy of further investigation. ATV is metabolized by the CYP3A4 isoenzyme, and many substrates of this drug metabolizing enzyme family are known to increase or reduce drug activity. When the complications of major polypharmacy (concurrent use of five or more drugs [10]) and complementary medicines are considered it would be reasonable to assume that many interactions have not been identified or their significance studied. Systemic availability of inhaled corticosteroids is generally low but considerable intersubject variability in the extent of systemic absorption and diurnal variation in fluticasone pharmacokinetics has been reported [23]. A drug interaction between a metabolic inhibitor of CYP3A4 (itraconazole) and inhaled corticosteroids causing Cushing Syndrome in a patient has been described [24]. The coadministration of RTV and fluticasone is not recommended due to the ability of RTV to inhibit CYP3A4 mediated fluticasone metabolism causing iatrogenic Cushing Syndrome [25–27]. Furthermore, some corticosteroids are known inducers of the P-gp efflux transporter protein [28] and cytochrome metabolizing enzymes that can cause decreased exposure to drugs [29]. The mechanism of the interaction between RTV, fluticasone and ATV is unclear. One possible explanation is that RTV inhibited the metabolism of fluticasone which caused an increased systemic accumulation of this agent subsequently leading to Cushing Syndrome and low exposure to ATV caused by metabolic induction or low systemic availability due to increased expression of P-gp. Interestingly, the subject had been receiving a combination of RTV, fluticasone and lopinavir for 11 months prior to the introduction of ATV without signs of Cushing Syndrome. The onset of Cushing Syndrome in patients who received RTV and fluticasone is variable (5–12 months [25–27]) and may be related to the frequency of use of fluticasone which we did not monitor in this study. We have reported two subjects with low ATV exposure who received either colchicine or nandrolone, two drugs that potentially interact with ATV. A reversible malabsorption syndrome has been reported for colchicine [30] and it is known to be an inducer of P-gp [31]. Few interactions have been reported for testosterone or nandrolone, two anabolic steroids that are regular pharmacotherapy in patients infected with HIV. Testosterone has been shown to induce P-gp activity 1.7-fold in vitro and the hydroxylation on the carbon at position 20 appears to be essential for this activity [28]. This hydroxylation is present in nandrolone [28]. Furthermore, endogenous steroids have been shown to activate CYP3A4 mediated metabolism and interactions by some drugs (e.g. nevirapine) with endogenous steroids in the active site of CYP3A4 can alter the activity of this isoenzyme [32]. For example, nevirapine 2-hydroxylation was strongly activated by many endogenous steroids including testosterone [32]. The effect of testosterone and nandrolone on ART metabolism is yet to be rigorously investigated.
ATV is a drug that is only slightly soluble in water and the aqueous solubility decreases when the pH of the solution is above pH 3.0 [15]. Adequate gastric acidity is required for dissolution and absorption of the drug. Reduced plasma concentrations of ATV are expected when the drug is used in combination with antacids, buffered medications, H2-receptor antagonists and proton-pump inhibitors [15]. Impaired absorption of ketoconazole secondary to achlorhydria in healthy subjects and patients with HIV has been reported [33]. The systemic absorption in achlorydrics was increased when ketoconazole was coadministered with a palatable acidic beverage (Coca-Cola Classic, pH 2.5) [33]. In this study we reported a similar response to ATV (400 mg daily dose) absorption after the administration of an acidic beverage in three people infected with HIV. While the extent of this problem is unknown, TDM is recommended in people who receive ATV to help identify people at risk of potential therapeutic failure.
A surprising finding in this study was the undetectable viral load in 10 of 11 people who had low exposure to ATV. All people in this study were highly treatment-experienced and had received ATV for at least 2 months before low ATV exposure was detected using TDM. These subjects had low viral loads for at least 1 year before ATV was commenced. The implications of this observation are unclear but perhaps people with undetectable viral loads can tolerate short periods of low drug exposure over a dosing interval for one drug within triple drug regimens. A ‘post antibiotic-like’ effect that is dependent on the intracellular pharmacokinetics of the protease inhibitor may be an important determinant of efficacy [34]. A second study simulating effect-relationships for dual combination therapy has shown that drug effect can persist for 3 h after the plasma concentration of the drug has fallen below the minimum effective concentration for viral suppression [35]. Furthermore, 2–6 months low exposure to ATV may be too short a period for viral rebound to occur in this cohort. It takes longer for virus to grow to above detection limits when the virus count is already suppressed to a very low level [36]. Huang et al.[36] also simulated HIV dynamics when every other dose of one drug in HAART was missed, a pattern of imperfect adherence not dissimilar to the low ATV exposure we reported in this cohort. In this simulation viral load did not rebound for at least 200 days [36]. Additional evidence of slow evolution of viral rebound in patients who have undetectable viraemia (<500 copies ml−1) and are adherent to HAART but have plasma drug concentrations below the minimum effective concentration suggests a period of approximately 32 weeks is necessary for virological rebound to occur [37]. Therefore, it would be reasonable to suggest that measuring both adherence and plasma drug concentrations in patients with suppressed viral load may be useful to predict future virological rebound. These data suggest that the clinical practitioner has two therapeutic strategies to consider. A ‘wait and see’ approach where a standard dose is given to all patients, regular monitoring is practised until a clinical change occurs (viral suppression, therapeutic failure or drug related toxicity). While this approach has proven effective, therapeutic failure, viral resistance and drug related toxicity remain serious concerns [1, 2]. Alternatively, a pre-emptive approach would apply TDM, consider this result with all relevant clinical information, intervene to educate the patient, educate the practitioner, change the regimen, change dose, etc. and monitor clinical outcome. Data from randomized-controlled trials suggest that the latter strategy is worthy of consideration [38, 39].
The results of this observational study in a cohort of people with HIV who received ATV suggest that TDM and applied clinical pharmacokinetic studies are fundamental tools for optimizing pharmacotherapy in people infected with HIV. Early intervention in the clinical development of new antiretroviral therapy using these tools can provide vital information to inform prescribers and facilitate the quality use of medicines.
Acknowledgments
The authors gratefully acknowledge the financial support of Bristol-Myers Squibb Pharmaceuticals for funding the development of the assay to quantify atazanavir in plasma and for the supply of atazanavir pure substance. We also thank Edna Pang and Dee Roan-Brown from the Division of Clinical Pharmacology & Toxicology, for their excellent technical assistance.
No conflicts of interests are declared.
References
- 1.Alexander CS, Asselin JJ, Ting SL, Montaner SG, Hogg RS, Yip B, O'Shaughnessey MV, Harrigan PR. Antiretroviral concentrations in untimed plasma samples predict therapy outcome in a population with advanced disease. J Infect Dis. 2003;188:541–8. doi: 10.1086/376835. [DOI] [PubMed] [Google Scholar]
- 2.Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, Angarano G, Colangeli V, De Luca A, Ippolito G. Insights into the reasons for discontinuation of the first highly active antriretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 3.Aarnoutse RE, Schapiro JM, Bouchner CA, Hekster YA, Burger DM. Therapeutic drug monitoring. An aid to optimising response to antriretroviral drugs? Drugs. 2003;63:741–53. doi: 10.2165/00003495-200363080-00002. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith DR, Perry CM. Atazanavir. Drugs. 2003;63:1679–93. doi: 10.2165/00003495-200363160-00003. [DOI] [PubMed] [Google Scholar]
- 5.O'Mara E, Cirincione B, Mummaneni V, Grasela T, Grasela D. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 2001. Chicago, IL: 2001. Population pharmacodynamic (PD) assessment of the safety and antiretroviral activity of atazanavir (BMS-232632) [Google Scholar]
- 6.Schapiro J, Winters M, Stewart F, Efron B, Norris J, Kozal M, Merigan T. The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann Int Med. 1996;124(12):1039–50. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Stein D, Fish D, Bilello J, Chodakewitz J, Emini E, Hildebrand C, Preston S, Martineau G, Drusano G. A 24-week open label phase I evaluation of the HIV protease inhibitor MK-639. AIDS. 1996;10:485–92. doi: 10.1097/00002030-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 8.McNicholl I. Drug interactions among the antiretrovirals. Curr Infect Dis Rep. 2004;6:159–62. doi: 10.1007/s11908-996-0013-9. [DOI] [PubMed] [Google Scholar]
- 9.Purdy B, Raymond A, Lesar T. Antriretroviral prescribing errors in hospitalised patients. Ann Pharmacother. 2000;34:833–8. doi: 10.1345/aph.19399. [DOI] [PubMed] [Google Scholar]
- 10.Bjerrum L, Sogaard J, Hallas J, Kragstrup J. Polypharmacy in general practice: differences between practitioners. Br J General Pract. 1999;49:195–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Martin K, Begaud B, Latry P, Miremont-Salame G, Fourrier A, Moore N. Differences between clinical trials and postmarketing use. Br J Clin Pharmacol. 2003;57:86–92. doi: 10.1046/j.1365-2125.2003.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasela T. Population approaches to pharmacokinetics and risk management. In: Schumacher GE, editor. Therapeutic Drug Monitoring. Connecticut: Appleton & Lange; 1995. pp. 119–41. [Google Scholar]
- 13.Gibaldi M, Perrier D. Pharmacokinetics. New York: Marcel Dekker; 1975. [Google Scholar]
- 14.Taburet A, Piketty C, Chazallon C, Vincent I, Gerard L, Calvez V, Clavel F, Aboulker J-P, Girard P-M. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:2091–6. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bristol-Myers Squibb Company. Reyataz (Atazanavir Sulfate) Product Information Guide. 2003.
- 16.Ray J, Pang E, Carey D. Simultaneous determination of indinavir, ritonavir and lopinavir (ABT 378) in human plasma by high-performance liquid chromatography. J Chromatogr B. 2002;775:225–30. doi: 10.1016/s1570-0232(02)00295-7. [DOI] [PubMed] [Google Scholar]
- 17.InnaPhase Corporation. Kinetica, Version 4.1 User Manual. Philadelphia: InnaPhase Corporation; 2002. [Google Scholar]
- 18.Agarwala S, Grasela D, Child M, Geraldes M, Geiger M, Meeker J, O'Mara E. International AIDS Society Conference on HIV Pathogenesis and Treatment 2003. Paris, France: 2003. Characterization of the steady-state pharmacokinetic (PK) profile of atazanavir (ATV) beyond the 24-hour dosing interval. [Google Scholar]
- 19.O'Mara E, Mummaneni V, Bifano M, Randall D, Uderman H, Knox L, Geraldes M. 8th Conference on Retroviruses and Opportunistic Infections 2001. Chicago, IL: 2001. Pilot study of the interaction between BMS-232632 and ritonavir. [Google Scholar]
- 20.Burger DM. Launch of guide to the use of drug level monitoring. HIV Treatment Bull. 2003;4(4):10. [Google Scholar]
- 21.Gisolf E, van Heeswijk R, Hoetelmans R, Danner S. Decreased exposure to saquinavir in HIV-1 infected patients after long-term antiretroviral therapy including ritonavir and saquinavir. AIDS. 2000;14:801–5. doi: 10.1097/00002030-200005050-00005. [DOI] [PubMed] [Google Scholar]
- 22.Taburet A, Raguin G, Tiec C, Droz C, Barrail A, Vincent I, Morand-Joubert L, Chene G, Clavel F, Girard P. Interactions between amprenavir and the lopinavir-ritonavir combination in heavily pretreated patients infected with human immunodeficiency virus. Clin Pharm Ther. 2004;75:310–23. doi: 10.1016/j.clpt.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Minto C, Li B, Tattum B, Brown K, Seale P, Donnelly R. Pharmacokinetics of epimeric budesonide and fluticasone propionate after repeated dose inhalation – intersubject variability in systemic absorption from the lung. Br J Clin Pharmacol. 2000;50:116–24. doi: 10.1046/j.1365-2125.2000.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollard M, Bagg W, Thomas M, Lucas J, Ticehurst R, Black P. Cushing's Syndrome due to interaction between inhaled corticosteroids and itraconazole. Ann Pharmacother. 2004;38:46–9. doi: 10.1345/aph.1D222. [DOI] [PubMed] [Google Scholar]
- 25.Hillebrand-Haverkort M, Prummel M, ten Veen J. Ritonavir–induced Cushing's syndrome in a patient treated with nasal fluticasone. AIDS. 1999;13:1803–6. doi: 10.1097/00002030-199909100-00038. [DOI] [PubMed] [Google Scholar]
- 26.Clevenbergh P, Corcostegui M, Gerard D, Hieronimus S, Mondain V, Chichmanian R, Sadoul J, Dellamonica P. Iatrogenic Cushing's Syndrome in an HIV-infected patient treated with inhaled corticosteroids (fluticasone propionate) and low dose ritonavir enhanced PI containing regimen. J Infect. 2002;44:194–5. doi: 10.1053/jinf.2001.0928. [DOI] [PubMed] [Google Scholar]
- 27.Rouanet L, Peyriere H, Mauboussin J, Vincent D. Cushing's syndrome in a patient treated by ritonavir/lopinavir and inhaled fluticasone. HIV Med. 2003;4:149–50. doi: 10.1046/j.1468-1293.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton K, Yazdanian M, Audus K. Modulation of P-glycoprotein activity in Calu-3 cells using steroids and β-ligands. Int J Pharmaceut. 2001;228:171–9. doi: 10.1016/s0378-5173(01)00836-5. [DOI] [PubMed] [Google Scholar]
- 29.Anglicheau D, Flamant M, Schlageter M, Martinez F, Cassinat B, Beaune P, Legendre C, Thervet E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18:2409–14. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- 30.Race T, Paes I, Faloon W. Intestinal malabsorption induced by oral colchicine. Comparison with neomycin and cathartic agents. Am J Med Sci. 1970;259:32–41. doi: 10.1097/00000441-197001000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Matheny C, Lamb M, Brouwer K, Pollack G. Pharmacokinetic and pharmacodynamic implications of P-glycoprotein modulation. Pharmacother. 2001;21:778–96. doi: 10.1592/phco.21.9.778.34558. [DOI] [PubMed] [Google Scholar]
- 32.Torimoto N, Ishii I, Hata M, Nakamura H, Imada H, Ariyoshi N, Ohmori S, Igarashi T, Kitada M. Direct interaction between substrates and endogenous steroids in the active site may change the activity of cytochrome P450 3A4. Biochemistry. 2003;42:15068–77. doi: 10.1021/bi034409n. [DOI] [PubMed] [Google Scholar]
- 33.Chin T, Loeb M, Fong I. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob Agents Chemother. 1995;39:1671–5. doi: 10.1128/aac.39.8.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nascimbeni M, Lamotte C, Peytavin G, Farinotti R, Clavel F. Kinetics of antiviral activity and intracellular pharmacokinetics of human immunodeficiency virus type 1 protease inhibitors in tissue culture. Antimicrob Agents Chemother. 1999;43:2629–34. doi: 10.1128/aac.43.11.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drusano G, D’Argenio D, Preston S, Barone C, Symonds W, LaFon S, Rogers M, Prince W, Bye A, Bilello J. Use of drug effect interaction modelling with Monte Carlo simulation to examine the impact of dosing interval on the projected antiviral activity of the combination of abacavir and amprenavir. Antimicrob Agents Chemother. 2000;44:1655–9. doi: 10.1128/aac.44.6.1655-1659.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Rosenkranz S, Wu H. Modelling HIV dynamics and antiviral response with consideration of time-varying drug exposures, adherence and phenotypic sensitivity. Math Biosci. 2003;184:165–86. doi: 10.1016/s0025-5564(03)00058-0. [DOI] [PubMed] [Google Scholar]
- 37.Antinori A, Cozzi-Lepri A, Ammassari A, Trotta M, Nauwelaers D, Hoetelmans RRM, Melzi S. Relative prognostic value of self-reported adherence and plasma NNRTI/PI concentrations to predict virological rebound in patients initially responding to HAART. Antivir Ther. 2004;9(2):291–6. [PubMed] [Google Scholar]
- 38.Fletcher C, Anderson P, Nakuda TC, Schacker T, Henry K, Gross C, et al. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS. 2002;16:551–60. doi: 10.1097/00002030-200203080-00006. [DOI] [PubMed] [Google Scholar]
- 39.Burger DM, Hugen P, Reiss P, Gyssens I, Schneider M, Kroon F. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive patients. AIDS. 2003;16:1157–65. doi: 10.1097/00002030-200305230-00007. [DOI] [PubMed] [Google Scholar]