Abstract
Aim
To quantify the effect of paracetamol on the anticoagulant effect of warfarin under normal clinical conditions.
Patients and methods
In a prospective double-blind, cross-over, placebo-controlled study, 11 patients on stable warfarin therapy received in random order two 14-day regimens of paracetamol 4 g day−1 or placebo, with a 14-day or more wash-out period in between, time necessary to fulfil the inclusion criteria.
Results
In patients on paracetamol, the mean maximum increase in the International Normalized Ratio (INR) observed was 1.04 ± 0.55 vs. 0.20 ± 0.32 in those on placebo (P = 0.003). The mean maximum INR observed was significantly higher with paracetamol than with placebo (3.47 vs. 2.61, P = 0.01). In patients receiving paracetamol, the mean observed INR was significantly increased after 4 days (+ 0.6 ± 0.6, P < 0.001).
Conclusion
Paracetamol at 4 g day−1 induces a significant increase in INR in patients receiving a stable regimen of warfarin, increasing the risk of bleeding associated with warfarin.
Keywords: anticoagulant, bleeding, INR, interaction, paracetamol, warfarin
Warfarin is the drug of choice in the prevention and treatment of venous thromboembolic disease and atrial fibrillation [1]. Paracetamol is recommended as a first-line analgesic therapy in patients receiving oral anticoagulation, as aspirin and nonsteroidal anti-inflammatory drugs (NSAIDS) enhance the risk of oral anticoagulation-associated bleeding by inhibiting platelet function and can also produce gastric erosions that increase the risk of upper gastrointestinal bleeding [1, 2]. The maximum recommended dosage of paracetamol is 4 g day−1[3]. Indications for both oral anticoagulation and analgesic therapy increase with age, leading to a widely used coprescription of oral anticoagulation/paracetamol. The occurrence of a potentiation of the anticoagulant effect of oral anticoagulation by acetaminophen has been subject to controversy leading, in the UK and France, to a warning in the summary of product characteristics that the anticoagulant effect of warfarin may be enhanced by the use of paracetamol. This question remains open to debate due to the lack of prospective and methodologically correct clinical studies assessing the effect of paracetamol administration on International Normalized Ratio (INR) in patients receiving chronic oral anticoagulation under normal clinical conditions [4, 5]. Most of the available prospective randomized double-blind studies were performed either in healthy volunteers receiving warfarin [6] or coumarins [7], or in patients receiving warfarin or anisindione, coumarin, phenprocoumon [8, 9] with evaluation of PT or thrombotest or with low doses of paracetamol [9].
Measurement of INR is of clinical relevance since enhanced INR is the major determinant of the bleeding risk [10]. The primary objective of this study was to assess whether or not paracetamol potentiates the anticoagulant effect of warfarin under normal clinical conditions.
Patients and methods
In the Outpatient Department of the Internal Medicine Unit of Lariboisière Hospital (Paris, France), ambulatory patients who had been taking warfarin (at 2–9 mg day−1) for more than 1 month with a target INR of between 2 and 3 and no recent or ongoing disease, were selected for the study. They were included in each study period when two INR values were in the target range during the last 5 days on the same dosage of warfarin. All treatments had to be unchanged and free from drugs containing paracetamol for the 14 days preceding inclusion and for the duration of the study. Each volunteer gave written informed consent. An inclusion register was made.
The prospective study was carried out using a two-phase, double-blind, randomized, cross-over design. The generation of the randomization sequence was performed by the Hospital Pharmacy using random number tables, concealed in sealed envelopes. Patients were randomized by blocks of four. This study was approved by the Research Ethics Committee of La Pitié-Salpétrière (Paris, France).
During each period (14 days), patients had to follow the same regimen of warfarin (intake of the same dosage orally at the same hour). In the first phase, each patient received a 14-day regimen of paracetamol (Doliprane®; Aventis Pharma, Théralpix, France) 1 g administered orally four times a day. In the second phase, each patient received a 14-day regimen of placebo administered orally four times a day. The order of phases 1 and 2 was assigned randomly with a 14-day or more wash-out period in between, time necessary to fulfil the inclusion criteria.
Because of the spontaneous fluctuation of INR in otherwise stable patients (0.3) [11], a change of at least 0.5 was considered significant. In order to show a change of INR of 0.5 or more on paracetamol, with an α level of 0.05 and a β level of 20%, 20 patients had to be included. An interim analysis was planned a priori after 10 inclusions in order to determine whether there was an interaction between warfarin and paracetamol and whether ethically the study should be continued.
Baseline INR was taken as the mean of the last three INR values before inclusion, in patients on the same dosage of warfarin. Blood samples were collected at inclusion (day 0) and then at the home of patients on days 2, 4, 7, 9, 11 and 14, 12 h after warfarin intake for measurement of INR. INR measurements were all performed in the same laboratory (Haematology, Lariboisière Hospital, Professor L. Drouet). At each visit, subjects were asked about any adverse event or missed treatment dose and underwent a physical examination. It was decided that in the interests of patient safety, the treatment would be discontinued if INR values rose higher than 3.5. Warfarin and paracetamol concentrations were not quantified since these measurements are not available in Paris. The primary aim of the study was to determine whether paracetamol increased or not the anticoagulant effect of warfarin.
Results
From January to June 2003, 31 patients were selected at consultation; 11 patients [mean age 66.0 ± 19.2 (24–89)] were included and completed the study. Characteristics of included patients were as follows: body weight 78.3 ± 23.3 kg; body mass index 28.0 ± 7.7 kg m−2; duration of oral anticoagulant therapy before inclusion 8 ± 8 (1–24) months; dosage of warfarin at inclusion before placebo period 3.75 ± 1.97 (2–9) mg day−1 and before paracetamol period 3.80 ± 1.96 (2–9) mg day−1. Indications for anticoagulation were: venous thromboembolism (45%), atrial fibrillation (55%). Reasons for non-inclusion were as follows: warfarin discontinuation (n = 8), consent reject (n = 2), protocol incomprehension (n = 3), address of domicile (n = 6), death (n = 1). The paracetamol regimen was stopped early in three patients because of enhanced INR (respectively on days 4, 9 and 9). The wash-out period lasted 3.5 ± 2 weeks. No bleeding events were noted.
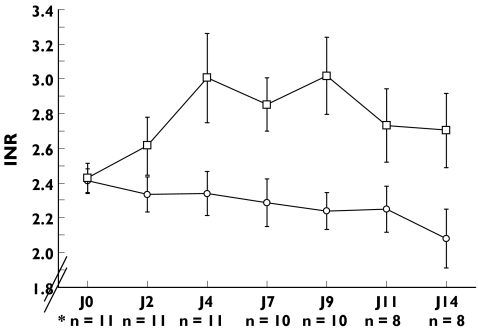
In patients on paracetamol, the mean observed INR was significantly increased after 4 days (+0.6 ± 0.6, P < 0.001) and for the duration of the study, while it did not significantly change with placebo (P = 0.20). The changes in mean INR over time are presented in Figure 1.
Figure 1.
Changes in INR over time in patients receiving placebo or paracetamol (4 g per day).
# paracetamol therapy was stopped in 3 patients because of 2 consecutive INR measurements above 3.5. There was no treatment interruption during the placebo period. Mean INR ± SD. *p < 0.05, INRji versus INRj0. Placebo n = 11 (○), paracétamol* (□)
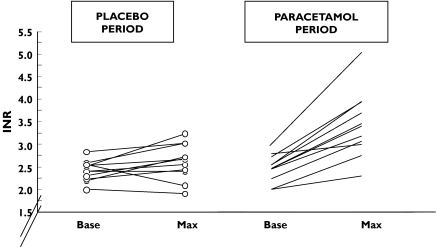
The mean maximum INR observed was 3.47 ± 0.75 in patients on paracetamol vs. 2.61 ± 0.40 in those on placebo (P = 0.001). After the paracetamol period, INR was higher than 3 in nine patients compared with three patients after the placebo period (P = 0.03) (Figure 2).
Figure 2.
Maximal INR variations observed in 11 patients on a stable regimen of warfarin receiving in random order placebo and paracetamol 4 g/d. Base: INR before study treatment; Max: maximal INR on study treatment
The mean maximum increase in INR observed in 11 patients was 1.04 ± 0.55 with paracetamol vs. 0.20 ± 0.32 with placebo (P = 0.003). There was no order effect or period effect.
Discussion
This prospective study confirms the occurrence of an interaction between warfarin and paracetamol at 4 g day−1. It is of importance to know whether there is an interaction between oral anticoagulation and paracetamol since paracetamol is the first-line analgesic and antipyretic therapy in patients receiving oral anticoagulation. A recent literature review shows that the methodology used varies widely: retrospective, observational or case–control studies, case reports or experimental studies in healthy subjects suggest the occurrence of an increase in the anticoagulant effect of oral anticoagulation by paracetamol [4, 6–8, 13, 14], while others do not [12, 15, 16]. But all these studies are subject to caution due to methodological biases (retrospective studies, few patients, patients not reflecting those encountered in normal clinical practice) and variable therapies (warfarin but also fluindione, acenocoumarol, phenprocoumon, coumarins). The most demonstrative is the study by Hylek, who conducted a prospective case–control study in an anticoagulation therapy unit in order to identify factors associated with an INR >6 in case patients (n = 93) and matched controls (n = 196, mean age 70 years) on warfarin for more than 1 month, whose target INR was 2–3 [4]. There was a dose-dependent relationship between the intake of more than seven tablets per week of paracetamol and an increased risk for an INR >6. That is why we undertook a prospective randomized study which included patients taking warfarin under normal clinical conditions. No study has demonstrated as yet the existence or not of a significant interaction between warfarin and paracetamol under normal clinical conditions in relation to the gold standard parameter (INR) [4–6].
Our data demonstrate that the intake of paracetamol at 4 g day−1 induces a significant increase in INR in patients receiving a stable regimen of warfarin. This interaction is clinically relevant since the mean increase in INR is as high as 1.04 with paracetamol and is significant after only 4 days of prescription of both drugs.
The risk of occurrence of bleeding, the most serious complication of oral anticoagulation, is closely related to the intensity of anticoagulation [10]. The haemorrhagic risk of oral anticoagulation could therefore be increased on a 4 g day−1 regimen of paracetamol. These findings are clinically relevant and have clinical implications since paracetamol is the first-line analgesic therapy for patients receiving oral anticoagulation.
One limitation of our study is the lack of determination of plasma warfarin and paracetamol levels. In fact, our primary aim was to determine whether there was a significant interaction between warfarin and paracetamol in terms of INR and under normal conditions (blood was collected at the home of patients). We cannot conclude as to the mechanism of this interaction.
In fact, the mechanism by which the anticoagulant effect of warfarin (and other oral anticoagulants) may be enhanced by paracetamol is unclear [3–5]. The mechanism could theoretically be mediated through the cytochrome P450 enzyme system, leading to higher warfarin plasma concentrations and therefore higher INR when paracetamol is coadministered. Commercially available warfarin is a racemic mixture of R- and S-enantiomers. S-warfarin is the most potent enantiomer of warfarin and is predominantly metabolized to 7-hydroxywarfarin by CYP2C9, while R-warfarin is metabolized by CYP1A2 and CYP2C19. At therapeutic dosages, the majority of paracetamol is eliminated by conjugation and the last part is metabolized by CYP1A2, CYP2E1. Since paracetamol is neither a substrate nor an inhibitor of CYP2C9, the predominant pathway of warfarin metabolism, the findings of an increase of anticoagulant effect by paracetamol in this way is subject to caution [5].
The effect could be nonpharmacokinetic: the warfarin plasma concentration remains constant but the INR increases in response to paracetamol. Gebauer reported that a patient on warfarin experienced an abrupt increase in INR after taking paracetamol, which was reversible after stopping paracetamol and which occurred again after the reintroduction of paracetamol, while the warfarin plasma concentration remained quite constant [17]. The factor VII concentration decreased when the INR was elevated, but the interaction cannot be explained by a change in warfarin kinetics.
In conclusion, as is indicated in the summary of product characteristics, all patients on warfarin and prescribers should be aware of the increase in the anticoagulant effect of warfarin by paracetamol at 4 g day−1. This interaction can be minimized by regular monitoring of the INR and there is no need to reduce the dose if this is kept constant. Since paracetamol does not inhibit platelet function and cause gastrointestinal bleeding, it is still much safer than aspirin and other NSAIDs in patients taking warfarin. Due to the lack of a safer alternative, if a patient on oral anticoagulation requires an analgesic or antipyretic drug, paracetamol should still be chosen, but the dose and duration of therapy should be as low as possible. In any case, it is recommended that the anticoagulant effect be closely monitored when regular paracetamol is either started or stopped when the patient is on warfarin.
Acknowledgments
Competing interests: This study was supported by a grant for Independent Research from Aventis Pharma, Théralpix, France.
References
- 1.Hirsch J, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119:8S–21S. doi: 10.1378/chest.119.1_suppl.8s. [DOI] [PubMed] [Google Scholar]
- 2.Levine MN, Raskob G, Landefeld S, Kearon C. Haemorrhagic complications of anticoagulant treatment. Chest. 2001;119:108S–121S. doi: 10.1378/chest.119.1_suppl.108s. [DOI] [PubMed] [Google Scholar]
- 3.American College of Rheumatology Subcommittee on osteoarthritis guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee 2000 update. Arthritis Rheum. 2000;43:1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE. Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA. 1998;279:657–62. doi: 10.1001/jama.279.9.657. [DOI] [PubMed] [Google Scholar]
- 5.Mahé I, Caulin C, Bergmann JF. Does paracetamol (acetaminophen) potentiate the effects of oral anticoagulants. A literature review. Drug Safety. 2004;27:283–52. doi: 10.2165/00002018-200427050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Rubin RN, Metzer RL, Budzynski AZ. Potentiation of anticoagulation effects of warfarin by acetaminophen (tylenol) Clin Res. 1984;32:698a. [Google Scholar]
- 7.Boeijinga JJ, Boerstra EE, Ris P, Breimer DD, Jeletich-Bastiaanse A. Interaction between paracetamol and coumarin anticoagulants. Lancet. 1982;1:506. doi: 10.1016/s0140-6736(82)91473-8. [DOI] [PubMed] [Google Scholar]
- 8.Antlitz AM, Mead JA, Jr, Tolentino MA. Potentiation of oral anticoagulant therapy by acetaminophen. Curr Ther Res Clin Exp. 1968;10:501–7. [PubMed] [Google Scholar]
- 9.Gadisseur AP, Van der Meer FJ, Rosendaal RF. Sustained intake of paracetamol (acetaminophen) during oral anticoagulant therapy with coumarins does not cause clinically important IRN changes: a randomised double-blind clinical trial. J Thromb Haemost. 2003;1:714–7. doi: 10.1046/j.1538-7836.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 10.Hylek E, Singer DE. Risk factors for intracranial haemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lassen JF, Kjeldsen J, Antonsen S, Hyltoft Petersen P, Brandslund I. Interpretation of serial measurements of international normalized ratio for prothrombin times in monitoring oral anticogulant therapy. Clin Chem. 1995;41:1171–6. [PubMed] [Google Scholar]
- 12.Kwan D, Bartle WR, Walker SE. The effects of acetaminophen on pharmacokinetics and pharmacodynamics of warfarin. J Clin Pharmacol. 1999;39:68–75. doi: 10.1177/00912709922007570. [DOI] [PubMed] [Google Scholar]
- 13.Andrews FJ. Retroperitoneal haematoma after paracetamol increased anticoagulation. Emerg Med J. 2002;19:84–5. doi: 10.1136/emj.19.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whyte IM, Buckley NA, Reith DM, et al. Acetaminophen causes an increased International Normalized Ratio by reducing functional factor VII. Ther Drug Monit. 2000;22:742–8. doi: 10.1097/00007691-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Antlitz AM, Awalt LF. A double blind study of acetaminophen used in conjunction with oral anticoagulant therapy. Curr Ther Res Clin Exp. 1969;11:360–1. [PubMed] [Google Scholar]
- 16.Fattinger K, Frisullo R, Masche U, et al. No clinically relevant drug interaction between paracetamol and phenprocoumon based on a pharmacoepidemiological cohort study in medical inpatients. Eur J Clin Pharmacol. 2002;57:863–7. doi: 10.1007/s00228-001-0404-7. [DOI] [PubMed] [Google Scholar]
- 17.Gebauer MG, Nyfort-Hansen K, Henschke PJ, Gallus AS. Warfarin and cetaminophen interaction. Pharmacotherapy. 2003;23:109–12. doi: 10.1592/phco.23.1.109.31913. [DOI] [PubMed] [Google Scholar]