Abstract
Aim
Lipid lowering therapy with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors is increasingly used for the prevention of cardiovascular events, but they should be used with caution in patients with impaired liver function. We therefore studied the pharmacokinetics of pitavastatin in patients with liver cirrhosis.
Methods
Plasma concentrations of pitavastatin were determined after administration of 2 mg single-dose pitavastatin to 12 male patients with liver cirrhosis (six Child-Pugh grade A and six grade B). These results were compared with the single-dose pharmacokinetic results obtained from six male volunteers without liver disease.
Results
Administration of 2 mg single-dose pitavastatin to patients with Child-Pugh grade A and grade B cirrhosis resulted in a 1.19- and 2.47-fold increase in Cmax and 1.27- and 3.64-fold increase in AUCt, respectively, when compared with normal subjects. The geomean Cmax of pitavastatin was 59.5 ng ml−1, 70.7 ng ml−1 and 147.1 ng ml−1 in the control, Child-Pugh grade A and Child-Pugh grade B groups, respectively. The geomean AUCt of pitavastatin in the three groups was 121.2 ng h−1 ml−1, 154.2 ng h−1 ml−1 and 441.7 ng h−1 ml−1, respectively. The geomean Cmax of pitavastatin lactone was 20.3 ng ml−1, 19.1 ng ml−1 and 9.9 ng ml−1 in the control, Child-Pugh grade A and grade B groups, respectively. The AUCt of pitavastatin lactone was 120.2 h−1 ml−1, 108.8 h−1 ml−1 and 87.5 h−1 ml−1, respectively.
Conclusion
The plasma concentration of pitavastatin is increased in patients with liver cirrhosis. In such patients, caution is required, although dose reduction may not be necessary in Child-Pugh A cirrhosis.
Keywords: Liver, cirrhosis, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, statin, pharmacokinetics, pitavastatin
Introduction
Coronary heart disease (CHD) is a major cause of morbidity and the leading cause of death in many countries. Risk factors for CHD include diabetes, hypertension, smoking, obesity and hypercholesterolaemia [1]. Of these, hypercholesterolaemia, characterized by high levels of low-density-lipoprotein (LDL) cholesterol, is considered one of the most important predictors of CHD [2, 3].
As 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in the liver is the rate-limiting and key-regulating enzyme of cholesterol biosynthesis, HMG-CoA reductase inhibitors, or statins, have been widely used to decrease cholesterol biosynthesis in patients with CHD [4]. The major biochemical effect of HMG-CoA reductase inhibitors or statins is unquestionably the inhibition of cholesterol biosynthesis, which leads to an up-regulation of LDL receptors on the cells surface and an increased clearance of LDL cholesterol from the circulation. In view of their efficacy, they have been widely used to treat hypercholesterolaemia, and thereby prevent CHD [5–7].
Pitavastatin (Kowa Company Ltd, Tokyo, Japan), a newly developed statin, is a potent, synthetic competitive inhibitor of HMG-CoA reductase [8]. It can reduce plasma levels of LDL cholesterol by 40% in hypercholesterolaemic patients [9]. Pitavastatin has been launched in Japan, and is becoming available in Europe, the USA, and Asia. With its high efficacy in reducing cholesterol synthesis and good safety profile, pitavastatin is expected to be widely prescribed in patients with hypercholesterolaemia or CHD.
As statins are metabolized in the intestine and the liver after administration, the plasma level of statins are bound to be higher in patients with impaired hepatic function. The pharmacokinetics of pravastatin [10], fluvastatin [11] and atorvastatin [12] has been reported in patients with hepatic impairment.
Pitavastatin is excreted predominantly into bile and thereby enters the enterohepatic circulation [13]. Very little is excreted into the urine. However, pitavastatin is not metabolized by the common liver cytochrome P-450 (CYP) 3A4 enzyme [13]. Although CYP 2C9 is the major CYP isoform associated with the metabolism of pitavastatin, it plays only a minor role in the metabolism of this drug [14]. The main membrane transport protein for pitavastatin in the liver is LST-1 (OATP2). Even though pitavastatin is taken up by the liver after absorption, it is rarely metabolized there. The pharmacokinetics of pitavastatin is also unaltered in subjects with fatty liver [15]. In view of this, we hypothesize that pitavastatin can be safely prescribed to patients with mild hepatic dysfunction.
We have therefore conducted a study on the pharmacokinetics of pitavastatin in patients with hepatic impairment. The objective of this study is to compare the pharmacokinetics of pitavastatin and its lactone, following the administration of a single dose of pitavastatin (2 mg tablets) in male patients with liver cirrhosis (Child-Pugh grade A, which corresponds to mild cirrhosis, and grade B, which corresponds to moderate cirrhosis) to male volunteers without liver disease.
Methods
This was a single-centre, open-labelled study, conducted at the Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR. The study was approved by the local Institutional Review Board, and written informed consent was obtained from all participants before their enrolment.
Subjects
A total of 18 male patients were recruited into this study, six with Child-Pugh grade A liver cirrhosis, six with Child-Pugh grade B liver cirrhosis and six normal controls without liver disease. Subjects were included as normal controls if they have no clinically significant diseases as determined by medical history, physical examination, ECG and laboratory tests. Liver cirrhosis was diagnosed with either liver biopsy, computerized tomography or ultrasound performed within the past 3 years. Subjects with liver cirrhosis had no other clinically significant diseases, as determined by medical history, physical examination, ECG and laboratory tests. Any stable chronic diseases should be adequately controlled. Exclusion criteria included carcinoma, oesophageal variceal bleeding in the past 6 months, portosystemic shunt, history of organ transplantation, pancreatic diseases, surgery or transfusion or blood donation within 4 weeks of enrolment, drug or alcohol abuse, cerebral thrombosis, myocardial infarction or significant cardiac diseases in the past 3 months, renal impairment with plasma creatinine >133 µmol l−1, international normalized ratio >2.0, bilirubin ≥190 µmol l−1, transaminases >10 times the upper limit of normal, use of systemic steroids in the past 3 months, use of agents known to affect hepatic metabolism in the past month, and use of herbal medications in the past week.
Study design
A single dose of pitavastatin (2 mg tablet) was administered to all subjects after overnight fasting. Blood samples were obtained prior to and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, 60, and 72 h after the administration of pitavastatin. During the study, drug safety and tolerability were also assessed. Any adverse event occurring throughout the study period was recorded. Use of any herbal preparations, including those containing St. John's Wort and Milk Thistle (Sylmarin), any liver enzyme and/or cytochrome P450-altering agents known to affect hepatic metabolic function, and alcohol, caffeine- or xanthine-containing beverages or substances were restricted during the period of the study.
Drug assays
The plasma concentration of pitavastatin and its lactone, which is the inactive main metabolite of pitavastatin, were measured with high performance liquid chromatography (HPLC) [16] at Adme Bioanalyses (Vergeze, France). The lower limit of quantification for pitavastatin and its lactone was 1.0 ng ml−1.
Calibration standards were obtained by adding blank human lithium-heparinized plasma with known concentrations of pitavastatin and lactone. Each calibration curve was constructed using seven calibration points. The concentrations used for the calibration were: 1, 2, 5, 10, 25, 50 and 100 ng ml−1. A blank sample with neither the product to be analysed nor the internal standard was analysed with each calibration curve. The samples for quality control were prepared at three concentrations using specific standard solutions: 2.5, 40 and 75 ng ml−1. These controls were prepared before each analytical series, with pitavastatin lactone being added into the extract solvent. Two quality control samples for each concentration level were measured during each analytical series. Each sample was discarded after each run in order to decrease the possibility of cross contamination. The precision and accuracy of the calibration standard concentration of pitavastatin during the analyses of the test samples were 3.0–7.0%and 99–101%, respectively. The precision and accuracy of the calibration standard concentration of pitavastatin lactone were 3.2–7.8% and 95–102%, respectively.
Pharmacokinetic parameters
Pharmacokinetic parameters were calculated using noncompartmental methods. Pharmacokinetic measurements for pitavastatin and its lactone included the maximum concentration of the drug in plasma (Cmax) and the time to reach the maximum concentration of drug in plasma (Tmax), the area under the plasma concentration-time curve from administration to last observed concentration of time (AUCt), total area under the plasma concentration-time curve extrapolated from administration to infinite time (AUCinf), and the elimination half-life (t1/2). AUCt and AUCinf were calculated using the trapezoidal rule.
Statistical analyses
All the statistical analysis was performed using Kinetica (version 4.0, Innaphase, Champs-sur-Marne, France). Subjects' baseline characteristics were compared using Wilcoxon's Rank Sum test. Pharmacokinetic parameters were compared using anova. All statistics were performed on the intention-to-treat population, which included all patients recruited into the study initially. Statistical significance was defined as P < 0.05 (2 tailed).
Results
The demographic data for the subjects are presented in Table 1. The median age of the control group was lower than the group of patients with Child-Pugh grade A and B liver cirrhosis (37.5 years, 42.5 and 46.5 years, respectively). The median weight of the control group (63.7 kg) was within ±15% of the median weight of the liver cirrhosis groups (69.7 kg and 75.0 kg, respectively). The baseline values of aspartate transaminase, alanine transaminase, gamma glutamyltranspeptidase, and total bilirubin were increased in relation to the degree of liver function impairment. The baseline value of albumin was decreased in relation to the severity of liver function impairment.
Table 1.
Demographic data
| Control (n = 6) Median (Range) | Child-Pugh A (n = 6) Median (Range) | Child-Pugh B (n = 6) Median (Range) | |
|---|---|---|---|
| Age (years) | 37.5 (29–48) | 42.5 (26–61) | 46.5 (33–61) |
| Weight (kg) | 63.7 (62.0–74.8) | 69.7 (56.2–85.2) | 75.0 (57.8–82.8) |
| AST (U l−1) | 16.5 (15–19) | 34.0 (20–211)* | 65.5 (40–155)* |
| ALT (U l−1) | 18.0 (12–28) | 49.0 (15–223)* | 45.5 (33–162)* |
| GGT (U l−1) | 19.0 (8–30) | 33.0 (14–123) | 77.0 (26–182)* |
| Total bilirubin (µmol l−1) | 8.0 (4–22) | 15.5 (4–44) | 36.5 (30–134)* |
| Albumin (g l−1) | 44.5 (43–46) | 40.5 (32–45) | 31.5 (27–35)* |
AST- aspartate transaminase, ALT- alanine transaminase, GGT- gamma glutamyl transpeptidase.
P < 0.05 compared with control.
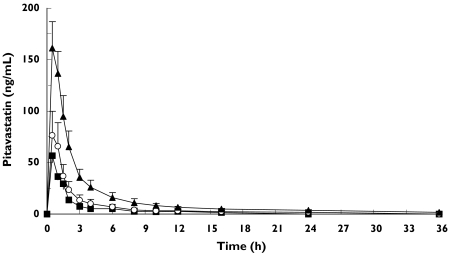
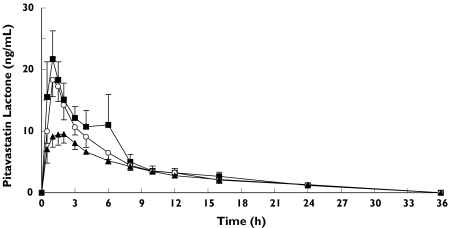
All 18 subjects completed the study. The mean plasma concentration-time curves following the administration of pitavastatin are shown in Figure 1 and Figure 2. A summary of the pharmacokinetic parameters for pitavastatin and its lactone are shown in Tables 2 and 3.
Figure 1.
Plasma concentrations (mean ±SEM) of pitavastatin after single-dose in volunteers without liver disease (n = 6) (▪), in patients with Child-Pugh grade A cirrhosis (n = 6) (○), and in patients with Child-Pugh grade B cirrhosis (n = 6) (▴)
Figure 2.
Plasma concentrations (mean ±SEM) of pitavastatin lactone after single-dose in volunteers without liver disease (n = 6) (▪), in patients with Child-Pugh grade A cirrhosis (n = 6) (○), and in patients with Child-Pugh grade B cirrhosis (n = 6) (▴)
Table 2.
Pitavastatin pharmacokinetic parameters
| Control (n = 6) | Child-Pugh A (n = 6) | Child-Pugh B (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median, range | Geomean | Median, range | Geomean | Geomean ratio 95% CI | Median, range | Geomean | Geomean ratio 95% CI | ||
| Cmax (ng ml−1) | 62.3 | 59.5 | 68.1 | 70.7 | 1.19 | 180.5 | 147.1 | 2.47 | P = 0.010 |
| 41.4–75.7 | 37.8–18.3 | 0.74–1.90 | 47.1–208.4 | 1.39–4.39 | |||||
| AUCt (ng ml−1) | 114.7 | 121.2 | 138.5 | 154.2 | 1.27 | 477.9 | 441.7 | 3.64 | P = 0.003 |
| 93.8–205.4 | 55.9–574.0 | 0.63–2.58 | 155.1–863.9 | 1.80–7.39 | |||||
| AUCinf (ng h ml−1) | 136.4 | 135.9 | 166.3 | 174.1 | 1.28 | 505.4 | 481.1 | 3.54 | P = 0.002 |
| 101.5–221.7 | 65.7–601.0 | 0.66–2.49 | 185.4–876.4 | 1.82–6.88 | |||||
| Tmax (h) | 0.5 | 0.67 | 0.5 | 0.56 | 0.83 | 0.5 | 0.56 | 0.83 | P = 0.608 |
| 0.5–1.5 | 0.5–1.0 | 0.53–1.30 | 0.5–1.0 | 0.53–1.30 | |||||
| t1/2 (h) | 10.0 | 7.0 | 9.0 | 8.3 | 1.18 | 13.8 | 14.4 | 2.06 | P = 0.06 |
| 2.8–11.1 | 3.2–19.3 | 0.64–2.19 | 12.2–18.8 | 1.11–3.82 | |||||
Geomean ratio = (Child-Pugh A or B geomean)/(Control geomean)
Cmax: maximum concentration of the drug in plasma, AUCt– the area under the plasmaconcentration-time curve from administration to last observed concentration of time, AUCinf: total area under the plasma concentration-time curve extrapolated from administration to infinite time, Tmax: time to reach maximum concentration of drug in plasma, t½: elimination half-time, SD: standard deviation, 95%CI: 95% confidence interval.
Table 3.
Pitavastatin lactone pharmacokinetic parameters
| Control (n = 6) | Child-Pugh A (n = 6) | Child-Pugh B (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median, range | Geomean | Median, range | Geomean | Geomean ratio 95% CI | Median, range | Geomean | Geomean ratio 95% CI | ||
| Cmax (ng ml−1) | 18.2 | 20.3 | 18.1 | 19.1 | 0.94 | 9.1 | 9.9 | 0.49 | P = 0.008 |
| 14.0–42.7 | 14.1–30.2 | 0.59–1.49 | 6.7–17.4 | 0.31–0.77 | |||||
| AUCt (ng h−1 ml−1) | 111.5 | 120.2 | 129.3 | 108.8 | 0.91 | 96.2 | 87.5 | 0.73 | P = 0.488 |
| 65.6–333.4 | 55.2–168.5 | 0.51–1.59 | 47.4–126.1 | 0.41–1.28 | |||||
| AUCinf (ng h−1 ml−1) | 130.1 | 152.5 | 146.9 | 130.4 | 0.85 | 126.0 | 116.4 | 0.76 | P = 0.510 |
| 92.6–358.8 | 71.8–195.3 | 0.53–1.39 | 80.0–152.0 | 0.47–1.24 | |||||
| Tmax (h) | 1.0 | 1.07 | 1.0 | 0.95 | 0.89 | 1.25 | 1.20 | 1.12 | P = 0.688 |
| 1.0–1.5 | 0.5–2.0 | 0.51–1.56 | 0.5–2.0 | 0.64–1.97 | |||||
| t1/2 (h) | 10.7 | 12.4 | 9.3 | 10.5 | 0.85 | 12.1 | 13.1 | 1.06 | P = 0.68 |
| 6.0–45.6 | 7.1–16.4 | 0.48–1.49 | 10.7–19.2 | 0.60–1.86 | |||||
Geomean ratio = (Child-Pugh A or B geomean)/(Control geomean)
Cmax: maximum concentration of the drug in plasma, AUCt– the area under the plasma concentration-time curve from administration to last observed concentration of time, AUCinf: total area under the plasma concentration-time curve extrapolated from administration to infinite time, Tmax: time to reach maximum concentration of drug in plasma, t1/2: elimination half-time, SD: standard deviation, 95%CI: 95% confidence interval.
Pitavastatin
The geometric mean (geomean) Cmax of pitavastatin was 59.5 ng ml−1, 70.7 ng ml−1 and 147.1 ng ml−1 in the control, Child-Pugh grade A and Child-Pugh grade B groups, respectively. The geomean AUCt of pitavastatin was 121.2 ng h−1 ml−1, 154.2 ng h−1 ml−1 and 441.7 ng h−1 ml−1 in the control, Child-Pugh grade A, and Child-Pugh grade B groups, respectively. The mean Tmax did not vary significantly among the groups. The elimination phase could not be quantified in the control group due to the limit of the quantification of assay. This might account for the differences in t1/2.
Pitavastatin lactone
The geomean Cmax of pitavastatin lactone was 20.3 ng ml−1, 19.1 ng ml−1 and 9.9 ng ml−1 in the control, Child-Pugh grade A and Child-Pugh grade B groups, respectively. The AUCt of pitavastatin lactone was 120.2 ng h−1 ml−1, 108.8 ng h−1 ml−1 and 87.5 ng h−1 ml−1 in the control, Child-Pugh grade A and Child-Pugh grade B groups, respectively. The mean Tmax did not vary significantly among the groups.
Discussion
This study compares the pharmacokinetic parameters of pitavastatin and its lactone following the administration of a single dose of pitavastatin (2 mg tablets) between patients with liver cirrhosis (Child-Pugh grade A and grade B) and a group of patients without liver disease (Control). Although there is 9 years of age difference between the Child-Pugh grade B groups, the pharmacokinetics of pitavastatin (Tmax, Cmax, AUC, and t½) has been shown to be unaffected by age in a previous study [17].
The plasma concentration of pitavastatin in patients with cirrhosis is higher than that in subjects with a normal liver function after a single dose of pitavastatin (2 mg tablet). The AUCt of pitavastatin is increased 1.27-fold in Child-Pugh grade A compared with the normal liver group, and 3.64-fold in Child-Pugh grade B (P = 0.003). The Cmax of pitavastatin is increased in Child-Pugh grade A up to 1.19-fold compared with normal liver subjects, and up to 2.47-fold in Child-Pugh grade B (P = 0.010). On the other hand, Cmax and AUCt of pitavastatin lactone are 0.94 and 0.91-fold lower in Child-Pugh grade A, and 0.49 and 0.73-fold lower in Child-Pugh grade B, respectively, compared with normal liver subjects (P = 0.008 for Cmax, P = 0.488 for AUCt). The results of this study indicate that the metabolism of pitavastatin is affected by the degree of hepatic impairment. The absorption rate of pitavastatin remained constant since the Tmax of pitavastatin and its lactone metabolite did not vary with the severity of hepatic impairment.
We believe that the blood concentration of pitavastatin is increased in relation to the degree of liver dysfunction because of reduced uptake by the liver. The decrease in blood concentration of pitavastatin lactone, the main metabolite of pitavastatin, in relation to the degree of liver dysfunction also indicates that the uptake of pitavastatin by the liver, where metabolism occurs, is reduced in the patients with cirrhosis. Further studies with repeated dosing would be helpful to ascertain plasma drug levels in the steady state and the extent of drug accumulation in patients with liver cirrhosis. Pitavastatin lactone is inactive, and therefore changes in its blood concentration would not be expected to change the therapeutic or toxic effect.
After oral administration of pravastatin, patients with alcoholic cirrhosis showed AUC values that were 1.3-fold higher than those in volunteers without liver disease matched for age and gender [10]. Administration of a single 40 mg dose of fluvastatin to a patient population with cirrhosis with fewer than 10 points in Child-Pugh score resulted in a 2.5-fold increase in both AUC and Cmax [11]. With 10 mg of atorvastatin, the Cmax is 5.5-fold greater in Child-Pugh grade A and 14.4-fold greater in Child-Pugh grade B patients. The AUC is 4.4-fold and 9.8-fold greater, respectively [12]. Although the degree of liver cirrhosis is not the same when compared with these studies, the increase in the plasma concentration of pitavastatin in patients with liver cirrhosis is similar to that observed with pravastatin and fluvastatin, but lower than that with atorvastatin.
This study showed that the plasma concentration of pitavastatin in cirrhotic patients is increased compared with subjects with a normal hepatic function. However, in the patients with Child-Pugh grade A cirrhosis, the ratio of both Cmax and AUC compared with subjects with normal hepatic function did not increase to the extent that requires a change of dosage.
In conclusion, the plasma concentration of pitavastatin is increased in patients with liver cirrhosis. Although it appears to be safe to prescribe this drug in such patients, caution is needed. Dose reduction may not be necessary in those with Child-Pugh A cirrhosis. Further studies are needed to evaluate the safety of repeated administration of pitavastatin in those with hepatic impairment.
Acknowledgments
The help from Dr Dan Weng and Dr Masahiko Kitagawa is gratefully acknowledged.
Competing interests: Kowa Co. Ltd, Japan, sponsored this study. CK Hui and GKK Lau have no conflict of interest to declare. BMY Cheung has been reimbursed by MSD, Novartis, Pfizer and Sanofi at various times in the past 5 years for organizing, attending or speaking in scientific meetings.
References
- 1.Consensus conference. Lowering blood cholesterol to prevent heart disease. JAMA. 1985;253:2080–6. [PubMed] [Google Scholar]
- 2.Anderson KM, Castelli WP, Levy D Cholesterol. mortality. 30 years of follow-up from the Framingham study. JAMA. 1987;257:2176–80. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 3.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–45. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 4.Havel RJ, Rapaport E. Management of primary hyperlipidemia. N Engl J Med. 1995;332:1491–8. doi: 10.1056/NEJM199506013322207. [DOI] [PubMed] [Google Scholar]
- 5.The West Scotland Coronary Prevention Study Group. A coronary primary prevention study of Scottish men aged 45–64 years: trial design. J Clin Epidemiol. 1992;45:849–60. doi: 10.1016/0895-4356(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 6.The Scandinavian Simvastatin Survival Study (4S) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 7.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. for West Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolaemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 8.Aoki T, Nishimura H, Nakagawa S, Kojima J, Suzuki H, Tamaki T, et al. Pharmacological profile of a novel synthetic inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Arzneimittelforschung. 1997;47:904–9. [PubMed] [Google Scholar]
- 9.Saito Y, Yamada N, Teramoto T, Itakura H, Hata Y, Nakaya N, et al. A randomized, double-blind trial comparing the efficacy and safety of pitavastatin versus pravastatin in patients with primary hypercholesterolaemia. Atherosclerosis. 2002;162:373–9. doi: 10.1016/s0021-9150(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 10.Pan HY. Clinical pharmacology of pravastatin, a selective inhibitor of HMG-CoA reductase. Eur J Clin Pharmacol. 1991;40(Suppl 1):S15–8. [PubMed] [Google Scholar]
- 11.Deslypere JP. Clinical Implications of the Biopharmaceutical Properties of Fluvastatin. Am J Cardiol. 1994;73:12D–17D. doi: 10.1016/0002-9149(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 12.Gibson DM, Yang BB, Abel RB, Blum RA, Horton M, Stern RH, Sedman AJ, Whitfield LR. Effects of hepatic and renal impairment on pharmacokinetics (PK) and pharmacodynamics (PD) of atorvastatin. Pharm Res. 1996;13(Suppl 9):S428. [Google Scholar]
- 13.Fujino H, Yamada I, Kojima J, Hirano M, Katsumoto H, Yoneda M. Studies on the metabolic fate of NK-104, a new inhibitor of HMG-CoA reductase (5): In vitro metabolism and plasma protein binding in animals and human. Xenobio Metabol Dispos. 1999;14:415–24. [Google Scholar]
- 14.Nakai D, Nakagomi R, Furuta Y, Tokui T, Abe T, Ikeda T, et al. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J Pharmacol Exp Ther. 2001;297:861–7. [PubMed] [Google Scholar]
- 15.Mori H, Yamada M, Kataumi S, Mori M, Saito Y. Pharmacokinetics of Pitavastatin (NK-104) Administered for 7-days to Volunteers with Fatty Liver –Pharmacokinetics and Safety of Pitavastatin Administered 2 mg Daily for 7 days to 12 Volunteers, 6 with Fatty Liver, 6 Normal. Rinsho Iyaku. 2003;19:371–9. [Google Scholar]
- 16.Kojima J, Fujino H, Yosimura M, Morikawa H, Kimata H. Simultaneous determination of NK-104 and its lactone in biological samples by column-switching high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1999;724:173–80. doi: 10.1016/s0378-4347(98)00523-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakaya N, Tateno M, Nakamura T, Kojima J. Pharmacokinetics of Repeated Dose NK-104 (Pitavastatin) in Healthy Elderly and Non-elderly Volunteers. Rinsho Iyaku. 2001;17:957–70. [Google Scholar]