Abstract
Aims
We assessed the disposition of oral amodiaquine (AQ) and CYP2C8 polymorphism in 20 children with falciparum malaria.
Methods
AQ and DEAQ concentrations were determined with SPE-HPLC method. CYP2C8 genotypes were assessed by PCR-RFLP method.
Results
AQ was not detectable beyond day 3 postdose. Cmax for DEAQ was reached in 3.0 days. The mean values for t1/2, MRT, and AUCtotal were 10.1 days, 15.5 days and 4512.6 µg l−1 day, respectively. All the children were CYP2C8* homozygous.
Conclusion
Our data are consistent with those previously reported, and the AQ regimen seems pharmacokinetically adequate in the absence of CYP2C8 polymorphism.
Keywords: amodiaquine, desethylamodiaquine, CYP2C8
Introduction
Amodiaquine (AQ) has been used for the treatment of nonsevere malaria in Papua New Guinean children for the last three decades [1], but very little pharmacokinetic data exits. Available pharmacokinetic data of AQ, derived mainly from healthy adult volunteers, indicate that oral AQ is cleared rapidly from systemic circulation [2–4]. Of the three quantifiable metabolites of AQ metabolism, N-desethylamodiaquine (DEAQ) is quantitatively the major metabolite [2–4] and is therapeutically important in vivo[4, 5]. The primary route of AQ metabolism to DEAQ is via a polymorphic CYP2C8 enzyme [6, 7]. Assessing the CYP2C8 genotype status of this polymorphism in individuals may be of clinical value in predicting the therapeutic and/or toxicological outcome of AQ use. In this study we assessed the disposition of oral AQ regimen, and CYP2C8 polymorphism in Papua New Guinean children with falciparum malaria.
Methods
Paediatric patients and dose schedule
Patients between the ages of 1.0–10.0 years were enrolled in the study if they had fever (≥37.5 °C), asexual Plasmodium falciparum parasitaemia and were able to take oral medication. Exclusion criteria were severe or complicated malaria [8], including high parasitaemia requiring parenteral drug administration, history of diarrhoea or vomiting in the preceding 24 h, haemoglobin ≤8.0 g dl−1, history of AQ allergy, presence of other disease in association with malaria, refusal to give written consent, and failure to comply with study protocol. All patients received the total oral AQ (infant Camoquin®, Prawll Laboratories Ltd, India, 100 mg tablet) of 30 mg kg−1 (10 mg kg −1 day−1× 3 days) and a single dose of sulphadoxine-pyrimethamine combination (sulphadoxine 500 mg-pyrimethamine 25 mg, S-P), given on day 7 (25 mg kg−1, based on the sulfadoxine component of the S-P combination) [9]. All drug doses were administered under supervision (IH) and children who vomited twice following the first and repeated doses on the same day were excluded. All the drugs were obtained from the National Department of Health Pharmaceutical Services who procured these drugs from reliable sources. The National Department of Health Medical Research Advisory Committee and Tokyo Women's Medical University Ethical Committee approved the study.
Blood sample handling
Finger prick blood (75.0 µl) was collected on an ET31CHR filter paper (Whatman Limited, Kent UK) for the pharmacokinetic and genotypic analyses. For pharmacokinetic studies, children were hospitalized and blood was sampled on filter papers at times 0, 2, 4, 12, 24, 36 and 48 h as in-patient; and on days 3, 5, 7, and 14 as out-patient follow-up. The filter paper samples were dried and stored at room temperature until analysis. For CYP2C8 genotypic analysis, filter paper samples were air-dried and stored in sealed plastic bags at 4 °C until analysis. DNA was extracted and purified from blood spot on the filter paper using a QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instruction with some modifications [10]. CYP2C8 genotyping was done using a previously described PCR/RFLP method [7].
Analytical methods
Blood AQ and DEAQ were measured by the validated solid-phase extraction (SPE) high-performance liquid chromatographic (HPLC) method of Lindegardh et al.[11]. The limit of quantification for AQ and DEAQ was 35.6 and 32.8 µg l−1, respectively. The intraday coefficients of variation (CVs) at 35.6, 177.9 and 1067.6 µg l−1 of AQ and 32.8, 163.9 and 983.6 µg l−1 of DEAQ were 9.7, 6.9, and 7.1% (AQ) and 8.3, 10.0, and 7.6% (DEAQ), respectively. The respective interday CVs (n = 5) at the same concentrations were 11.8, 11.8, and 6.2% (AQ) and 13.8, 11.3, and 8.3% (DEAQ). The calibration curves in spiked whole blood samples were linear within 32.8–1067.6 µg l−1 (r2 ≥ 0.98) range. AQ and DEAQ concentrations were calculated using the peak height ratio of AQ and DEAQ to the internal standard.
Pharmacokinetic calculations
The peak concentrations (Cmax) and times to reach the Cmax (Tmax) were determined from the blood concentration-time curves. Other DEAQ pharmacokinetic parameters were calculated by noncompartmental analysis using a pharmacokinetic software program (KineticaTM, version 4.2, Inna Phase, USA). Spearman rank correlation coefficient (rs-value) test was used to assess relationship between oral AQ metabolism and CYP2C8 polymorphism. Results are expressed as mean with 95% confidence interval (CI) unless stated.
Results
The concentration-time data of AQ and DEAQ were available in 20 children. The median age for these children was 4.6 (range: 1.10–8.11) years. The median body-weight was 15.5 (range: 9.6–25.0) kg. The parasitaemia levels in all the children were greater than 1000 parasites µl−1 of blood.
Pharmacokinetic and genotype data
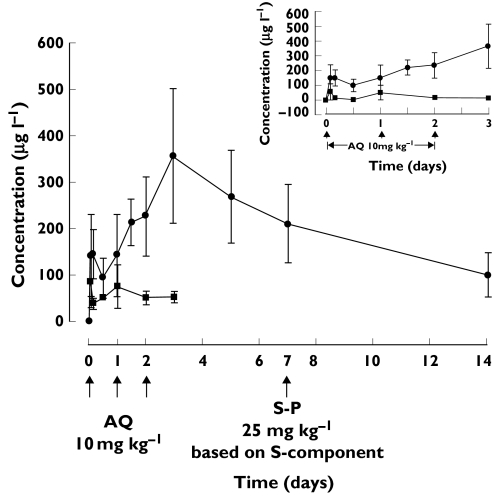
Figure 1 shows the mean (± SD) concentration vs. time profiles for AQ and DEAQ. The mean ± SD. capillary blood concentrations of AQ on days 1, 2, and 3 were 75.6 ± 47.2, 51.2 ± 14.0, 53.0 ± 11.9 µl−1, respectively; DEAQ at 2, 4, 12, 24, 36, and 48 h postdosing were 142.5 ± 88.4, 145.1 ± 53.0, 94.5 ± 41.6, 143.8 ± 88.8 (trough), 214.7 ± 50.8, and 227.5 ± 85.1 µg l−1 (trough), respectively. The mean (95%CI) Cmax of 368.8 (306.6–431.0) µg l−1 was reached in the median time of 3 days. The standard pharmacokinetic parameters (mean with 95% CI) for DEAQ are shown in Table 1. All 20 children were CYP2C8*1 homozygous.
Figure 1.
Mean (±SD) capillary blood concentrations of AQ (▪) and DEAQ (•) vs. time in 20 children after the oral administration of AQ (10 mg kg–1 day–1× 3 days). Inset indicates data of the first three days post-dosing; Error bars show ± SD
Table 1.
Pharmacokinetic parameters (mean, 95%CI) of DEAQ in 20 children with falciparum malaria who received oral amodiaquine (10 mg kg-1 day-1×3 days)
| Patient no. | Cmax (µg l–1) | Tmax (days) | AUC0-14 (µg l−1 day) | AUCtotal (µg l−1 day) | t1/2 (days) | MRT (days) |
|---|---|---|---|---|---|---|
| 01 | 735.1 | 3 | 2772.8 | 4361.4 | 3.5 | 6.8 |
| 02 | 351.8 | 3 | 2220.4 | 2407.1 | 3.3 | 6.6 |
| 03 | 399.7 | 3 | 1351.4 | 2827.7 | 6.7 | 10.5 |
| 04 | 407.8 | 3 | 3881.2 | 5863.7 | 8.4 | 13.0 |
| 05 | 226.0 | 3 | 1337.2 | 1445.9 | 3.4 | 6.1 |
| 06 | 590.5 | 3 | 4052.3 | 5825.2 | 7.7 | 11.9 |
| 07 | 282.4 | 1.5 | 224 8.9 | 3258.3 | 7.9 | 12.2 |
| 08 | 278.2 | 3 | 2198.4 | 3516.4 | 9.3 | 14.2 |
| 09 | 451.2 | 0.08 | 2988.5 | 10472.4 | 30.5 | 43.3 |
| 10 | 273.7 | 3 | 1416.6 | 3983.0 | 8.8 | 14.2 |
| 11 | 416.6 | 1 | 3154.5 | 3774.9 | 5.1 | 8.1 |
| 12 | 262.2 | 0.08 | 1752.5 | 5157.3 | 23.6 | 33.9 |
| 13 | 525.4 | 3 | 4505.3 | 5469.6 | 5.1 | 8.8 |
| 14 | 228.7 | 2 | 1158.6 | 4450.8 | 13.5 | 20.6 |
| 15 | 280.4 | 3 | 2438.1 | 8414.6 | 29.1 | 41.7 |
| 16 | 310.0 | 3 | 2673.6 | 3796.3 | 7.1 | 11.8 |
| 17 | 462.4 | 3 | 3949.6 | 5632.3 | 7.1 | 11.9 |
| 18 | 245.9 | 5 | 2125.0 | 2507.0 | 4.2 | 8.6 |
| 19 | 344.0 | 0.08 | 1835.1 | 2415.9 | 6.8 | 10.0 |
| 20 | 303.8 | 3 | 2674.8 | 4672.7 | 10.6 | 16.3 |
| Mean | 368.8 | 3.0* | 2536.7 | 4512.6 | 10.1 | 15.5 |
| 95% CI | 306.6–431.0 | 0.08–5.0* | 2078.2–2995.2 | 3517.3–5507.9 | 6.3–13.9 | 10.3–20.7 |
Median value with range.
Discussion
Our study confirms previous observations that AQ is rapidly and extensively metabolized to DEAQ upon oral administration [2–4]. The blood concentration-time profiles of AQ and DEAQ observed in the present study are similar to those previously reported from healthy adult volunteers [2–4] or malaria patients [5]. Therefore, it seems apparent from all these studies [2–5] including our study that AQ is a minor entity in vivo; implicating DEAQ as the major antimalarial component in both the paediatric and adult populations.
Although the blood concentration data obtained from these children are similar to those previously reported by Winstanley et al.[5], there are some differences between the two studies. For example, the Cmax and Tmax values reported for African patients are lower than those observed in our study (Table 1). The disparity may reflect differences in ethnicity, drug formulations, age, drug dosage, and methodologies between the two studies. It is difficult therefore to compare the extent to which the results of the two studies differ from each other. Clearly the small numbers (n = 20), with all expressing homozygous for the wild-type (CYP2C8*1) allele, have prevented any evaluation of the role of genetic polymorphisms in CYP2C8 in determining the disposition of amodiaquine. However, our data on genetic polymorphisms of CYP2C8 assessed in 173 individuals within the study area and 112 individuals randomly selected within Papua New Guinea population so far showed absence of CYP2C8 polymorphisms (authors’ unpublished data). Therefore, we are confident that the incidence of the genetic polymorphisms of CYP2C8 in Papua New Guinean population might be low. Nevertheless, since AQ-SP combination regimen continues to be the first-line treatment for nonsevere malaria in Papua New Guinean children [9], clinical implications of the oral AQ disposition in relation to the CYP2C8 polymorphism are still being investigated including determination of CYP2C8 (CYP2C8*1/*2/*3) allelic frequencies in Papua New Guinean population. We conclude, however, that our study confirms the pharmacokinetic evaluations of AQ and DEAQ carried out previously in healthy volunteers and malaria patients. Our data seem to suggest that the oral AQ dose regimen recommended in Papua New Guinean children may be pharmacokinetically adequate in the absence of any CYP2C8 polymorphisms.
Acknowledgments
The authors thank Japan International Cooperation Agency (JICA) for funding the study. I.T. was supported by a grant-in-aid (#99–2) from the Organization for Pharmaceutical Safety and Research (OPSR), Tokyo, Japan. We thank Dr Yngve Bergqvist at Dalarna University College, Sweden, for offering AQ, DEAQ and I.S to us.
References
- 1.Rieckmann KH. A field study on the effects of a combination of cycloguanil pamoate and amodiaquine against malaria in the Rabaul area of New Guinea. Am J Trop Med Hyg. 1966;15:833–7. doi: 10.4269/ajtmh.1966.15.833. [DOI] [PubMed] [Google Scholar]
- 2.Winstanley P, Edwards G, Orme M, Breckenridge A. The disposition of amodiaquine in man after oral administration. Br J Clin Pharmacol. 1987;23:1–7. doi: 10.1111/j.1365-2125.1987.tb03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent F, Saivin S, Chretien P, Magnaval JF, Peyron F, Sqalli A, Tufenkji AE, Coulais Y, Baba H, Campistron G. Pharmacokinetic and pharmacodynamic study of amodiaquine and its two metabolites after a single oral dose in human volunteers. Arzneimittelforschung. 1993;43:612–6. [PubMed] [Google Scholar]
- 4.Churchill FC, Patchen LC, Campbell CC, Schwartz IK, Nguyen-Dinh P, Dickinson CM. Amodiaquine as a pro-drug. the importance of metabolite(s) in the anti-malarial effect of amodiaquine in humans. Life Sci. 1985;36:53–62. doi: 10.1016/0024-3205(85)90285-1. [DOI] [PubMed] [Google Scholar]
- 5.Winstanley PA, Simooya O, Kofi-Ekue JM, Walker O, Salako LA, Edwards G, Orme ML, Breckenridge AM. The disposition of amodiaquine in Zambians and Nigerians with malaria. Br J Clin Pharmacol. 1990;29:695–701. doi: 10.1111/j.1365-2125.1990.tb03690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X-Q, Bjorkman A, Anderson TB, Ridderstrom M, Masimirembwa CM. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther. 2002;300:399–407. doi: 10.1124/jpet.300.2.399. [DOI] [PubMed] [Google Scholar]
- 7.Dai D. Allelic frequencies of human CYP2C8 and genetic linkage among different ethnic populations. FASEP. 2001a;15:A575. [Google Scholar]
- 8.World Health Organization. Assessment of Therapeutic Efficacy of Anti-Malarial Drugs: for Uncomplicated Falciparum Malaria in Areas with Intense Transmission. 1996. WHO/MAL/96.1077, Manila.
- 9.Department of Health. Standard treatment for common illness of children in Papua New Guinea. 10. Port Moresby: 2002. [Google Scholar]
- 10.Sakihama N, Mitamura T, Kaneko A, Horii T, Tanabe K. Long PCR amplification of Plasmodium falciparum DNA extracted from filter paper blots. Exp Parasitol. 2001;97:50–4. doi: 10.1006/expr.2000.4591. [DOI] [PubMed] [Google Scholar]
- 11.Lindegardh N, Forslund M, Green MD, Kaneko A, Bergqvist Y. Automated solid-phase extraction for determination of amodiaquine, chloroquine and metabolites in capillary blood on sampling paper by liquid chromatography. Chromatographia. 2002;55:5–12. [Google Scholar]