Abstract
Aims
The aim of this study was to compare the variability and sensitivity of impulse oscillometry (R5, X5 and RF), plethysmography (Raw and sGaw) and spirometry (FEV1, FVC and MMEF) in order to determine the most powerful technique for assessing bronchodilation in COPD clinical trials.
Methods
Twenty-four patients with COPD had impulse oscillometry, plethysmography and spirometry measured twice 30 mins apart, to determine variability. Then ascending doses of salbutamol (20, 50, 100, 200, 400 and 800 µg) were given and the same measurements made after each dose. Significant changes greater than variability were determined for each performed measurement (expressed as mean percentage improvement with 95% CI).
Results
Significant effects (P < 0.05) were detected after 20 µg by X5 (18.5% CI 9.8–27.2) RF (11.1% CI 7.2–15.0) and sGaw (21.5% CI 10.1–32.9), and after 50 µg by R5 (16.7% CI 10.8–22.5) and Raw (19.7% CI 13.0–26.4). FEV1 was less sensitive, detecting significant bronchodilation at 100 µg (10.2% CI 7.4–12.9).
Conclusions
We conclude that impulse oscillometry and plethysmography should be considered the preferred techniques for measuring bronchodilation in COPD clinical trials.
Keywords: Bronchodilation, COPD, clinical trials
Introduction
Clinical trials of novel or marketed bronchodilator drugs in COPD often investigate dose–response relationships, or compare the effects of different drugs [1–3]. The spirometric measurement of FEV1 is usually the primary outcome variable used to assess respiratory function in these studies. However, spirometry has limitations; it is effort dependent and the deep inspiration and forced expiration required can itself lead to changes in airway tone [4]. Furthermore, bronchodilator drugs can improve lung mechanics in COPD patients despite little change in FEV1, e.g. due to a decrease in hyperinflation [5].
Alternative techniques for assessing pulmonary function include body plethysmography and impulse oscillometry (IOS). Plethysmography measures airway resistance and conductance as well as lung volumes, while IOS measures airway resistance and lung reactance [6]. Unlike spirometry, these tests do not require effort-dependent forced expiration. Furthermore, they are known to be more sensitive than FEV1 for measuring the physiological effects of drugs in asthma [7]. However this has not been studied in COPD.
In clinical trials, the ability of a method to detect a pharmacological effect is dependent on its variability and sensitivity. The most powerful methods have low variability, but change greatly after administration of a drug that causes a therapeutic benefit. In contrast, less powerful methods show high variability but change little after the administration of an effective drug. There have been no studies comparing the variability and sensitivity of body plethysmography, IOS and spirometry in COPD patients. We compared these lung function techniques in order to determine the most powerful technique for assessing bronchodilation in COPD clinical trials.
Methods
Subjects
24 patients aged over 40 years with COPD diagnosed according to current GOLD guidelines [8] participated in the study (Table 1). Exclusion criteria were an exacerbation or any change in their COPD therapy within 4 weeks of the study. Historical data of FEV1 reversibility testing within the past 3 years with inhaled salbutamol (200 µg) was recorded. However patients were not selected according to any reversibility criteria. Written informed consent was obtained and the local ethics committee approved the study.
Table 1.
Subject characteristics
| Mean age (SD) | 63.6 (7.1) |
| Male | 16 (67%) |
| Current/Ex smoker | 9C/15Ex |
| Pack years | 43 (20–122) |
| Median (range) | |
| % predicted FEV1 | 58.4(12.8) |
| Mean (SD) | |
| % predicted TLC | 103.1 (11.8) |
| Mean (SD) | |
| % predicted RV | 145.9 (29.9) |
| Mean (SD) | |
| FEV1 Reversibility (%) | 9.2 (5.1) |
| Mean (SD) | |
Study design
Initial pulmonary function tests (Test 1) were performed in the following order; IOS, body plethysmography (including lung volume measurement) and spirometry. This order avoided any effect of spirometry on subsequent tests and was adhered to throughout the study. These measurements were repeated 30 min later (Test 2). Salbutamol was then administered in ascending doses of 20, 50, 100, 200, 400 and 800 µg, each dose separated by a 30 min interval. Pulmonary function was performed 15 min after each dose. Lung volumes were performed again only after the last dose of salbutamol, as it would not have been possible to perform all the measurements within the time permitted and patients would have found this too exhausting. Short acting bronchodilators were withheld for 6 h prior to the study day. Long acting beta agonists and tiotropium were withheld for 12 and 24 h, respectively.
Pulmonary function measurements and salbutamol administration
For IOS (Masterscreen IOS, Erich Jaeger, Hoechberg, Germany) subjects supported their cheeks to reduce upper airway shunting while impulses were applied during tidal breathing for 30 s. IOS measures airway resistance by sending a pulse-shaped sound wave produced by a loudspeaker to the patients lungs and listening for the reflection of that wave. The overall impedance of the pulse is due to the resistive and viscoelastic forces of the respiratory system. This is reported as R5, R20 (respiratory resistance at 5 and 20Hz, respectively) and X5 (reactance at 5 Hz). The point at which reactance is zero is known as the resonant frequency (RF) and is measured in Hertz. Raw, sGaw, functional residual capacity (FRC), vital capacity (VC) and inspiratory capacity (IC) were measured in a constant volume plethysmograph (Vmax 6200, Sensormedics, Bilthoven, The Netherlands). Total lung capacity (TLC) and residual volume (RV) were then calculated from these parameters. IOS and body plethysmograph measurements were performed in triplicate and the mean used for further analysis. Maximum expiratory flow volume measurements (FEV1, FVC and MMEF) were performed using the spirometry system on the Masterscreen. Readings were again performed in triplicate, with the highest FEV1, MMEF and FVC used in further analysis. Salbutamol (Ventolin nebules, Allen and Hanburys, Greenford, UK) was administered via a dosimeter (Mefar, Medicali, Brescia, Italy) calibrated to deliver 10 µl per inhalation. 1 mg ml−1 nebules were used to administer 20 and 50 µg doses while 2 mg ml−1 nebules were used for subsequent doses.
Statistical methods
Our sample size was chosen to allow power calculations for future clinical trials of bronchodilator drugs to be performed with 90% power using the within subject standard deviation (SD) observed. These estimates will have at least 73% power if the true within patient SD is 25% larger than observed in the present study. The within test variability was defined as the variation due to the method during three repeated measurements at the same time-point. This was assessed by the coefficient of variation (CV), derived by calculating (SD/mean), of the three readings. The within day variation (comparison of Tests 1 and 2) was assessed using the single determination SD to calculate the CV [9]. Pulmonary function measurements after each dose of salbutamol were compared with measurements at Test 2 and percentage change expressed a function of this baseline measurement. The physiological changes observed for each dose were compared to within day variability (i.e. the difference between Tests 1 and 2) using a paired student's t-test, as the data for the differences were normally distributed. This allowed physiological changes that were significantly greater than within day variability to be identified. The Bonferoni correction was applied to avoid bias from multiple comparisons. A P-value of <0.05 was considered statistically significant. The dose level at which significant bronchodilation was detected by each technique is presented. However the carry-over effect of previous doses should also be noted.
Results
Variability of spirometry, plethysmography and IOS
Within test variability was lower than within day variability for most measurements (Table 2). FEV1 and TLC were the most reproducible measurements. The most variable measurements were those related to airway resistance (R5, R20, Raw, sGaw) as well as MMEF and X5. The least variable IOS parameter was RF (Table 2).
Table 2.
Within test and within day variability
| Mean (SD) | Coefficient of variation (%) | |||
|---|---|---|---|---|
| Test 1 | Test 2 | Within test | Within day | |
| R5 (kPa l−1 s) | 0.58 (0.06) | 0.57 (0.05) | 8.4 | 13.5 |
| R20 (kPa l−1 s) | 0.35 (0.03) | 0.34 (0.02) | 7.7 | 11.6 |
| X5 (kPa l−1 s) | 0.28 (0.04) | 0.30 (0.03) | 11.9 | 36.0 |
| RF (Hz) | 24.44 (1.13) | 24.30 (1.26) | 5.0 | 7.4 |
| Raw (kPa l−1 s) | 0.40 (0.04) | 0.39 (0.04) | 10.0 | 10.3 |
| sGaw (kPa−1 s−1) | 0.64 (0.06) | 0.63 (0.05) | 9.3 | 7.8 |
| FEV1 (l) | 1.70 (0.06) | 1.75 (0.05) | 3.3 | 3.5 |
| FVC (l) | 3.21 (0.12) | 3.28 (0.08) | 3.3 | 6.3 |
| MMEF (l s−1) | 0.54 (0.07) | 0.58 (0.04) | 9.5 | 11.1 |
| TLC (l) | 6.24 (0.12) | 6.26 (0.13) | 2.1 | 3.4 |
| RV (l) | 3.27 (0.08) | 3.26 (0.08) | 2.4 | 7.7 |
| IC (l) | 2.22 (0.08) | 2.16 (0.06) | 3.2 | 6.6 |
Dose response effects of salbutamol
Salbutamol caused significant changes in X5, RF, and sGaw after 20 µg and R5 and Raw after 50 µg (Table 3). FEV1 was less sensitive, detecting significant salbutamol effects at 100 µg. MMEF and FVC demonstrated significant improvement over within day variability only at the 200 µg and 400 µg dose levels, respectively, but this improvement was not sustained at subsequent doses (Table 3). R20 failed to demonstrate significant improvement over within day variability at any dose. RV and IC showed significant improvement after 800 µg but TLC did not (Table 3). Figure 1 shows that for sGaw there was as initial steep improvement after low doses of salbutamol compared with spirometry and IOS parameters. However, after the 50 µg dose, the curves had similar gradients.
Table 3.
Mean (95% CI) percentage changes in pulmonary function variables following salbutamol administration
| Dose (mcg) | 20 Mean % change (95% CI) | 50 Mean % change (95% CI) | 100 Mean % change (95% CI) | 200 Mean % change (95% CI) | 400 Mean % change (95% CI) | 800 Mean % change (95% CI) | Within day Mean percentage difference between test 1 and test 2 (95% CI) |
|---|---|---|---|---|---|---|---|
| R5 | −9.0 | −16.7* | −16.0† | −17.9* | −20.0* | −22.4* | 0.4 |
| (kPa l−1 s) | (−14.0 to –4.0) | (−22.5 to –10.8) | (−23.3 to –8.6) | (−25.5 to –10.3) | (−28.3 to –11.6) | (−29.3 to –15.4) | (−5.6 to 6.4) |
| R20 | −2.7 | −5.8 | −4.6 | −6.2 | −7.4 | −11.0 | −1.21 |
| (kPa l−1 s) | (−7.0 to 1.7) | (−10.4 to –1.2) | (−11.6 to 2.3) | (−13.0 to 0.6) | (−13.9 to –0.9) | (−17.4 to –4.6) | (−6.7 to 4.3) |
| X5 | −18.5† | −32.0† | −26.7† | −28.6† | −32.8† | −36.2* | 6.7 |
| (kPa l−1 s) | (−27.2 to –9.8) | (−47.0 to –16.9) | (−42.7 to –10.7) | (−45.9 to –11.3) | (−48.8 to –16.8) | (−49.6 to –22.8) | (−6.0 to 19.5) |
| RF | −11.1* | −19.4* | −17.9* | −20.7* | −22.7* | −23.3* | −0.17 |
| (Hz) | (−15.0 to –7.2) | (−25.0 to –13.7) | (−23.3 to –12.4) | (−25.8 to –15.7) | (−29.1 to –16.3) | (−29.0 to –17.5) | (−4.4 to 4.0) |
| Raw | −10.9 | −19.7* | −19.5* | −20.8* | −21.2* | −20.7* | −1.2 |
| (kPa l−1 s) | (−16.9 to –4.8) | (−26.4 to –13.0) | (−25.8 to –13.3) | (−27.2 to –14.3) | (−27.9 to –14.5) | (−28.7 to –12.7) | (−5.8 to 3.4) |
| sGaw | 21.5† | 37.4* | 38.4* | 41.6* | 44.8* | 46.7* | −0.6 |
| (kPa−1 s−1) | (10.1 to 32.9) | (22.1 to 52.8) | (24.9 to 51.9) | (26.4 to 56.8) | (29.3 to 60.4) | (27.3 to 66.1) | (−5.9 to 4.7) |
| FEV1 | 2.8 | 8.0 | 10.2† | 11.9* | 13.7* | 16.3* | 3.4 |
| (l) | (0.8 to 4.9) | (5.2 to 10.7) | (7.4 to 12.9) | (8.6 to 15.1) | (10.2 to 17.2) | (12.2 to 20.4) | (1.5 to 5.4) |
| FVC | 4.0 | 6.8 | 9.2 | 9.0 | 12.2† | 11.7 | 3.1 |
| (l) | (1.5 to 6.5) | (3.3 to 10.4) | (5.5 to 12.9) | (5.5 to 12.4) | (8.6 to 15.8) | (6.0 to 17.3) | (−0.2 to 6.4) |
| MMEF | 3.6 | 12.8 | 13.6 | 21.3† | 19.3 | 25.0 | 3.4 |
| (l s−1) | (−1.8 to 8.9) | (4.2 to 21.4) | (5.2 to 22.1) | (11.1 to 31.6) | (7.9 to 30.7) | (11.8 to 38.2) | (−2.8 to 9.6) |
| TLC | −2.4 | 0.4 | |||||
| (l) | (−3.7 to –1.1) | (−1.5 to 2.3) | |||||
| RV | −10.9† | −0.5 | |||||
| (l) | (−14.2 to –7.5) | (−4.7 to 3.8) | |||||
| IC | 8.1† | −2.4 | |||||
| (l) | (4.0 to 12.3) | (−5.8 to 1.0) |
P < 0.05 or
P < 0.005 denotes significant change compared with variability using paired student's t-test with Bonferoni correction.
Figure 1.
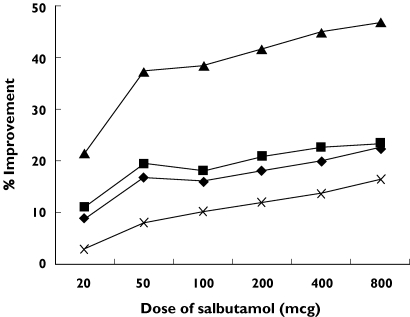
Dose–response curve of lung function parameters. R5 (♦), RF (▪), sGaw (▴), FEV1 (×)
Discussion
This is the first study to compare the ability of oscillometry, plethysmography and spirometry to detect bronchodilator effects exclusively in COPD patients. We found that IOS and plethysmography were able to detect significant bronchodilation after 20 µg salbutamol, whereas FEV1 was less sensitive.
Initially, we compared the variability of the methods and found that FEV1 and static lung volumes were the most reproducible. IOS and sGaw were relatively more variable. Similarly, Gimeno et al. found that Raw was more variable than FEV1 and IC in a group of COPD patients, although IOS was not studied [10]. Van Noord et al. compared the variability of oscillometry to spirometry and plethysmography, albeit in a mixed group of patients including asthmatics. The findings were similar to the current study; sGaw was the most variable measurement followed by oscillometry parameters, with spirometry being the least variable [11].
We then assessed the most sensitive measurements for detecting the physiological effects of salbutamol in COPD, and found that R5, X5, RF, Raw and sGaw were all more sensitive than FEV1. Our findings, supported by similar work by van Noord et al.[11] in a mixed group of patients, indicate that oscillometry and plethysmography should be used more often to assess the effects of pharmacological interventions in COPD clinical trials. Our results are also similar to our previous findings in asthma patients; namely that impulse oscillometry and plethysmography are more sensitive methods of detecting drug effects compared with FEV1[7]. The relevance of these physiological improvements to patient symptoms is not clear. Further work is required to investigate the relationship between improvements in IOS and plethysmography parameters and symptoms.
The methods compared in this study measure different aspects of lung function; FEV1 is a direct assessment of expired volume, body plethysmography measures airway resistance and lung conductance while controlling for the lung volume, whereas oscillometry measures resistance and compliance but does not control for volume changes. Bronchodilation in COPD patients causes an increase in airway diameter, which increases the expiratory capacity (hence an improvement in FEV1) and decreases airway resistance. Changes in airway resistance explain the improvements in sGaw, R5 and R20 observed in this study. The improvements in X5 and RF may be related to small airway bronchodilation causing a decrease in hyperinflation, thus leading to improved lung compliance. It is clear that bronchodilation in COPD patients results in complex changes in pulmonary physiology, and that body plethysmography, IOS and spirometry assess different aspects of these physiological changes.
The shape of the dose–response curves was different for sGaw compared with other measurements; there was an initial sharp improvement in sGaw at low doses of salbutamol, after which the gradient of the curve was similar to the other readings. This initial rapid improvement in airway conductance explains the increased sensitivity of plethysmography compared with the other methods at low bronchodilator doses. For the purposes of detecting small physiological effects in COPD, this may be a potential advantage, e.g. measuring physiological changes at 12 or 24 h after administration of a long acting bronchodilator [1–3].
COPD is currently defined by the presence of airflow limitation using FEV1 criteria that is not fully reversible to bronchodilator therapy [8]. Clinical COPD trials often exclude patients if they exceed set bronchodilator reversibility criteria [12, 13]. However, recent studies have shown that many COPD patients have significant reversibility to bronchodilators [14] and that reversibility can either increase or decrease with time [15]. For these reasons we did not select patients according to any reversibility criteria.
Many COPD patients, particularly those with hyperinflation, show significant improvements in TLC and RV after the administration of a bronchodilator despite little change in FEV1[5]. Although our patient group had normal TLC percentage predicted, there was evidence of hyperinflation as the mean RV was of 146% of the predicted value. We did not measure lung volumes after each salbutamol dose due to time constraints and anticipated patient fatigue. However, we did find that both RV and IC improved significantly after 800 µg salbutamol. Further studies are needed to compare the sensitivity of these lung volume measurements to IOS parameters and sGaw in COPD.
COPD patients demonstrate frequency dependence of resistance with IOS, i.e. the resistance at low frequencies (R5) is raised to a greater extent than that at high frequencies (R20) [16]. This may explain why R20 did not demonstrate significant improvement in this group of patients. We found R5 to be a more sensitive measurement of small airway changes in COPD patients than MMEF. MMEF showed a significant improvement compared to within day variability at the 200 µg dose, which was not sustained at subsequent doses. This may be because as the FVC of a COPD patient improves, their MMEF shifts along the flow volume loop and may even decline. For this reason it has been suggested that changes in MMEF should be corrected for changes in FVC [17]. However, previous similar studies have not used this method [7, 16, 18]. These results have significant implications for the measurement of the effects of drugs that target small airway function in COPD; our findings indicate that the best small airway measurement in pharmacological trials of COPD patients is R5 rather than MMEF.
There are important practical factors to consider when determining the optimum lung function test for use in clinical trials. While spirometry is easy to perform, it is also effort dependent, and can lead to a temporary alteration in bronchomotor tone due to the deep inspiration required, which has implications for repeated measurements. IOS requires only tidal breathing and is easy to perform. Plethysmography is a more complex procedure that some subjects find difficult to perform as it involves ‘panting’. However, in our experience most COPD patients can perform repeated plethysmography measurements without undue fatigue. These practical considerations, together with the results of the current study, indicate that IOS and plethysmography should be considered the preferred techniques for measuring bronchodilation in COPD clinical trials.
Acknowledgments
We would like to acknowledge the assistance of statistician Julie Morris with the data analysis of this study.
Competing interests: None declared.
References
- 1.Vincken W, van Noord JA, Greefhorst APM, Bantje TA, Kesten S, Korducki L, Cornelissen PJG. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19:209–16. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 2.Littner MR, Ilowite JS, Tashkin DP, Friedman M, Serby CW, Menjoge SS, Witek TJ. Long-acting bronchodilation with once-daily dosing of tiotropium (spiriva) in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1136–42. doi: 10.1164/ajrccm.161.4.9903044. [DOI] [PubMed] [Google Scholar]
- 3.Cazzola M, Santus P, Di Marco F, Boveri B, Castagna F, Carlucci P, Matera MG, Centanni S. Bronchodilator effect of an inhaled combination therapy with salmeterol + fluticasone and formoterol + budesonide in patients with COPD. Respir Med. 2003;97:453–7. doi: 10.1053/rmed.2002.1455. [DOI] [PubMed] [Google Scholar]
- 4.Burns GP, Gibson GJ. A novel hypothesis to explain the bronchoconstrictor effect of deep inspiration in asthma. Thorax. 2002;57:116–9. doi: 10.1136/thorax.57.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton MF, O'Donnell DE, Forkert L. Response of lung Volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121:1042–50. doi: 10.1378/chest.121.4.1042. [DOI] [PubMed] [Google Scholar]
- 6.Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther. 2001;14:341–50. doi: 10.1006/pupt.2001.0310. [DOI] [PubMed] [Google Scholar]
- 7.Houghton CM, Woodcock AA, Singh D. A comparison of lung function methods for assessing dose–response effects of salbutamol. Brit J Clin Pharm. 2004;58:134–41. doi: 10.1111/j.1365-2125.2004.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health, National Heart, Lung and. Blood Institute. Global Initiative for Chronic Obstructive Lung Disease. NHLBI/WHO, Workshop report. 2001. Publication no. 2701.
- 9.Chinn S. Repeatability and method comparison. Thorax. 1991;46:454–6. doi: 10.1136/thx.46.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimeno F, Postma DS, van Altena R. Plethysmographic parameters in the assessment of reversibility of airways obstruction in patients with clinical emphysema. Chest. 1993;104:467–70. doi: 10.1378/chest.104.2.467. [DOI] [PubMed] [Google Scholar]
- 11.van Noord JA, Smeets J, Clement J, van de Woestijne KP, Demedts M. Assessment of reversibility of airflow obstruction. Am J Respir Crit Care Med. 1994;150:551–4. doi: 10.1164/ajrccm.150.2.8049845. [DOI] [PubMed] [Google Scholar]
- 12.Gamble E, Grootendorst DC, Brightling CE, Troy S, Qiu Y, Zhu J, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:976–82. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- 13.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 14.Anthonisen NR, Wright EC. Bronchodilator response in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;133:814–9. [PubMed] [Google Scholar]
- 15.Calverley PMA, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–64. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjeldgaard JM, Hyde RW, Speers DM, Reichert WW. Frequency dependence of total respiratory resistance in early airways disease. Am Rev Resp Dis. 1976;114:501–8. doi: 10.1164/arrd.1976.114.3.501. [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft DW, Berscheid BA. Volume adjustment of maximal midexpiratory flow. Importance of changes in total lung capacity. Chest. 1980;78:595–600. doi: 10.1378/chest.78.4.595. [DOI] [PubMed] [Google Scholar]
- 18.Maesen FPV, Smeets JJ, Sledsens TJH, Wald FDM, Cornelissen PJG. Tiotropium bromide, a new long-acting antimuscarinic bronchodilator: a pharmacodynamic study in patients with chronic obstructive pulmonary disease (COPD) Eur Resp J. 1995;8:1506–13. [PubMed] [Google Scholar]


