Abstract
Aims
To assess the reproducibility of the forearm blood flow (FBF) response to intra-arterial infusion of calcitonin-gene related peptide (CGRP), measured by venous occlusion plethysmography. In addition, to compare different ways of expressing the FBF response and perform sample size calculations.
Methods
On two separate visits, CGRP (10 ng min−1 dl−1 forearm) was infused for 45 min into the brachial artery of six healthy subjects. Reproducibility was assessed by calculating mean difference, repeatability coefficient, within-subject coefficient of variation (WCV) and intraclass correlation coefficient.
Results
CGRP increased FBF from 2.8 ± 0.4 and 3.2 ± 0.7 (at baseline) to 15.4 ± 1.4 and 15.2 ± 1.5 ml min−1 dl−1 forearm (at 45 min) on visits 1 and 2, respectively (P < 0.0001 for both visits). Mean difference in FBF at 45 min between both visits was 0.3 ml min−1 dl−1 forearm (repeatability coefficient: 4.1 ml min−1 dl−1 forearm). This FBF response appeared to be more reproducible when expressed as absolute FBF in the infused arm (WCV 11%) compared with absolute FBF-ratio between both arms (WCV 37%), percentage change from baseline in FBF in the infused arm (WCV 29%) and percentage change from baseline in FBF-ratio (WCV 40%). When expressed as absolute FBF, a sample size of five (95% confidence interval: 2–12) subjects gives 90% power at a type I error probability of 0.05 to detect a 25% shift in FBF response.
Conclusions
Intra-arterial infusion of CGRP results in a forearm vasodilator response which is reproducible between days. This response is most reproducible when expressed as absolute FBF. The presented methodology provides a suitable pharmacodynamic model to assess the in vivo activity of CGRP-receptor antagonists in a small number of subjects.
Keywords: forearm blood flow, venous occlusion plethysmography, calcitonin gene-related peptide, reproducibility, vasodilation
Introduction
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide, which is broadly distributed in the cardiovascular system through a dense peripheral sensory network innervating arteries, veins and the heart [1]. It is also present in sensory neurones of the central nervous system, originating from the trigeminal ganglion and innervating the cerebral circulation [2]. CGRP is one of the most potent endogenous vasodilatory peptides [3] and exerts its vasorelaxant effects through interaction with CGRP-receptors, present on the vascular endothelium and vascular smooth muscle cells [4].
CGRP is suggested to play a major role in the pathophysiology of primary headaches, in particular migraine and cluster headache [5]. This observation fuelled research leading to the development of small-molecule CGRP-receptor antagonists [6–8]. Recently, one of these compounds proved to be effective in the acute treatment of migraine [9]. The mechanism of action of this potential new class of acute antimigraine drugs is still unclear. Both vascular mechanisms, i.e. inhibition of CGRP-induced vasodilation [6] and nonvascular mechanisms, i.e. inhibition of pain transmission in the trigeminocervical complex [10], have been proposed.
Venous occlusion plethysmography combined with brachial artery infusion provides a powerful tool for the assessment of vascular effects of vasoactive compounds [11]. Using this technique, local vasodilation of human forearm blood vessels during intra-arterial infusion of CGRP has repeatedly been demonstrated [12–17]. The technique has been used previously to evaluate endothelin-1 receptor antagonists [18–21] and a neurokinin-1 (substance P) receptor antagonist [22]. Likewise, we intend to use the technique to assess the pharmacodynamic effects of CGRP-receptor antagonists in an early phase of clinical development. To that end, the reproducibility of CGRP-induced vasodilation assessed by venous occlusion plethysmography needs to be established. The aim of the present study was to assess the reproducibility of the forearm blood flow (FBF) response to intrabrachial infusions of CGRP in healthy subjects. In addition, different ways of expressing the FBF response were compared and sample size calculations were performed.
Methods
Subjects
After approval by the ethics committee of the University Hospital, written informed consent was obtained. After a screening visit, six healthy nonsmoking subjects (three male, three female) participated in this study. Subjects attended on two occasions (designated as visit 1 and visit 2) separated by a wash-out period of at least 5 days and were instructed to abstain from any drug (except oral contraceptives) during 3 days and from chocolate-, alcohol- and caffeine-containing beverages and food during 12 h preceding each experiment. Subjects fasted for at least 3 h before measurements were performed. Measurements were performed at the same time of day for each subject.
Drugs
The dose of CGRP (10 ng min−1 dl−1 forearm) used in this study was selected based on previous work [17]. CGRP (Clinalfa, Läufelfingen, Switzerland) was dissolved in 0.9% saline (B Braun, Melsungen, Germany) on the day of the experiment and a final solution of CGRP (100 ng ml−1) was made immediately before each experiment. CGRP or 0.9% saline was infused continuously using automated infusion pumps (Ivac® P1000, Ivac Medical Systems, Brussels, Belgium) into the brachial artery of the nondominant arm through a 27 gauge mounted needle (Sterican®, B Braun, Melsungen, Germany). Doses were normalized to forearm volume (measured by water displacement) by keeping the rate of all intra-arterial infusions constant at 100 µl min−1 dl−1 forearm.
Measurements
Forearm blood flow
The response to intrabrachial infusions of CGRP was assessed by measuring FBF in both the infused and noninfused arm using strain gauge venous occlusion plethysmography [11]. Electrically calibrated mercury-in-silastic strain gauges (D E Hokanson, Bellevue, USA) were placed at the point of maximal forearm circumference. The hands were occluded from the circulation during measurements through rapid inflation of wrist cuffs to ∼200 mmHg. Wrist cuffs were inflated at least 60 s before starting plethysmographic recordings in order to allow FBF to stabilize. Upper arm cuffs were intermittently inflated to 40 mmHg for the first 10 s in every 15 s to block temporarily venous return of blood from the forearm and thus obtain multiple plethysmographic tracings (EC6 Plethysmograph, D E Hokanson, Bellevue, USA). Analogue voltage output from the plethysmograph was processed by an analogue-to-digital converter (Powerlab®/8SP, AD Instruments, Castle Hill, Australia) and appropriate software (Chart® v4.0 for Windows, AD Instruments, Castle Hill, Australia) and recorded on to a computer (Dell Optiplex® G1, Dell Computer Corporation, Limerick, Ireland). Calibration was achieved using the internal standard of the plethysmograph.
Blood pressure and heart rate
Systolic (SBP), diastolic blood pressure (DBP) and heart rate were measured in the noninfused arm at 15 min intervals using a validated semiautomated oscillometric device (OMRON 705 CP, OMRON Healthcare, Hamburg, Germany). Measurements were performed immediately after the preceding FBF recording.
Forearm blood flow experiments
All experiments were performed in a quiet, temperature-controlled room maintained at 24 ± 1 °C. Each experiment lasted ∼1.5 h. Subjects rested supine on a comfortable bed with their forearms positioned above the level of the heart by resting the elbows on foam pads and supporting the hands with pillows. The brachial artery of the nondominant arm was cannulated at the antecubital crease. After arterial cannulation, 0.9% saline was infused for a period of 30 min to allow for equilibration. Baseline FBF was measured at the end of this period by recording plethysmographic tracings for 3 min. Immediately after baseline measurements, infusion of CGRP was started and continued for 45 min. During this infusion, plethysmographic tracings were recorded for 2 min, every 5 min
Data analysis and statistics
FBF was determined from the slope of the initial part of each plethysmographic tracing. Plethysmographic data were extracted from the Chart® data files and FBF responses were calculated for individual plethysmographic tracings using a template spreadsheet (Microsoft Excel 2000 v9.0, Microsoft Corporation, USA). For each recording period, the mean of the last five consecutive tracings was used. Tracings unsuitable for analysis due to motion artefacts were manually rejected. All tracings were analysed by the same investigator (FV).
In order to compare different ways of expressing data, FBF was expressed as:
absolute FBF in the infused arm (FBFinfused, in ml min−1 dl−1 forearm);
absolute FBF-ratio between the infused and noninfused arm (FBF-ratio);
percentage change from baseline in FBF in the infused arm (FBFinfused%, in percentage); and
percentage change from baseline in FBF-ratio (FBF-ratio%, in percentage).
FBF was calculated for each time point and time-response curves were constructed. In addition, the area under the curve (AUC0−45) and the change in FBF from baseline to 45 min (ΔFBF0−45) were calculated as summary responses.
The reproducibility of baseline measurements, FBF at 45 min (FBF45) and the summary responses AUC0−45 and ΔFBF0−45 was assessed as follows. First, for each subject the difference in response between visits was compared with the mean response of both visits according to Bland and Altman [23]. Using this approach, the mean difference and the repeatability coefficient, i.e. 1.96 times the standard deviation of the differences, were calculated [24]. Secondly, the mean within-subject coefficient of variation (WCV) was calculated by dividing the within-subject standard deviation by the mean and expressing it as a percentage. The within-subject standard deviation was calculated as the square root of the residual mean square using two-way analysis of variance (anova) [25]. Finally, the intraclass correlation coefficient (ICC) was calculated according to Deyo et al. [26].
Sample size calculations for a paired study design with continuous response measures were performed using the mean response of visit 1 and the standard deviation of the difference in response between visits 1 and 2 [27]. Sample sizes required to detect a predetermined difference of 10, 25, 33, 50, 75 and 100% in FBF response with 80 and 90% power were calculated given a type I error probability (α) of 0.05.
All responses are expressed as mean ± standard error of the mean (SEM), unless indicated otherwise. For measures of reproducibility and sample sizes, 95% confidence intervals (CI) were calculated if applicable. Blood pressure, heart rate, baseline measurements and summary measures were compared using Wilcoxon's matched-pairs signed-rank test. FBF data were examined by repeated-measures anova. P < 0.05 was considered statistically significant.
Results
All subjects successfully completed the study without any adverse events of note. Mean ± SD (range) for age, weight, height and forearm volume was 29 ± 8 (22–40) years, 65.7 ± 6.9 (56–74) kg, 173 ± 7 (162–182) cm and 910 ± 177 (708–1216) ml, respectively. There were no differences between baseline measurements of visits 1 and 2 (Table 1). Compared with baseline, a small increase in SBP (both visits) and DBP (only visit 2) was detected at the end of the 45 min infusion (P < 0.05). However, heart rate or FBF in the noninfused arm did not change (Table 1).
Table 1.
Comparison of haemodynamic parameters between visits
| Visit 1 | Visit 2 | |
|---|---|---|
| SBP (mmHg) | ||
| ″″Baseline | 117 ± 3 | 115 ± 3 |
| ″″45 min | 120 ± 4* | 118 ± 2* |
| DBP (mmHg) | ||
| ″″Baseline | 69 ± 1 | 65 ± 2 |
| ″″45 min | 71 ± 1 | 69 ± 2* |
| Heart rate (bpm) | ||
| ″″Baseline | 61 ± 5 | 62 ± 4 |
| ″″45 min | 64 ± 6 | 66 ± 5 |
| FBF (ml min−1dl−1forearm) | ||
| ″″Non-infused arm | ||
| ″″Baseline | 2.5 ± 0.3 | 2.3 ± 0.2 |
| ″″45 min | 2.8 ± 0.4 | 2.3 ± 0.3 |
| ″″Infused arm | ||
| ″″Baseline | 2.8 ± 0.4 | 3.2 ± 0.7 |
| ″″45 min | 15.4 ± 1.4 | 15.2 ± 1.5 |
| FBF-ratio | ||
| ″″Baseline | 1.2 ± 0.2 | 1.3 ± 0.2 |
| ″″45 min | 6.4 ± 1.0 | 7.1 ± 0.8 |
| ″″AUC0–45(ml dl−1forearm) | 571 ± 51 | 556 ± 64 |
| ″″ΔFBF0−45 | 12.6 ± 1.2 | 12.0 ± 1.5 |
SBP and DBP, systolic and diastolic blood pressure; FBF, forearm blood flow; FBF-ratio, FBF-ratio between the infused and noninfused arm; AUC0−45, the area under the curve from baseline to 45 min; ΔFBF0−45, increase in FBF from baseline to 45 min. Data are mean ± SEM.
P < 0.05 vs. baseline assessed by Wilcoxon matched-pairs signed-rank test.
CGRP infusions were well tolerated. Local flushing and an increase in temperature of the infused forearm were observed. These changes disappeared within 1–2 h after the end of the infusion.
Forearm blood flow response
CGRP infusions gradually increased FBF by about 5-fold at 45 min (P < 0.0001 for both visits and for all methods of data expression, Figure 1 and Table 1). No difference was observed between the mean response on visit 1 and visit 2. Individual time-response curves expressed as absolute FBF are shown in Figure 2. In addition, no difference was detected between visits in the summary responses AUC0−45 and ΔFBF0−45(Figure 3).
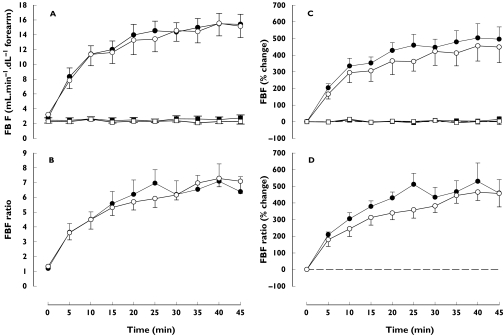
Figure 1.
Response of forearm blood flow (infused arm, circles; noninfused arm, squares) to intrabrachial infusion of CGRP (45 min continuous infusion, 10 ng min−1 dl−1 forearm) on visit 1 (closed symbols) and visit 2 (open symbols). Data (mean ± SEM) are expressed as (A) absolute FBF in the infused arm (B) FBF-ratio between the infused and noninfused arm (C) percentage change from baseline in FBF in the infused arm and (D) percentage change from baseline in FBF-ratio
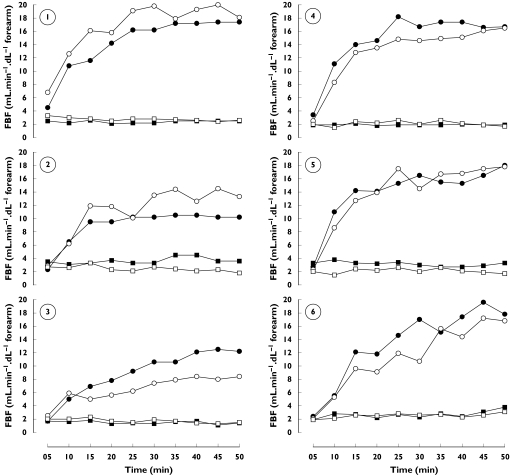
Figure 2.
Individual (n = 6) time-response curves (infused arm, circles; noninfused arm, squares) to intrabrachial infusion of CGRP (45 min continuous infusion, 10 ng min−1 dl−1 forearm) on visit 1 (closed symbols) and visit 2 (open symbols)
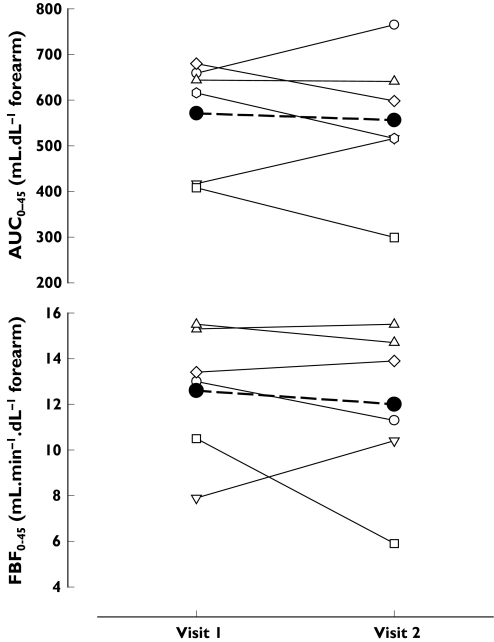
Figure 3.
Summary responses (AUC0−45 and ΔFBF0−45) to intrabrachial infusion of CGRP (45 min continuous infusion, 10 ng min−1 dl−1 forearm) on visit 1 and visit 2. Individual responses (n = 6) are shown as thin lines with open symbols. A thick dashed line with closed circles indicates the mean response
FBF in the noninfused arm changed neither when expressed as absolute blood flow nor when expressed as percentage change from baseline (Figure 1).
Reproducibility and sample size calculations
Results of the reproducibility analyses for baseline measurements and FBF responses are shown in Table 2. The number of subjects needed to detect a predetermined shift in FBF response was smaller when data were expressed as FBFinfused compared with the other methods of data expression (Table 3). When expressed as FBFinfused, a sample size of five (95% CI 2–12) subjects gives 90% power at a type I error probability of 0.05 to detect a 25% shift in FBF45.
Table 2.
Reproducibility of baseline measurements and forearm blood flow (FBF) responses
| Baseline measurement | Mean difference (95% CI) | Repeatability coefficient | WCV (%) (95% CI) | ICC |
|---|---|---|---|---|
| SBP (mmHg) | 2.3 (−4.8, 9.5) | 13.4 | 4 (1–8) | 0.57 (−0.45, 0.94) |
| DBP (mmHg) | 3.3 (−1.7, 8.4) | 9.5 | 4 (1–7) | 0.42 (−0.59, 0.92) |
| Heart rate (bpm) | −1.0 (−6.3, 4.3) | 10.0 | 6 (1–12) | 0.90 (0.31, 0.99) |
| FBFinfused (ml min−1 dl−1 forearm) | −0.4 (−1.5, 0.8) | 2.2 | 27 (5–49) | 0.70 (−0.26, 0.96) |
| FBFnon-infused (ml min−1 dl−1 forearm) | 0.2 (−0.6, 0.9) | 1.4 | 23 (4–41) | 0.36 (−0.64, 0.91) |
| FBF-ratio | −0.1 (−0.5, 0.3) | 0.7 | 20 (4–36) | 0.68 (−0.29, 0.96) |
| FBF response | Method of data expression | Mean difference (95% CI) | Repeatability coefficient | WCV (%) | ICC |
|---|---|---|---|---|---|
| FBF45 | FBFinfused | 0.3 (−1.9, 2.4) | 4.1 | 11 (2–19) | 0.82 (0.02, 0.95) |
| FBF-ratio | −0.7 (−4.1, 2.7) | 6.4 | 37 (7–66) | −0.22 (−0.88, 0.72) | |
| FBFinfused% | 29 (−154, 212) | 342 | 29 (5–52) | 0.56 (−0.46, 0.94) | |
| FBF-ratio% | −8 (−253, 237) | 458 | 40 (7–72) | 0.24 (−0.71, 0.88) | |
| AUC0−45 | FBFinfused | 15 (−81, 110) | 178 | 12 (2–22) | 0.78 (−0.07, 0.98) |
| FBF-ratio | 4 (−83, 90) | 162 | 26 (5–46) | 0.18 (−0.74, 0.87) | |
| FBFinfused% | 1613 (−3932, 7158) | 10 359 | 25 (5–45) | 0.56 (−0.46, 0.94) | |
| FBF-ratio% | 2144 (−3908, 8197) | 11 307 | 26 (5–47) | 0.24 (−0.71, 0.88) | |
| ΔFBF0−45 | FBFinfused | 0.6 (−1.7, 3.0) | 4.4 | 14 (3–25) | 0.75 (−0.15, 0.97) |
| FBF-ratio | −0.6 (−3.8, 2.6) | 6.0 | 43 (8–77) | −0.23 (−0.88, 0.71) | |
| FBFinfused% | 29 (−154, 212) | 342 | 29 (5–52) | 0.56 (−0.46, 0.94) | |
| FBF-ratio% | −8 (−253, 237) | 458 | 40 (7–72) | 0.24 (−0.71, 0.88) |
WCV, within-subject coefficient of variation; ICC, intraclass correlation coefficient; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBFinfused, forearm blood flow in the infused arm; FBFnon-infused, forearm blood flow in the noninfused arm; FBF-ratio, ratio of forearm blood flow between the infused and noninfused arm. FBF45, FBF at 45 min; AUC0−45, the area under the curve from baseline to 45 min; ΔFBF0−45, change in FBF from baseline to 45 min. FBF response is expressed as: (1) FBFinfused (ml min−1 dl−1 forearm) (2) FBF-ratio (3) percentage change from baseline in FBF in the infused arm (FBFinfused%,%) and (4) percentage change from baseline in FBF-ratio (FBF-ratio%, %).
Table 3.
Sample size calculations
| FBFinfused | FBF-ratio | FBFinfused% | FBF-ratio% | |||||
|---|---|---|---|---|---|---|---|---|
| % shift | 90% power | 80% power | 90% power | 80% power | 90% power | 80% power | 90% power | 80% power |
| FBF45 | ||||||||
| 10 | 21 (3–66) | 16 (3–50) | 276 (12–901) | 207 (9–673) | 212 (9–693) | 159 (8–518) | 419 (17–1377) | 313 (13–1029) |
| 25 | 5 (2–12) | 4 (2–10) | 46 (4–146) | 35 (3–109) | 36 (3–114) | 27 (3–85) | 69 (5–224) | 52 (4–168) |
| 33 | 4 (1–8) | 4 (1–6) | 27 (3–84) | 21 (3–64) | 21 (3–65) | 16 (3–49) | 41 (4–131) | 31 (3–98) |
| 50 | 3 (1–5) | 3 (1–4) | 13 (2–38) | 10 (2–29) | 11 (2–30) | 8 (2–23) | 19 (3–58) | 15 (3–43) |
| 75 | 2 (1–3) | 2 (1–3) | 7 (2–18) | 6 (2–14) | 6 (2–14) | 5 (2–11) | 10 (2–27) | 8 (2–20) |
| 100 | 2 (1–3) | 2 (1–3) | 5 (2–11) | 4 (2–9) | 4 (2–9) | 4 (1–7) | 6 (2–16) | 5 (2–12) |
| AUC0−45 | ||||||||
| 10 | 29 (3–90) | 22 (3–68) | 115 (6–373) | 86 (5–279) | 160 (8–520) | 120 (6–389) | 181 (8–590) | 136 (7–441) |
| 25 | 6 (2–16) | 5 (2–13) | 20 (3–61) | 16 (3–46) | 27 (3–85) | 21 (3–64) | 31 (3–96) | 23 (3–73) |
| 33 | 5 (2–10) | 4 (2–8) | 13 (2–36) | 10 (2–27) | 17 (2–50) | 13 (2–38) | 19 (3–56) | 14 (3–42) |
| 50 | 3 (1–6) | 3 (1–5) | 7 (2–17) | 6 (2–13) | 8 (2–23) | 7 (2–18) | 9 (2–26) | 7 (2–20) |
| 75 | 3 (1–4) | 2 (1–3) | 4 (2–9) | 4 (1–7) | 5 (2–11) | 4 (2–9) | 5 (2–13) | 5 (2–10) |
| 100 | 2 (1–3) | 2 (1–3) | 3 (1–6) | 3 (1–5) | 4 (1–7) | 3 (1–6) | 4 (2–8) | 4 (1–7) |
| ΔFBF0−45 | ||||||||
| 10 | 36 (3–113) | 27 (3–85) | 371 (15–1215) | 278 (12–908) | 212 (9–693) | 159 (8–518) | 419 (17–1377) | 313 (13–1029) |
| 25 | 8 (2–20) | 6 (2–15) | 61 (4–197) | 46 (4–147) | 36 (3–114) | 27 (3–85) | 69 (5–224) | 52 (4–168) |
| 33 | 5 (2–12) | 5 (2–10) | 36 (3–114) | 27 (3–85) | 21 (3–65) | 16 (3–49) | 41 (4–131) | 31 (3–98) |
| 50 | 4 (1–7) | 3 (1–5) | 17 (3–51) | 13 (3–38) | 11 (2–30) | 8 (2–23) | 19 (3–58) | 15 (3–43) |
| 75 | 3 (1–4) | 3 (1–4) | 9 (2–24) | 7 (2–18) | 6 (2–14) | 5 (2–11) | 10 (2–27) | 8 (2–20) |
| 100 | 2 (1–3) | 2 (1–3) | 6 (2–14) | 5 (2–11) | 4 (2–9) | 4 (1–7) | 6 (2–16) | 5 (2–12) |
Sample sizes (95% confidence intervals) required to detect a 10, 25, 33, 50, 75 and 100% shift in forearm blood flow (FBF) response were calculated with a power of 90% and 80% and a type I error probability of 0.05. FBF responses are expressed as: (1) absolute FBF in the infused arm (FBFinfused, ml min−1 dl−1 forearm) (2) absolute FBF-ratio between the infused and noninfused arm (FBF-ratio) (3) percentage change from baseline in FBF the infused arm (FBFinfused%, %) and (4) percentage change from baseline in FBF-ratio (FBF-ratio%,%). FBF45, FBF at 45 min; AUC0−45, the area under the curve from baseline to 45 min; ΔFBF0−45, increase in FBF response from baseline to 45 min.
Discussion
The present study convincingly demonstrates that a 45 min intrabrachial infusion of CGRP is well tolerated and results in a reproducible forearm blood flow increase in healthy subjects. However, reproducibility and sample size calculations are clearly affected by the way FBF response is expressed.
The magnitude of the FBF response to CGRP (10 ng min−1 dl−1 forearm) observed in the present study is consistent with previously reported responses to comparable doses, but with much shorter durations of infusion ranging from 3 to 12 min [13, 15–17]. This relates to the fact that most of the FBF increase is achieved within the first minutes of infusion. Indeed, when McEwan et al. [12] infused CGRP for 30 min in three healthy subjects at a dose of 2.8 ng min−1 dl−1 forearm, they observed a rapid increase in FBF during the initial 5 min followed by a more gradual increase, achieving a maximum flow at 25 min and about 80% of the maximum increase at 8 min. Results from pilot experiments (n = 6, data not shown), in which CGRP was infused continuously for 90 min at the dose used in the present study, showed a stable maximal FBF response from 60 min onwards. About 80% of the maximal increase was achieved at 20 min, 90% at 30 min and 95% at 45 min, respectively. The discrepancy between our results and those of McEwan et al. with respect to the speed with which the maximum response is achieved, probably relates to the substantial difference in dose. Local forearm venous plasma concentrations of CGRP during intrabrachial infusion at a dose of 10 ng min−1 dl−1 forearm [17] or 100 ng min−1[15] are in the range of those reported during a migraine attack in the internal [28] and external jugular vein [29]. The selected dose therefore is considered to be clinically relevant.
There is some debate concerning the quantification of drug dose during intrabrachial infusion. Usually, a fixed mass of drug is administered per unit of time [30]. Chin-Dusting et al. [31] recommended dose correction for the drug-induced change in blood flow by normalizing the dose to forearm volume, but this has been contested by others [32]. Although correction for forearm volume does not influence within-subject variability of FBF responses and adds some complexity to the study design, it may result in smaller between-subject variability of FBF responses, which is important when making comparisons between groups. However, this should be further investigated.
Several authors have assessed the reproducibility of FBF measurements by venous occlusion plethysmography, both in resting conditions and during infusion of vasodilating substances. As various measures of reproducibility are reported in the medical literature and measurement protocols often differ between studies, it is difficult to compare reproducibility data between studies. We chose to report the mean difference and repeatability coefficient [23], the within-subject coefficient of variation (WCV) [25] and the intraclass correlation coefficient (ICC) [26]. The majority of studies report at least the within-subject coefficient of variation, a measure of reproducibility which can be readily compared across studies. We found a mean interday WCV of 23–27% for unilateral resting FBF, which is within the range of those reported in the literature [33, 34]. When we expressed resting FBF as the FBF-ratio between both arms, reproducibility seemed to improve (WCV 20%), which is in agreement with Petrie et al. [34], who observed a better interday reproducibility for baseline FBF-ratio (WCV 19%) compared with unilateral FBF (WCV 31–39%).
How should FBF responses be expressed? Several authors have recommended expressing FBF responses to intra-arterial infusion of vasoactive drugs as FBF-ratio [30, 31, 34, 35]. Expressing responses as FBF-ratio would have the advantages that all data collected are used to their full advantage and that systemic changes unrelated to the local stimulus are compensated for. However, some authors have advocated the expression of FBF responses as FBF in the infused arm only, rather than as FBF-ratio [32, 36]. In this case, the data of the noninfused arm are used to confirm that there are no systemic drug effects. It seems therefore that there is no ‘gold standard’ for expressing FBF responses.
In order to identify the best way to express FBF data for future clinical trials making use of intrabrachial infusions of CGRP, we compared the reproducibility of four alternative ways of expressing FBF responses:
absolute FBF in the infused arm
absolute FBF-ratio between the infused and noninfused arm
percentage change from baseline in FBF in the infused arm and
percentage change from baseline in FBF-ratio.
Regardless of the selected FBF response, i.e. FBF at the end of the 45 min infusion (FBF45) or a summary response such as AUC0−45 and ΔFBF0−45, within subject coefficients of variation were consistently lower and intraclass correlation coefficients consistently higher when expressed as absolute FBF in the infused arm compared with the other methods (Table 2). Consequently, it is more likely that a shift of a given size is statistically significant when responses are expressed as absolute FBF in the infused arm (Table 3). These results are in agreement with Walker et al. who showed that forearm vasodilator responses were significantly less variable when expressed as absolute flows rather than as percentage increase in blood flow ratio [32]. Petrie et al. examined the reproducibility of vasoconstrictor responses as well as of vasodilator responses [34]. Interestingly, they showed that contrary to vasoconstrictor responses, which were less variable when expressed as percentage change in FBF-ratio, vasodilator responses were less variable when expressed as absolute blood flow in the infused arm. This was confirmed by Strachan et al. who showed that the reproducibility of vasoconstrictor responses to endothelin-1 was better when expressed as percentage change in FBF-ratio as compared with percentage change in FBF in the infused arm [37]. A possible explanation for the difference in reproducibility between vasodilator and vasoconstrictor responses is that vasoconstrictor drugs produce relatively small absolute changes in FBF in the infused arm which may be subject to small variations in basal flow to the same degree as FBF in the noninfused arm. However, vasodilator drugs cause large changes in FBF in the infused arm, which are unlikely to be affected to the same extent by these small variations in basal flow.
FBF responses are often reported as percentage change from baseline. Reporting a percentage change from baseline allows investigators to present results in clinically relevant terms. However, percentage change from baseline should not be used in statistical analysis [38]. Analysis of covariance (ancova) has been suggested as the method of choice for analysing results of trials with baseline and post-treatment measurements. With small samples, however, absolute change from baseline (i.e. ΔFBF0−45) and absolute post-treatment score (i.e. FBF45) are acceptable alternatives [38]. We found that FBF responses were more reproducible when expressed as absolute values as compared with percentage change from baseline (Table 2), even when there were no differences between both visits in baseline FBF in the infused arm or baseline FBF-ratio (Table 1). This is in accordance with Lind et al. [36], who demonstrated that reproducibility was very good for the FBF response during intra-arterial infusion of the vasodilators metacholine and sodium nitroprusside, whereas reproducibility was not as good for basal FBF. Based on these observations, they hypothesized that FBF responses during infusions with vasodilating substances would show the least variation when expressed as absolute FBF, rather than expressed in relation to basal FBF.
The use of summary measures to analyse serial measurements is a useful and simple tool in medical research [39]. Summary measures may be more clinically relevant, allow presentation of responses over time as a single value and may reduce within-subject variability. In the present study, reproducibility of the summary responses AUC0−45 and ΔFBF0−45 was not better compared with FBF at 45 min, when expressed as absolute FBF in the infused arm (Table 2). This is in agreement with Strachan et al. who calculated sample sizes based on FBF responses to endothelin-1 and reported sample sizes that were not consistently smaller for AUC compared with single time point measures [37].
There are several limitations to this study. The presented model is intended to be used in early clinical studies which are often restricted to men. As in the present study both male and female subjects were included, it could be argued that the results are not representative for early clinical studies. However, as the responses seemed more reproducible in men compared with women, the calculated sample sizes might even be smaller for studies including male subjects only. Furthermore, the precision of the calculated measures of reproducibility and sample sizes is limited as calculations are based on a small sample (n = 6). However, when investigating the reproducibility of venous occlusion plethysmography, small numbers of subjects are frequently used due to the complexity, duration and cost of the experiments [32, 34, 37, 40]. Notwithstanding the use of small groups of subjects (typically 6–8 subjects), local inhibition of vasoactive compounds can be shown convincingly using this technique [18–20, 22].
In conclusion, intrabrachial administration of CGRP to healthy subjects results in a FBF increase which is reproducible between days. The FBF response to CGRP is most reproducible when expressed as absolute FBF in the infused arm. The presented methodology provides a suitable pharmacodynamic model to assess the in vivo activity of CGRP-receptor antagonists in a small number of subjects.
Acknowledgments
This work was supported by a grant from the Research Foundation-Flanders (FWO. Vlaanderen). FV is a Junior Research Fellow of the Research Foundation-Flanders.
References
- 1.van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–78. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 2.Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab. 1987;7:720–8. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- 3.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–6. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 4.Bell D, McDermott BJ. Calcitonin gene-related peptide in the cardiovascular system: characterization of receptor populations and their (patho) physiological significance. Pharmacol Rev. 1996;48:253–88. [PubMed] [Google Scholar]
- 5.Edvinsson L. Calcitonin gene-related peptide (CGRP) and the pathophysiology of headache: therapeutic implications. CNS Drugs. 2001;15:745–53. doi: 10.2165/00023210-200115100-00001. [DOI] [PubMed] [Google Scholar]
- 6.Doods H. Development of CGRP antagonists for the treatment of migraine. Curr Opin Invest Drugs. 2001;2:1261–8. [PubMed] [Google Scholar]
- 7.Doggrell SA. Migraine and beyond: cardiovascular therapeutic potential for CGRP modulators. Expert Opin Invest Drugs. 2001;10:1131–8. doi: 10.1517/13543784.10.6.1131. [DOI] [PubMed] [Google Scholar]
- 8.Edvinsson L. New therapeutic target in primary headaches – blocking the CGRP receptor. Expert Opin Ther Targets. 2003;7:377–83. doi: 10.1517/14728222.7.3.377. [DOI] [PubMed] [Google Scholar]
- 9.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 10.Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol. 2004;142:1171–81. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631–46. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwan JR, Benjamin N, Larkin S, Fuller RW, Dollery CT, MacIntyre I. Vasodilatation by calcitonin gene-related peptide and by substance P. a comparison of their effects on resistance and capacitance vessels of human forearms. Circulation. 1988;77:1072–80. doi: 10.1161/01.cir.77.5.1072. [DOI] [PubMed] [Google Scholar]
- 13.Thom SM, Hughes AD, Goldberg P, Martin G, Schachter M, Sever P. The actions of calcitonin gene-related peptide and vasoactive intestinal peptide as vasodilators in man in vivo and in vitro. Br J Clin Pharmacol. 1987;24:139–44. doi: 10.1111/j.1365-2125.1987.tb03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernbeck J, Dalsgaard C-J, Pernow B. The effect of calcitonin gene-related peptide (CGRP) on human forearm blood flow. Clin Physiol. 1990;10:335–43. doi: 10.1111/j.1475-097x.1990.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 15.Ando K, Ito Y, Ogata E, Fujita T. Vasodilating actions of calcitonin gene-related peptide in normal man: comparison with atrial natriuretic peptide. Am Heart J. 1992;123:111–6. doi: 10.1016/0002-8703(92)90754-j. [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft JR, Noon JP, Gardner MJ, Bennett T. Hemodynamic effects of adrenomedullin in human resistance and capacitance vessels. Br J Clin Pharmacol. 1997;44:57–60. doi: 10.1046/j.1365-2125.1997.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Hoon JN, Pickkers P, Smits P, Struijker-Boudier HA, Van Bortel LM. Calcitonin gene-related peptide: exploring its vasodilating mechanism of action in humans. Clin Pharmacol Ther. 2003;73:312–21. doi: 10.1016/s0009-9236(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 18.Haynes WG, Ferro CJ, O'Kane KP, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–70. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- 19.Ferro CJ, Haynes WG, Johnston NR, Lomax CC, Newby DE, Webb DJ. The peptide endothelin receptor antagonist, TAK-044, produces sustained inhibition of endothelin-1 mediated arteriolar vasoconstriction. Br J Clin Pharmacol. 1997;44:377–83. doi: 10.1046/j.1365-2125.1997.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhaar MC, Grahn AY, Van Weerdt AW, et al. Pharmacokinetics and pharmacodynamic effects of ABT-627, an oral ETA selective endothelin antagonist, in humans. Br J Clin Pharmacol. 2000;49:562–73. doi: 10.1046/j.1365-2125.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spratt JC, Goddard J, Patel N, Strachan FE, Rankin AJ, Webb DJ. Systemic ETA receptor antagonism with BQ-123 blocks ET-1 induced forearm vasoconstriction and decreases peripheral vascular resistance in healthy men. Br J Pharmacol. 2001;134:648–54. doi: 10.1038/sj.bjp.0704304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newby DE, Sciberras DG, Ferro CJ, et al. Substance P-induced vasodilatation is mediated by the neurokinin type 1 receptor but does not contribute to basal vascular tone in man. Br J Clin Pharmacol. 1999;48:336–44. doi: 10.1046/j.1365-2125.1999.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bland MJ, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 24.British Standards Insistute Precision of Test Methods. London: BSI; 1979. 1: Guide for the Determination and Reproducibility of a Standard Test Method (BS5497, Part 1) [Google Scholar]
- 25.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12:142S–158S. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 27.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 28.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–18. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 29.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–23. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 31.Chin-Dusting JPF, Cameron JD, Dart AM, Jennings GLR. Human forearm venous occlusion plethysmography: methodology, presentation and analysis. Clin Sci. 1999;96:439–40. [PubMed] [Google Scholar]
- 32.Walker HA, Jackson G, Ritter JM, Chowienczyk PJ. Assessment of forearm vasodilator responses to acetylcholine and albuterol by strain gauge plethysmography: reproducibility and influence of strain gauge placement. Br J Clin Pharmacol. 2001;51:225–9. doi: 10.1046/j.1365-2125.2001.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altenkirch HU, Koch G, Koralewski HE. Variability and reproducibility of arterial and venous circulation parameters in the forearm and calf measured at one-week intervals. Vasa. 1990;19:21–5. [PubMed] [Google Scholar]
- 34.Petrie JR, Ueda S, Morris AD, Murray LS, Elliott HL, Connell JM. How reproducible is bilateral forearm plethysmography? Br J Clin Pharmacol. 1998;45:131–9. doi: 10.1046/j.1365-2125.1998.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb DJ. The pharmacology of human vessels in vivo. J Vasc Res. 1995;32:2–15. doi: 10.1159/000159072. [DOI] [PubMed] [Google Scholar]
- 36.Lind L, Sarabi M, Millgard J. Methodological aspects of the evaluation of endothelium-dependent vasodilatation in the human forearm. Clin Physiol. 1998;18:81–7. doi: 10.1046/j.1365-2281.1998.00077.x. [DOI] [PubMed] [Google Scholar]
- 37.Strachan FE, Newby DE, Sciberras DG, McCrea JB, Goldberg MR, Webb DJ. Repeatability of local forearm vasoconstriction to endothelin-1 measured by venous occlusion plethysmography. Br J Clin Pharmacol. 2002;54:386–94. doi: 10.1046/j.1365-2125.2002.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newby DE, Sciberras DG, Mendel CM, Gertz BJ, Boon NA, Webb DJ. Intra-arterial substance P mediated vasodilatation in the human forearm: pharmacology, reproducibility and tolerability. Br J Clin Pharmacol. 1997;43:493–9. doi: 10.1046/j.1365-2125.1997.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]