Abstract
Aim
To analyse the pharmacokinetics of melphalan in 52 children (0.3–18 years) and determine whether any clinical factors affect the pharmacokinetic parameters Additionally, to examine whether a test melphalan dose can predict the pharmacokinetics of a full dose, when there are 5 intervening days of carboplatin therapy.
Methods
Melphalan concentrations were measured in 14 blood samples collected from each child following doses ranging from 30 to 180 mg m−2. The pharmacokinetics were analysed with Kinetica 4.0.
Results
Children who did not have carboplatin (n = 27) had median melphalan clearance (CL) of 15.5 l h−1 m−2 (interquartile range: 12.4–19.9 l h−1 m−2) and steady state volume of distribution (Vss) of 14.9 l m−2 (interquartile range: 12.7–18.3 l m−2). Children who had carboplatin (n = 25) had 34% lower median CL (10.2 l h−1 m−2) and 18% lower median Vss (12.2 l m−2) (P < 0.001). Melphalan elimination was impaired in a separate group of three children given concomitant carboplatin and etoposide. Stepwise multiple linear regression indicated that weight, carboplatin, glomerular filtration rate (GFR) and total body irradiation (TBI) significantly affected CL, while weight and carboplatin influenced Vss. A test dose (10 mg m−2) tended to underpredict the area-under-the-concentration-vs.-time-curve for a full (180 mg m−2) dose in 19 individuals given carboplatin.
Conclusions
In children, melphalan CL is influenced by weight, carboplatin, TBI and GFR. Vss is influenced by weight and carboplatin.
Keywords: Melphalan pharmacokinetics, children, weight, carboplatin, renal function
Introduction
Melphalan is an alkylating agent that has demonstrated activity against a number of malignant diseases in children [1]. High dose melphalan has been used as a single agent or in combination with other anticancer agents (e.g. carboplatin) before autologous haematopoietic stem cell transplantation in the treatment of solid tumours including advanced stage neuroblastoma, rhabdomyosarcoma, soft tissue sarcoma and Ewings sarcoma [2]. Haematological malignancies, including acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL), have also been treated with high-dose melphalan alone or in combination with other anticancer drugs and followed by autologous or allogeneic bone marrow transplantation (BMT) [3, 4]. In these regimens, melphalan is usually administered at doses ranging from 140 to 200 mg m−2. Dose escalation beyond 200 mg m−2 is limited by myelosuppression and gastro-intestinal tract toxicity [5–7], the severity of which has been related to melphalan exposure [8, 9].
The main mechanisms of melphalan elimination are renal excretion and spontaneous degradation to its mono and di-hydroxy metabolites [10, 11]. However, there is some debate about the relative importance of these two modes of elimination. While some previous publications have indicated that spontaneous hydrolysis is the most important elimination pathway [11, 12], Gera et al.[13] provide evidence that this pathway is relatively minor: after 24 h of incubation at 37 °C in whole blood and plasma, the decrease in melphalan concentration due to hydrolysis was only 5%. Human plasma proteins have been found to retard the hydrolysis rate of melphalan [14]. In water and in urine, however, melphalan undergoes rapid decomposition [11, 14]. The chemical instability of melphalan in urine has made it difficult to study the 24 h urinary excretion of melphalan and to assess the importance of renal excretion as an elimination pathway. Alberts et al.[11] found that the mean percentage of the dose excreted unchanged in the urine over 24 h in nine patients was only 13 ± 5.4% (range: 1.5–21.6%), while Reece et al.[10] obtained highly variable results of 34.2 ± 32.9% (mean ± SD, n = 9) and a range of 2.5–92.8%. These latter authors paid particular attention to freezing the urine specimens rapidly. However, they suggest that some losses could have occurred prior to the collection and freezing of specimens, particularly in the bladder, explaining the wide variability in their results. The 24 h urinary excretion of melphalan was probably underestimated in both of these studies. However, in three patients Reece et al.[10] recovered greater than 60% of the dose in the urine over 24 h, suggesting that renal excretion could be a very important elimination pathway for melphalan.
A number of researchers have investigated the pharmacokinetics of melphalan in children [15–17] and they have observed wide intersubject variability in the pharmacokinetic parameters, with clearance estimates varying up to 10-fold. Most of these studies included only small numbers of patients and did not identify sources of variability in the pharmacokinetic parameters, although some adult studies noted correlations with glomerular filtration rate (GFR) [9, 18, 19]. Melphalan pharmacokinetics have been previously shown to be linear with dose in patients not receiving carboplatin [9, 20], and a test dose was successfully used to determine the dose required to provide a desired AUC [9]. However, linear pharmacokinetics and successful pharmacokinetically-guided dosing have not been demonstrated in patients receiving carboplatin.
The main aim of this investigation was to analyse the pharmacokinetics of melphalan in children and to determine whether clinical factors such as weight, renal function, carboplatin therapy and total body irradiation (TBI) affect the pharmacokinetic parameters. A second aim was to examine whether a test dose of melphalan can predict the pharmacokinetics of a full dose when the two doses are separated by 5 days of carboplatin therapy.
Materials and methods
Materials
Melphalan (Alkeran) for clinical administration and for use as standards in the assay was obtained from Glaxo Wellcome Australia Ltd.
Patients
A total of 52 children aged between 0.3 and 18 years undergoing BMT for malignant diseases were involved in the study. The Children's Hospital at Westmead's Ethics Committee approved the study and the parents of all children involved gave informed consent. Table 1 gives the clinical details for these children. Glomerular filtration rate (GFR) was determined for all patients by measuring the plasma clearance of 43Tc99-diethylenetriaminepentacetic acid.
Table 1.
Clinical data of 52 children who received melphalan prior to bone marrow transplantation
| Age (years) | 5.6 |
| Median (interquartile range) | (3.2–9.9) |
| Weight (kg) | 19.1 |
| Median (interquartile range) | (13.6–25.9) |
| Surface area (m2) | 0.77 |
| Median (interquartile range) | (0.60–0.96) |
| GFR (absolute) (ml min−1) | 50 |
| Median (interquartile range) | (39–70) |
| GFR (normalized) (ml min−1 1.73 m−2) | 110 |
| Median (interquartile range) | (94–137) |
| Diagnosis (number of patients) | |
| Acute myeloid leukaemia | 7 |
| Acute lymphoblastic leukaemia | 9 |
| Non-Hodgkins lymphoma | 3 |
| Neuroblastoma | 18 |
| Rhabdomyosarcoma | 6 |
| Soft tissue sarcoma | 3 |
| Ewings sarcoma | 2 |
| Chondrosarcoma | 1 |
| Hepatoblastoma | 1 |
| Retinoblastoma | 1 |
| Mediastinal large cell lymphoma | 1 |
| BMT conditioning (Number of patients) | |
| Mel (4 × 30 mg m−2) | 1 |
| Mel (4 × 30 mg m−2) + TBI + etoposide | 2 |
| Mel (3 × 70 mg m−2) + BU | 4 |
| Mel (100 mg m−2) + BU | 1 |
| Mel (140 mg m−2) + BU | 5 |
| Mel (140 mg m−2) + TBI | 8 |
| Mel (140 mg m−2) + TBI + Thiotepa | 3 |
| Mel (180 mg m−2) | 2 |
| Mel (180 mg m−2) + CPT | 16 |
| Mel (180 mg m−2) + CPT + TBI | 8 |
| Mel (180 mg m−2) + TBI + Thiotepa | 1 |
| Mel (180 mg m−2) + CPT + etoposide | 1 |
BMT: Blood or marrow transplant; Mel: Melphalan; CPT: carboplatin; TBI: total body irradiation; BU: busulphan.
Melphalan doses and BMT conditioning regimens
The chemotherapy regimens for the 52 children are summarized in Table 1. A total of 27 children had no carboplatin as part of their conditioning regimen (No Carboplatin Group), with melphalan given as single high doses of 140 or 180 mg m−2, or as part of divided dose schedules (3 days of 70 mg m−2 or 4 days of 30 mg m−2 melphalan). Of these, 10 children, following the regimens of Valteau-Couanet et al.[21] or Watanabe et al.[4], had busulphan prior to melphalan, while 14 had prior TBI. One child, who was scheduled for busulphan and melphalan (140 mg m−2) had impaired renal function (GFR was 55 ml min−1 1.73 m−2) and was given a reduced melphalan dose of 100 mg m−2.
A total of 25 children received a 180 mg m−2 melphalan dose following 5 days of carboplatin according to the regimen of Shaw et al.[2] (Carboplatin Group). In 19 of these, a test dose of melphalan (10 mg m−2) was administered prior to the carboplatin. The daily carboplatin dose was determined using a formula based on GFR and surface area which aimed to achieve an area-under-the-concentration-time-curve (AUC) of 4 mg ml−1 min−1. Carboplatin was given as a 1 h intravenous infusion in the evening (approximately 18:00 h) with overnight hydration. Pre-transplant conditioning also included TBI for eight children (given prior to melphalan) and etoposide for one child.
Melphalan administration
All patients had a double lumen central line, so that one lumen could be used for drug administration and one for sampling. To avoid contamination, 5 ml of blood was withdrawn and discarded prior to taking each sample.
Melphalan was administered intravenously as a 15–20-min injection in the majority of children (n = 50), but in two children the drug was administered over 20 min using a burette with 10 min of flushing. Melphalan was administered with double maintenance fluids.
Blood samples
Heparinized whole blood samples (2–3 ml) for the measurement of melphalan concentrations were collected prior to the infusion and then at 0 min, 5 min, 10 min, 15 min, 20 min, 30 min, 40 min, 50 min, 1 h, 2 h, 3 h, 4 h, 6 h, 12 h and 24 h after the infusion end. Plasma samples were separated by centrifugation for 10 min at 4 °C at 1500 g, then frozen and stored at −40 °C until analysis. Samples were analysed within 1 week of collection. Melphalan has previously been shown to be stable in plasma for 3 weeks at −20 °C [22].
Melphalan assay
Melphalan was determined in plasma using a modified version of a previous method [23]. Melphalan was extracted from plasma (0.1 ml) by protein precipitation with methanol (0.2 ml). After vortexing for 10 s and centrifuging for 2 min at 12000 g, a methanolic extract was obtained which was injected into the high performance liquid chromatography (HPLC) system. The HPLC system consisted of an ICI LC1100 pump (GBC Scientific Equipment P/L, Dandedong, VIC, Australia) and a Waters model 2487 dual wavelength UV detector set at 254 nm (Waters Australia P/L, Rydalmere, NSW, Australia). Melphalan was eluted with a retention time of 13 min at ambient temperature using a Phenomenex (25 cm × 4.1 mm, 5 micron, Spheri-5) column (Phenomenex Australia, Lane Cove, NSW, Australia) fitted with a Brownlee (1.5 cm × 3.2 mm, 7 micron RP-18) precolumn (Alltech Associates P/L, Baulkham Hills, NSW, Australia), a flow rate of 1 ml min−1 and a mobile phase consisting of 0.02 m sodium phosphate pH 3.75 buffer and acetonitrile in 77 : 23 proportions. The plasma melphalan concentration was determined by comparing the sample peak height with those of extracted plasma standards containing 0, 0.5, 1.0, 2.5, 5.0, 7.5, 10 and 15 µg ml−1 melphalan. The between-day coefficient of variation of the assay was 15% for a 3.2 µm concentration (n = 25), 10% for a 8.3 µm concentration (n = 12), 6% for a 16.3 µm concentration (n = 25) and 5% for a 24.1 µm concentration (n = 12). The limit of detection of the assay was approximately 0.1 µm. The limit of quantification was approximately 0.5 µm. The calibration curve was linear over the range 1.6–131 µm melphalan.
Pharmacokinetic analysis
Melphalan pharmacokinetic parameters were determined using the computer software, Kinetica 4.0 (Innaphase, Philadelphia, USA). The area-under-the-melphalan concentration-time-curve (AUC0–∞) was determined using mixed log linear rule. Additional pharmacokinetic parameters were also generated including clearance (CL), volume of distribution during the terminal phase (Vz), volume of distribution at steady state (Vss), mean residence time (MRT) and elimination half-life (t1/2).
Investigating the influence of patient clinical factors on melphalan pharmacokinetics
Simple regression (implemented in spss version 10.0), incorporating only single covariates, was used to determine the relative importance of different patient covariates in explaining the variability in the pharmacokinetic parameters. These covariates included weight, weight0.75, age, height, surface area, normalized GFR, carboplatin therapy, busulphan therapy and TBI. In this analysis normalized GFR was converted to a categorical variable where GFR values below the 20th percentile (<87 ml min−1 1.73 m−2) were given the value of 1, and GFR values = 88 ml min−1 1.73 m−2) were given the value zero. Stepwise multiple linear regression was then used to identify the most important, independent covariates that significantly affected melphalan pharmacokinetic parameters. In this analysis the probability of F-to-enter was ≤0.05, while the probability of F-to-remove was ≥0.1. Regression equations describing the relationships were generated.
The Mann–Whitney test (implemented in spss version 10.0) was used to examine the effect of carboplatin therapy on melphalan pharmacokinetics, but could not be used for other categorical covariates (e.g. TBI and busulphan therapy) because the treatment groups were not matched in weight or age with the nontreatment groups.
Linearity of melphalan pharmacokinetics within individuals given carboplatin
A total of 19 children received a test dose of 10 mg m−2 melphalan followed by 5 days of carboplatin and then a full dose of 180 mg m−2 melphalan. Melphalan concentrations were measured after administration of both the test and full doses and values for AUC(0−∞) determined. The test dose AUC was used to calculate a predicted AUC for the full dose by multiplying the test dose AUC by 18. This predicted AUC was then compared with the observed full dose AUC using the paired Wilcoxon Signed Ranks test.
Concomitant carboplatin and etoposide
Three children (separate from those in the main study) received 3 days of melphalan concomitant with 24 h intravenous infusions of carboplatin (dose range: 240–425 mg m−2) and etoposide (dose range: 200–338 mg m−2) with prior TBI, according to the LA6 protocol [24]. The melphalan daily dose was 70 mg m−2 for two of the children, while the other child, who was younger than 2 years, received a weight-based dose of 2.3 mg kg−1 (or 52 mg m−2). Melphalan was given as a 2-min intravenous bolus injection. Carboplatin was started immediately following the injection on day 1 and continued throughout the 3 days of melphalan therapy. Melphalan concentrations were monitored for a 24-h period on day 3 of melphalan therapy and AUC(0−24 h) determined using trapezoidal rule (implemented in Kinetica 4.0). The shapes of the concentration vs. time curves were compared with those obtained by five children in the No Carboplatin Group receiving similar 70 mg m−2 doses.
Results
Melphalan concentrations
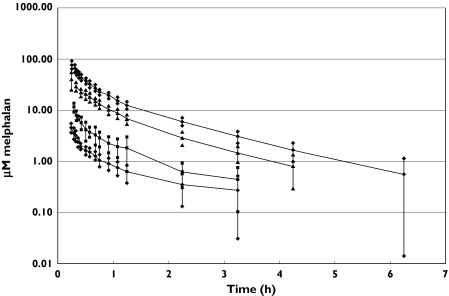
Figure 1 shows a log-plot of mean plasma concentration vs. time curves for children who had a 180 mg m−2 dose with prior carboplatin and for those who had 140, 30 and 10 mg m−2 doses with no carboplatin. Melphalan was either not detected or very low at 6 h after dose administration. Mean plasma melphalan concentrations declined in multiple log-linear segments, indicative of multicompartmental pharmacokinetics. Plots from individual patients (data not shown) showed that the decline in melphalan concentrations with time was either biphasic (n = 36) or triphasic (n = 16).
Figure 1.
Melphalan concentration vs. time curves for children receiving different melphalan doses with or without prior carboplatin. For each time point mean concentrations and the lower and upper limits of the 95% confidence interval are shown. 180 mg/m2, n = 25, carboplatin group (•), 140 mg/m2, n = 16, no carboplatin group (▴), 30 mg/m2, n = 3, no carboplatin group (▪), 10 mg/m2, n = 19, no prior carboplatin (♦)
Comparison with other pharmacokinetic studies
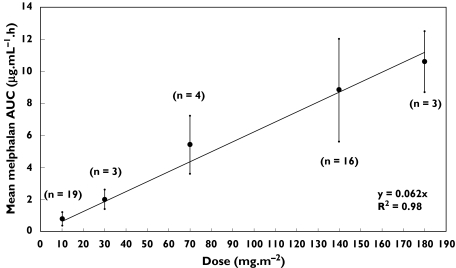
Our mean values for melphalan AUC(0–∞), CL and Vss from children who did not have carboplatin are generally in good agreement with those from previous studies which also involved children who did not have carboplatin (Table 2). However, the group of 25 children in our study who had prior carboplatin stand out as having much higher AUC(0–∞) values (for equivalent doses) and lower clearance values. In Table 2 and in Figure 2 it can be seen that, in the No Carboplatin Group, mean AUC tends to increase linearly with dose.
Table 2.
Comparison of pharmacokinetic studies performed in children
| Study | Children(n) | Age range(years) | Dose(mg m−2) | AUC(0–∞)*(µg ml−1 h−1) | CL*(l h−1 m−2) | Vss*(l m−2) |
|---|---|---|---|---|---|---|
| This study | 19 | 2–11 | 10 | 0.8 ± 0.4 | 16.6 ± 10.5 | 9.0 ± 2.6 |
| No CPT | 3 | 4–11 | 30 | 2.0 ± 0.6 | 14.9 ± 3.7 | 11.5 ± 1.1 |
| 4 | 0.3–6 | 70 | 5.4 ± 1.8 | 14.6 ± 5.5 | 16.3 ± 7.3 | |
| 16 | 1–18 | 140 | 8.8 ± 3.2 | 17.6 ± 5.1 | 15.8 ± 3.2 | |
| 3 | 5–13 | 180 | 10.6 ± 1.9 | 17.3 ± 3.3 | 17.6 ± 7.1 | |
| This study, prior CPT | 25 | 1–12 | 180 | 19.0 ± 5.5 | 10.4 ± 3.5 | 11.4 ± 3.2 |
| Tranchard et al. 1989 [20] | 5 | 4–13 | 140 | 6.3 ± 2.0 | 19.9 ± 5.2 | 11.8 ± 2.8 |
| Ardiet et al. 1986 [15] | 15 | 1–14 | 140 | 6.6 ± 2.4 | 24.0 ± 9.0 | 19.6 ± 9.7 |
| Gouyette et al. 1986 [16] | 15 | 1–14 | 140 | 10.7 ± 5.5 | 15.4 ± 5.8 | |
| 7 | 2–11 | 180 | 7.2 ± 3.0 | 29.9 ± 14.8 |
Mean ±SD; CPT: carboplatin.
Figure 2.
Melphalan dose vs. dose group mean AUC (±SD) in children who did not have prior carboplatin therapy. The number of children (n) in each dose group is also shown
Effect of carboplatin on melphalan pharmacokinetics
Children who had prior carboplatin therapy (n = 25) were found to have 34% lower median melphalan clearance (10.2 vs. 15.5 l h−1 m−2, P < 0.001), 18% lower median Vss (12.2 vs. 14.9 l m−2, P < 0.001), 12% lower median Vz (15.4 vs. 17.5 l m−2, P < 0.05) and a 32% higher median elimination half-life (1.0 vs. 00.92 h, P < 0.005) than 27 children who did not have carboplatin (Table 3). The two groups of children did not differ significantly in age.
Table 3.
Effect of prior carboplatin therapy on melphalan pharmacokinetic parameters
| Parameter (units) | No carboplatin | Carboplatin | Difference (95% CI)‡ | Significance |
|---|---|---|---|---|
| Children (n) | 27 | 25 | ||
| Age (years) Median (interquartile range) | 5.7 (3.3–11.4) | 5.0 (3.0–8.7) | −0.9, 3.6 | NS* |
| Prior TBI (no. children) Yes/No | 4/13 | 8/17 | NS† | |
| CL (l h−1) Median (interquartile range) | 13.1 (9.1–20.4) | 7.3 (5.7–9.8) | 4.3, 12.7 | P < 0.001* |
| CL (l h−1 m−2) Median (interquartile range) | 15.5 (12.4–19.9) | 10.2 (7.7–12.4) | 3.6, 8.4 | P < 0.001* |
| Vss (l) Median (interquartile range) | 11.9 (8.4–18.5) | 8.9 (6.8–10.3) | 2.4, 11.7 | P < 0.005* |
| Vss (l m−2) Median (interquartile range) | 4.9 (12.7–18.3) | 12.2 (8.5–13.4) | 2.0, 6.3 | P < 0.001* |
| Vz (l) Median (interquartile range) | 13.9 (9.7–19.8) | 11.6 (8.8–13.6) | 1.7, 12.3 | P < 0.05* |
| Vz (l m−2) Median (interquartile range) | 17.5 (14.6–20.5) | 15.4 (11.1–17.8) | 0.6, 5.9 | P < 0.05* |
| t1/2 (h) Median (interquartile range) | 0.76 (0.67–0.99) | 1.0 (0.81–1.25) | −0.36, −0.04 | P < 0.005* |
| MRT (h) Median (interquartile range) | 0.92 (0.81–1.18) | 1.12 (0.91–1.27) | −0.32, 0.07 | NS* |
Mann–Whitney test;
Chi square test, NS: Not significant;
Lower and upper limits of the 95% confidence interval of the difference
Regression models describing the relationships between melphalan pharmacokinetic parameters and patient clinical factors
It was possible to develop significant (P < 0.001) simple regression models for CL, Vss and Vz that incorporated only weight, weight0.75, age, height or surface area. Of these, the models incorporating only weight explained the greatest amount of the variability in CL (80%), Vss (85%) and Vz (84%). Weight0.75 explained an equivalent proportion of the variability in CL (80%) but a slightly lower proportion of the variability in V (83%) and Vss (83%). Models with only carboplatin explained 23% of the variability in CL (P < 0.001), 14% of the variability in Vss (P < 0.005) and 10% of the variability in Vz (P < 0.05). Those incorporating only TBI explained 16% of the variability in CL (P < 0.005), 8% of the variability in Vss (P < 0.05) and 7% of the variability in Vz (P < 0.05). Normalized GFR (treated as a categorical variable) explained 9% of the variability in CL (P < 0.05) using simple regression. Therapy with busulphan was not significant in any regression model.
Regression equations describing multiple linear regression models for CL, V and Vss are shown in Table 4. The model for CL incorporated weight, carboplatin therapy, normalized GFR and TBI. The model for Vss incorporated weight and carboplatin therapy, while the model for Vz incorporated only weight. The proportion of variability in the pharmacokinetic parameters explained by these regression models was 89% for CL, 86% for Vss and 84% for Vz. The standardized residuals from the regression models each had means of zero and distributions which were close to normal.
Table 4.
Regression models describing the relationships between the influential patient covariates and the pharmacokinetic parameters of melphalan
| Regression model | Regression coefficients (B) Mean (95% CI) | R2 | F | Significance |
|---|---|---|---|---|
| CL (l h−1) = B1 + B2WT + B3CPT + B4GFR + B5TBI | B1: 4.77 (2.56, 6.98) B2: 0.37 (0.32, 0.42) | 0.89 | 95 | P < 0.001 |
| B3: −3.99 (−5.80, −2.17) B4: −2.84 (−5.08, −0.59) | ||||
| B5: 2.10 (0.25, 3.94) | ||||
| Vz (l) = B1 + B2WT | B1: 2.36 (0.38, 4.33) B2: 0.52 (0.46, 0.59) | 0.84 | 260 | P < 0.001 |
| Vss (l) = B1 + B2 WT + B3CPT | B1: 2.50 (0.31, 4.68) B2: 0.45 (0.39, 0.50)B3: −2.24 (−4.29, −0.19) | 0.86 | 151 | P < 0.001 |
WT: weight (kg); CPT: Prior carboplatin therapy (code: no = 0, yes = 1); TBI: Prior total body irradiation (code: no = 0, yes = 1); GFR: GFR (code: 0 for GFR ≥ 88 ml min−1 1.73 m2, 1 for GFR < 87 ml min−1 1.73 m−2); 95% CI: lower and upper limits of the 95% confidence interval.
Melphalan dose reduction in one child with impaired renal function
One child with impaired renal function was given a reduced melphalan dose of 100 mg m−2 instead of 140 mg m−2 and achieved an AUC(0–∞) of 13.9 µg ml−1 h−1. This level of exposure was comparable with that of five other children on the same regimen who received 140 mg m−2 melphalan and achieved a median AUC(0–∞) of 10.2 mg l−1 h−1 (interquartile range: 7.0–16.9 mg l−1 h−1).
Linearity of melphalan pharmacokinetics within individuals given carboplatin
The median full dose AUC that was predicted from a test dose given to 19 children before starting carboplatin was 14.3 µg ml−1 h−1 (interquartile range: 9.4–17.1 µg ml−1 h−1). The actual observed AUC after 5 days of carboplatin was 24% higher with a median value of 17.7 µg ml−1 h−1 and an interquartile range of 14.8–23.9 µg ml−1. This difference was significant (P < 0.005) using the paired Wilcoxon Signed ranks test.
Concomitant carboplatin and etoposide
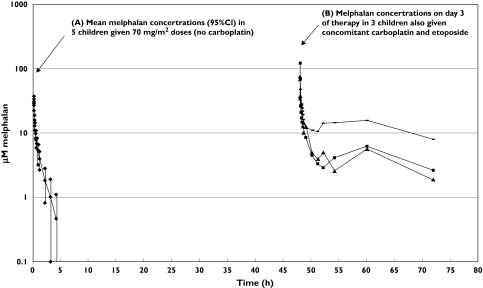
Figure 3 shows log-plots of melphalan concentration vs. time curves from three children who had concomitant carboplatin and etoposide as well as mean levels from five children who had similar 70 mg m−2 doses, but no carboplatin. It can be seen that the shapes of these curves differ markedly. During the terminal elimination phase, melphalan concentrations in the children who had concomitant carboplatin and etoposide remained static, instead of declining in a log-linear fashion. It was therefore impossible to determine the elimination half-life. Melphalan persisted in the plasma of these children, remaining at detectable levels even at 24 h after the dose. In contrast, melphalan concentrations in children receiving comparable 70 mg m−2 doses but no carboplatin were generally below 1 µm at 4 h after the dose.
Figure 3.
(A) Mean melphalan concentration vs. time-curve (and 95% confidence intervals) in five children who had 70 mg m−2 doses, and no carboplatin; (B) melphalan concentration vs. time curves in three children who had concomitant carboplatin and etoposide. Patient 1: 70 mg/m2 melphalan with carboplatin and etoposide (▄), patient 2: 70 mg/m2 melphalan with carboplatin and etoposide (▪), patient 3: 23 mg/kg melphalan with carboplatin and etoposide (▴), 70 mg/m2 melphalan, mean of 5 children (96% CI), no carboplatin (♦)
The mean peak concentration for the three children receiving concomitant carboplatin and etoposide was 86.2 µm (range: 66–119 µm), which was 3.6-fold higher than the mean peak concentration of 23.8 µm (range: 13.7–35.8 µm), obtained for the five children in the No Carboplatin Group who had 70 mg m−2 melphalan. Concomitant carboplatin and etoposide result in high and variable exposure to melphalan: AUC(0−24 h) values were 25.9, 43.0 and 96.2 µg ml−1 h−1 for melphalan doses of 2.3 mg kg−1, 70 mg m−2 and 70 mg m−2, respectively. These values are 4.8–17.8-fold higher than the mean AUC(0–∞), of 5.4 ± 1.8 µg ml−1 h−1, obtained from children receiving similar doses of 70 mg m−2 melphalan, but no carboplatin.
Discussion
In this study, the decline in melphalan concentrations with time was observed to be either biphasic or triphasic. This suggests that melphalan distributes into multiple compartments as has been previously observed [15, 25].
Rather than using a combination of two and three compartment models to determine the pharmacokinetic parameters, we used a noncompartmental pharmacokinetic analysis method. AUC(0–∞) was calculated for all patients except the three who had concomitant carboplatin and etoposide, where the failure to observe a linear decline in melphalan concentrations during the terminal elimination phase made it impossible to extrapolate the AUC to infinity. In these children AUC(0−24 h) was therefore determined using trapezoidal rule.
Patient weight was found to be the most important clinical factor influencing melphalan pharmacokinetic parameters. Using simple regression it was shown that weight alone explained a large proportion of the variability in CL (80%), Vss (85%) and Vz (84%). If carboplatin, normalized GFR and TBI, in addition to weight, are included in a multiple linear regression model for CL, then there is only an additional 9% variability explained when compared with the weight-only model. Similarly, the inclusion of carboplatin explained only 2% more of the variability in Vss than the weight-only model. In children, weight is related to age, height, surface area and absolute glomerular filtration rate. Thus, simple regression analysis also identified these other covariates as factors that significantly influence melphalan clearance and volume of distribution. As children grow and increase in size, there is an increase in the size of the kidneys, in glomerular filtration rate and in urine output, an increase in the volume of different compartments in the body (e.g. the total body water compartment). These changes may explain the increases in melphalan clearance and volume of distribution with weight and age.
Melphalan pharmacokinetic parameters were strongly affected by prior carboplatin therapy. Children who had prior carboplatin had 34% lower median estimates of melphalan clearance and 12–18% lower median estimates of volume of distribution than children who did not have any carboplatin therapy. Additionally, a test dose of melphalan failed to accurately predict the AUC of a full dose when the two doses were separated by 5 days of carboplatin therapy, providing further evidence of an effect of prior carboplatin therapy upon melphalan pharmacokinetics. Tranchard et al.[26] also showed that 5 days of carboplatin administration prior to a test dose of melphalan on day 6 and the full dose on day 7 affected the linearity of melphalan pharmacokinetics and the test dose did not accurately predict the pharmacokinetics of the full dose. Pharmacokinetically-guided dosing is therefore not possible in patients receiving carboplatin.
In children not receiving carboplatin, the use of a test dose of melphalan to predict the AUC of the full dose is feasible as we found a linear relationship between dose and mean AUC (Figure 2). The correlation between dose and AUC was very close (r2 = 0.98), especially considering the dose-groups consisted of different individuals and that there is considerable intersubject variability in melphalan clearance. In this study the ability of a test dose to predict the AUC of a full dose was not investigated in children not receiving carboplatin because previous researchers have already demonstrated linear melphalan pharmacokinetics in the same individuals [20] and have successfully used the test dose method to adjust the dose to achieve a target AUC [9].
In this study low normalized GFR (<87 ml min−1 1.73 m−2) was identified as a significant determinant of melphalan clearance using stepwise multiple linear regression. The negative sign in the regression equation indicates that clearance tends to be lower when normalized GFR is low. This finding is consistent with previous studies showing increased toxicity [5, 27, 28] and decreased clearance of melphalan [28] when renal function is impaired. Dose reduction in renal impairment may therefore be necessary and is suggested by the results of a large Clinical Cancer and Leukaemia Group B study where patients with renal dysfunction were found to have significantly increased toxicity which was improved by a 50% reduction in dose [5]. In the present study, one child had a very low GFR (55 ml min−1 1.73 m−2) prior to busulphan/melphalan conditioning [21] and received a 29% reduced dose of melphalan (100 mg m−2 instead of 140 mg m−2). Exposure to melphalan was still more than adequate in this child, as the AUC obtained (13.9 µg ml−1 h−1) was comparable with that achieved by the five other children on a similar regimen, who had a median AUC of 10.2 µg ml−1 h−1. This result suggests that melphalan doses should be reduced in children with impaired renal function. However, it will be necessary to study a greater number of children with renal impairment to confirm this.
Regression analysis showed that prior TBI was associated with higher clearance of melphalan. Kergueris et al.[18] also noted that clearance of melphalan tended to be higher after TBI, but significance was not demonstrated because of the small number of patients in that study.
The children who had 24 h intravenous carboplatin and etoposide concomitant with melphalan according to the LA6 protocol were found to have impaired elimination of melphalan and achieved high concentrations of melphalan in plasma. On day 3 of melphalan therapy when carboplatin and etoposide are presumably at steady state, exposure after 24 h (AUC(0−24 h)) ranged from 25.9 to 96.2 mg l−1 h−1 and were 5–20-fold higher than the mean AUC(0–∞) of 5.4 mg l−1 h−1, that was achieved by children receiving comparable doses of 70 mg m−2 melphalan but no carboplatin. It is likely that this very high exposure to melphalan may result in severe toxicity. Patients with poor renal function may be particularly vulnerable on this protocol since impaired renal function, in addition to concomitant carboplatin therapy, may lead to increased exposure to melphalan. Indeed, in one report all toxic deaths occurring in children treated according to the LA6 protocol were in children with low GFRs [24]. Another problem with this conditioning regimen is the high degree of variability in melphalan exposure, which may lead to variable and unpredictable outcomes. It should be noted that it is unclear from our study whether it is carboplatin or etoposide which interacts pharmacokinetically with melphalan. However, Peters et al.[29] observed altered pharmacokinetics and a prolonged plasma melphalan elimination half-life in adult patients receiving concomitant cisplatin, a drug similar to carboplatin. This, and our results showing altered melphalan pharmacokinetics with prior carboplatin therapy, strongly suggest that it is carboplatin which alters the pharmacokinetics of melphalan but further studies are needed to clarify this.
An interaction between melphalan and carboplatin could occur through a number of mechanisms. Evidence from three studies [10–12] suggest that renal clearance is an important route for melphalan excretion. Carboplatin is also primarily eliminated by the kidneys [30] and may compete with melphalan for renal clearance. Platinum therapy has also been known to induce renal damage [31]. In one series, half the patients developed reduced GFR during carboplatin therapy [32]. It is therefore possible that 5 days of carboplatin therapy may have an immediate effect on renal function, resulting in reduced elimination of melphalan. Plasma protein binding interactions are another possible source of interaction between melphalan and carboplatin. Further studies are required to determine which mechanisms are responsible.
This preliminary study on melphalan pharmacokinetics uses a traditional two-stage analysis method. After acquiring additional data we intend to develop a population pharmacokinetic model for melphalan that will use the mean estimates for CL and V from this study as prior estimates for the population analysis. A population pharmacokinetic model will ultimately provide more reliable estimates of pharmacokinetic variability.
In this study we found that weight explained a high proportion of the variability in clearance and volume of distribution. Prior carboplatin therapy and low GFR values (<87 ml min−1 1.73 m−2) were found to decrease melphalan clearance, while prior TBI increased clearance. In a limited number of children, we also observed extremely high and variable exposure to melphalan when it was administered concomitantly with carboplatin and etoposide. This has the potential to cause severe toxicity in some patients. A pharmacokinetic interaction between melphalan and carboplatin or etoposide needs to be investigated further.
Acknowledgments
C.E. Nath is supported by the Leukaemia Research and Support Fund. K Montgomery was responsible for collection of the detailed pharmacokinetic sampling for most of the study patients. We would like to thank the patients and their families for taking part in the study and the nursing staff in the oncology unit for their care of the patients including taking samples for pharmacokinetic analysis.
Competing Interests: None to declare.
References
- 1.Samuels BL, Bitran JD. High dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–99. doi: 10.1200/JCO.1995.13.7.1786. [DOI] [PubMed] [Google Scholar]
- 2.Shaw PJ, Pinkerton CR, Yaniv I. Melphalan combined with carboplatin dose based on glomerular filtration rate followed by autologous stem cell rescue for children with solid tumours. Bone Marrow Transplant. 1996;16:401–5. [PubMed] [Google Scholar]
- 3.Michel G, Maraninchi D, Demeocq F, et al. Repeated courses of high dose melphalan and unpurged autologous bone marrow transplantation in children with acute non-lymphoblastic leukemia in first complete remission. Bone Marrow Transplant. 1998;3:105–11. [PubMed] [Google Scholar]
- 4.Watanabe T, Kajiume T, Abe T, et al. Allogeneic peripheral blood stem cell transplantation in children with haematological malignancies from HLA-matched siblings. Med Paediatr Onc. 2000;34:171–6. doi: 10.1002/(sici)1096-911x(200003)34:3<171::aid-mpo2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Cornwell GG, III, Pajak TF, McIntyre OR, et al. Influence of renal failure on myelosuppressive effects of melphalan: cancer and leukemia group B experience. Cancer Treat Rep. 1982;66:475–81. [PubMed] [Google Scholar]
- 6.Moreau P, Kergueris M-F, Milpied N, et al. A pilot study of 220 mg/m2 melphalan followed by autologous stem cell transplantation in patients with advanced haematological malignancies: pharmacokinetics and toxicity. Br J Haematol. 1996;95:527–30. doi: 10.1046/j.1365-2141.1996.d01-1932.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarosy G, Leyland-Jones B, Soochan P, Cheson BD. The systemic administration of intravenous melphalan. J Clin Oncol. 1988;6:1768–82. doi: 10.1200/JCO.1988.6.11.1768. [DOI] [PubMed] [Google Scholar]
- 8.Vassal G, Tranchard B, Valteau-Couanet D, et al. Pharmacodynamics of tandem high-dose melphalan with peripheral blood stem cell transplantation in children with neuroblastoma and medulloblastoma. Bone Marrow Transplant. 2001;27:471–7. doi: 10.1038/sj.bmt.1702806. [DOI] [PubMed] [Google Scholar]
- 9.Ploin DY, Tranchard B, Guastalla JP, et al. Pharmacokinetically guided dosing for intravenous melphalan: a pilot study in patients with advanced ovarian adenocarcinoma. Eur J Cancer. 1992;28:1311–5. doi: 10.1016/0959-8049(92)90506-w. [DOI] [PubMed] [Google Scholar]
- 10.Reece PA, Hill HS, Green RM, et al. Renal clearance and protein binding of melphalan in patients with cancer. Cancer Chemother Pharmacol. 1988;22:348–52. doi: 10.1007/BF00254244. [DOI] [PubMed] [Google Scholar]
- 11.Alberts DS, Chang SY, Chen HS, et al. Kinetics of intravenous melphalan. Clin Pharmacol Ther. 1979;26:73–80. doi: 10.1002/cpt197926173. [DOI] [PubMed] [Google Scholar]
- 12.Tattersall MHN, Jarman M, Newlands ES, et al. Pharmacokinetics of melphalan following oral or intravenous administration in patients with malignant disease. Eur J Cancer. 1978;14:507–13. doi: 10.1016/0014-2964(78)90253-0. [DOI] [PubMed] [Google Scholar]
- 13.Gera S, Musch E, Osterheld HK, Loos U. Relevance of the hydrolysis and protein binding of melphalan to the treatment of multiple myeloma. Cancer Chemother Pharmacol. 1989;23:76–80. doi: 10.1007/BF00273521. [DOI] [PubMed] [Google Scholar]
- 14.Chang SY, Alberts DS, Farquhar D, et al. Hydrolysis and protein binding of melphalan. J Pharm Sci. 1978;67:682–4. doi: 10.1002/jps.2600670530. [DOI] [PubMed] [Google Scholar]
- 15.Ardiet C, Tranchard B, Biron P, et al. Pharmacokinetics of high-dose intravenous melphalan in children and adults with forced diuresis. Cancer Chemother Pharmacol. 1986;16:300–4. doi: 10.1007/BF00293997. [DOI] [PubMed] [Google Scholar]
- 16.Gouyette A, Hartmann O, Pico JL. Pharmacokinetics of high dose melphalan in children and adults. Cancer Chemother Pharmacol. 1986;16:184–9. doi: 10.1007/BF00256174. [DOI] [PubMed] [Google Scholar]
- 17.Taha AK, Ahmad RA, Rogers DW, et al. Pharmacokinetics of melphalan in children following high-dose intravenous injection. Cancer Chemother Pharmacol. 1983;10:212–6. doi: 10.1007/BF00255766. [DOI] [PubMed] [Google Scholar]
- 18.Kergueris MF, Milpied N, Moreau P, et al. Pharmacokinetics of high-dose melphalan in adults: influence of renal function. Anticancer Res. 1994;14:2379–82. [PubMed] [Google Scholar]
- 19.Pinguet F, Martel P, Fabbro M, et al. Pharmacokinetics of high-dose intravenous melphalan in patients undergoing peripheral blood hematopoietic progenitor-cell transplantation. Anticancer Res. 1997;17:605–12. [PubMed] [Google Scholar]
- 20.Tranchard B, Ploin DY, Minuit MP, et al. High-dose melphalan adjustment: possibility of using a test dose. Cancer Chemother Pharmacol. 1989;23:95–100. doi: 10.1007/BF00273524. [DOI] [PubMed] [Google Scholar]
- 21.Valteau-Couanet D, Benhamou E, Vassal G, et al. Consolidation with a busulfan-containing regimen followed by stem cell transplantation in infants with poor prognosis stage 4 neuroblastoma. Bone Marrow Transplant. 2000;25:937–42. doi: 10.1038/sj.bmt.1702376. [DOI] [PubMed] [Google Scholar]
- 22.Chang SY, Alberts DS, Farquhar D, et al. High pressure liquid chromatographic analysis of melphalan in plasma. J Pharm Sci. 1978;67:679–82. doi: 10.1002/jps.2600670529. [DOI] [PubMed] [Google Scholar]
- 23.Springolo V, Borella F, Finardi GP, et al. High-performance liquid chromatographic determination of m-bis (chloroethyl) aminophenyl-L-alanine in plasma. J Chromatogr. 1989;490:224–9. doi: 10.1016/s0378-4347(00)82779-6. [DOI] [PubMed] [Google Scholar]
- 24.Villablanca J, Matthay K, Swift P, et al. Phase 1 trial of carboplatin, etoposide, melphalan and local irradiation (CEM-L1) with purged autologous bone marrow transplantation (ABMT) for high risk neuroblastoma. Med Pediatr Onc. 1999;33:170–Abstract O-114. [Google Scholar]
- 25.Ninane J, Baurain R, de Selys A, et al. High dose melphalan in children with advanced malignant disease: a pharmacokinetic study. Cancer Chemother Pharmacol. 1985;15:263–7. doi: 10.1007/BF00263898. [DOI] [PubMed] [Google Scholar]
- 26.Tranchard B, Ardiet C, Bouffet E, et al. Effect of carboplatin on the pharmacokinetics of melphalan administered by the intravenous route. Bull Cancer. 1994;81:43–6. [PubMed] [Google Scholar]
- 27.Schuh A, Dandridge J, Haydon P, Littlewood TJ. Encephalopathy complicating high-dose melphalan. Bone Marrow Transplant. 1999;24:1141–3. doi: 10.1038/sj.bmt.1702041. [DOI] [PubMed] [Google Scholar]
- 28.Alberts DS, Chen H-SG, Benz D, Mason NL. Effect of renal dysfunction in dogs on the disposition and marrow toxicity of melphalan. Br J Cancer. 1981;43:330–4. doi: 10.1038/bjc.1981.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters WP, Stuart A, Klotman M, et al. High-dose combination cyclophosphamide, cisplatin and melphalan with autologous bone marrow support. Cancer Chemother Pharmacol. 1989;23:377–83. doi: 10.1007/BF00435840. [DOI] [PubMed] [Google Scholar]
- 30.Olver IN, Webster LK, Millward MJ, et al. A phase I and pharmacokinetics study of prolonged ambulatory-infusion carboplatin. Cancer Chemother Pharmacol. 1995;37:79–85. doi: 10.1007/BF00685632. [DOI] [PubMed] [Google Scholar]
- 31.de Gislain C, Dumas M, d’Athis P, et al. Urinary β2-microglobulin: early indicator of high dose cisdiamminedichloroplatinum nephrotoxicity? Influence of furosemide. Cancer Chemother Pharmacol. 1986;18:276–9. doi: 10.1007/BF00273402. [DOI] [PubMed] [Google Scholar]
- 32.Shea TC, Flaherty M, Elias A, et al. A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support. J Clin Oncol. 1989;7:651–61. doi: 10.1200/JCO.1989.7.5.651. [DOI] [PubMed] [Google Scholar]