Abstract
Aims
To compare the pharmacokinetics of lopinavir/ritonavir (LPV/r) 800/200 mg administered once daily in the morning compared with the evening.
Methods
This was a randomized, two-way, cross-over study in HIV+ subjects. In each subject the pharmacokinetics of each drug were characterized after 2 weeks of LPV/r 800/200 mg administered once daily at 08.00 h and 19.00 h. On study days, LPV/r was taken with a standardized meal (800 kCal, 25% from fat) after fasting for at least 5 h. LPV/r concentrations were measured by LC-MS/MS, and the data were analyzed by noncompartmental pharmacokinetic analysis.
Results
Fourteen subjects completed the study (all men, mean age/weight 44 year/81 kg). The median (interquartile range) LPV AUC(0,24 h), maximum plasma concentration (Cmax) and concentration at the end of the dosing interval (C24 h) after am and pm dosing was, respectively, 143 (116–214) mg l−1 h, 12.8 (10.3–17.2) mg l−1, 1.34 (0.58–3.25) mg l−1, and 171 (120–232) mg l−1 h, 12.9 (8.22–16.3) mg l−1, 1.15 (0.59–1.98) mg l−1. The geometric mean ratio (GMR, am : pm) and 95% CI of the LPV AUC(0,24 h), Cmax, and C24 h was 0.91 (0.79, 1.06), 1.11 (0.94, 1.32), and 1.19 (0.72, 1.96), respectively. The median ritonavir Cmax after am and pm dosing was 1.05 and 0.90 mg l−1, respectively. The GMR (95% CI) of the RTV AUC(0,24 h), Cmax, and C24 h was 0.93 (0.80, 1.08), 1.27 (1.00, 1.63), and 1.04 (0.68, 1.60), respectively. Administration of LPV/r in a once-daily regimen was generally well tolerated.
Conclusions
No differences were observed in the pharmacokinetics of LPV/r after am or pm dosing with food, which suggests that this once daily combination, can be taken in the morning or evening. Such flexibility in dosing may improve adherence.
Keywords: circadian, HIV, lopinavir, pharmacokinetics, ritonavir
Introduction
The absorption, distribution, metabolism and elimination of drugs are influenced by many different physiological functions, which may vary with the time of the day [1]. For example, gastrointestinal motility, liver blood flow, and hepatic enzyme activity have been shown to follow a circadian rhythm [2–4], which affects the pharmacokinetics of the human immunodeficiency virus (HIV) protease inhibitor ritonavir. An average 32% lower value for the area under the plasma concentration vs time curve (AUC) was observed after ingestion of the evening dose compared with the morning dose [5]. A circadian phase dependency has also been reported for the pharmacokinetics of other protease inhibitors, as well as other widely used drugs (e.g. digoxin, doxorubicin, gentamicin, nifedipine) [6–10].
Where the therapeutic window of the drug is narrow, circadian variation in plasma concentrations may be clinically important. Evaluation of circadian variation in protease inhibitor pharmacokinetics is warranted to optimize the time of administration and maximize drug exposure, particularly in developing a once daily dosing regimen. The latter may enhance patient adherence to anti-HIV treatment, and therefore is increasingly popular in clinical practice. Furthermore maintaining therapeutic drug concentrations is of paramount importance for a sustained virological response to therapy [11].
Lopinavir is a potent protease inhibitor that requires co-administration of low dose ritonavir to enhance its systemic profile through inhibition of intestinal and hepatic cytochrome P450 3A4 (CYP3A4)-mediated metabolism [12]. For this purpose, lopinavir is coformulated with low dose ritonavir (lopinavir/ritonavir 133/33 mg per capsule) allowing for a convenient dosing regimen of lopinavir/ritonavir 400/100 mg twice daily, which has been shown to be safe and effective for the treatment of HIV-1-infected patients [13, 14]. Lopinavir/ritonavir is currently licensed for twice daily dosing, but once daily dosing has been evaluated in randomized clinical studies and shown to give a similar virological and immunological response [15, 16]. With respect to pharmacokinetics, no differences were observed between the 800/200 mg once daily and the 400/100 mg twice daily regimen for lopinavir AUC(0,24 h) (mean ± SD 164.9 ± 67.5 and 185.2 ± 73.4 µg ml−1 h, respectively, P = 0.68), or for Cmax (10.94 ± 2.81 and 9.81 ± 3.66 µg ml−1, respectively, P = 0.19) [15]. However, the mean lopinavir predose concentration was about 50% lower during once daily dosing compared with twice daily dosing (3.63 ± 3.38 and 7.13 ± 2.93 µg ml−1, respectively, P ≤ 0.05) [15].
As the pharmacokinetics of lopinavir are dependent on ritonavir, we hypothesized that the previously reported circadian variation in the pharmacokinetics of ritonavir would translate into clinically relevant circadian variation in the pharmacokinetics of lopinavir. This would be of particular relevance for once daily administration of lopinavir/ritonavir. Accordingly, the objective of the current study was to investigate the steady-state pharmacokinetics of lopinavir/ritonavir 800/200 mg once daily after morning compared with evening dosing in HIV-1-infected patients.
Methods
Patients and ethics
HIV-1-infected adult patients using lopinavir/ritonavir (Kaletra®; Abbott Laboratories, Chicago, IL) in the licensed dosage of 400/100 mg twice daily in combination with two nucleoside reverse-transcriptase inhibitors, with an undetectable plasma HIV-1 RNA concentration (<50 copies ml−1), and with a CD4+ lymphocyte cell count of at least 200 cells µl−1 were eligible for this study. Patients were recruited if they had no abnormal laboratory tests, and no history of acute or chronic renal, hepatic or pancreatic disease. Comedication known to induce CYP3A4 activity was withheld for safety reasons. All participants were recruited at The Ottawa Hospital (Ottawa, Ontario, Canada). The study protocol was approved by the Institutional Review Board, and written informed consent was obtained from all patients before enrolment.
Study design and assessments
This was a randomized, balanced, two-way cross-over study. At the start patients were randomized 1 : 1 to switch from lopinavir/ritonavir 400/100 mg twice daily to lopinavir/ritonavir 800/200 mg once daily, administered in the morning (08.00 h) or in the evening (19.00 h) with a meal. There were no changes to any of the co-administered drugs during the study period. After 2 weeks of once daily dosing the pharmacokinetics of lopinavir and ritonavir were assessed over a 24 h dosing interval. Subsequently, patients were switched to the alternate time of administration and blood sampling was repeated after a further 2 weeks. Patients were asked to abstain from alcohol from 24 h before, and during each study day. On both study days lopinavir/ritonavir was ingested within 10 min after completion of a standardized meal (800 kCal, 25% from fat), after fasting for at least 5 h. Any other medication was taken as prescribed. Venous blood samples (5 ml) were collected in heparinized tubes from an indwelling catheter or venepuncture immediately before and 1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 20, and 24 h after ingestion of lopinavir/ritonavir. Plasma was isolated by centrifugation (900 g for 10 min) on the same day and was stored in polypropylene tubes at −70 °C until analysis. Samples were heated in a waterbath at 60 °C for 1 h to inactivate HIV, prior to analysis. After the second study day, patients were switched back to their initial dosage of lopinavir/ritonavir 400/100 mg twice daily.
Drug analysis
Concentrations of lopinavir and ritonavir in plasma were measured simultaneously by high-performance liquid chromatography coupled to tandem mass-spectroscopy (LC-MS/MS) at The Ottawa Hospital. Analytical reference standards for lopinavir and ritonavir were obtained from Abbott Laboratories (Chicago, IL). All samples from a single subject were analyzed in one run. Briefly, samples were thawed and the analytes were extracted from 250 µl of plasma with 5 ml methyl-tert-butyl-ether after addition of 2 ml ammonium hydroxide 2.5%, and dimethyl-dipyridylquinoxaline (internal standard). The organic extract was evaporated to dryness under a gentle stream of nitrogen at 40 °C, and the residue was dissolved in 300 µl of freshly prepared n-hexane : methanol : acetonitrile (10 : 25 : 25 v : v : v). Aliquots of 10 µl were injected onto the LC-MS/MS. Chromatographic separation was performed on a Supelcosil® ABZ+-plus column (150 × 4.6 mm, Supelco, Bellefonte, PA) with isocratic elution using a mixture of 5 m m ammonium hydroxide buffer (pH 4.15) : methanol : acetonitrile (30 : 35 : 35 v : v : v) on a HP1100 series HPLC system (Agilent Technologies, Palo Alto, CA). Detection of lopinavir and ritonavir was carried out by electrospray ionization mass spectrometry on an API2000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). Lopinavir and ritonavir were detected by positive mode multiple reaction monitoring using the reactions 628.8 to 429.4 m/z, and 720.9 to 296.2 m/z, respectively. The intra- and interassay variability for both lopinavir and ritonavir at low (100 ng ml−1), medium (3000 ng ml−1) and high concentrations (7500 ng ml−1) was less than 11.9% and 7.6%, respectively, as determined from analysis of six quality control samples at each concentration in four batches (a total of 24 samples per concentration). This bioanalytical method has been externally validated in the International Quality Control Program for Therapeutic Drug Monitoring in HIV Infection [17].
Pharmacokinetic analysis
The plasma concentration (C) vs time (t) data for lopinavir and ritonavir were analyzed by standard noncompartmental methods using WinNonlin Pro (version 4.0, Pharsight Corp, Cary, NC). The concentration at the end of the dosing interval was defined as C24 h, the highest observed plasma concentration as Cmax, and the corresponding sampling time as tmax. The elimination rate constant (λz) was determined by least squares linear regression analysis (log C vs t) of all measurable concentrations from tmax until the end of the dosing interval. The plasma elimination half-life (t1/2·z) was calculated from the expression ln2/λz. The area under the plasma concentration vs time curve from 0 to 24 h (AUC(0,24 h)) was determined using the linear-linear trapezoidal rule. The apparent oral clearance (CL/F, where F represents the oral bioavailability) was calculated from the expression dose/AUC, and the volume of distribution (V/F) from (CL/F)/λz.
Statistical analysis
This study was designed to have 80% power at the 5% significance level to detect a minimum 30% difference in the lopinavir AUC(0,24 h) after morning vs evening dosing, assuming a sample size of 14 subjects and an intrasubject variability of 25%. Pharmacokinetic parameters are presented as median values with interquartile ranges. Values after morning vs evening dosing, and laboratory parameters at baseline vs the end of the study were compared using the Wilcoxon signed-rank test. A P value ≤ 0.05 was considered to be statistically significant in all analyses. Furthermore, geometric mean ratios (GMR) and 95% confidence intervals (95% CI) were calculated for the AUC(0,24 h), Cmax, and C24 h of lopinavir and ritonavir. Spearman's correlation coefficient (rs) was used to test for any association between the pharmacokinetic parameters. Statistical calculations were performed with SPSS for Windows, version 11.0 (SPSS Inc., Chicago, IL).
Results
Fourteen male HIV-1-infected patients completed this study (three Black, two Hispanic, and nine Caucasian). Prior to randomization all patients used lopinavir/ritonavir 400/100 mg twice daily in combination with two nucleoside reverse-transcriptase inhibitors (lamivudine/stavudine (n = 9), lamivudine/zidovudine (Combivir®, n = 4), or lamivudine/abacavir (n = 1)). The median age was 40 years (range 32–76 years), and the median weight was 80 kg (range 64–95 kg). The median CD4+ cell count during screening was 550 cells µl−1 (range 270–953 cells µl−1). All subjects were negative for HBV and HCV, and had no signs of abnormal liver or kidney function.
All subjects maintained an undetectable plasma viral load (< 50 HIV-1 RNA copies ml−1; Chiron 3.0, Chiron Corp. Emeryville, CA) throughout the study. Lopinavir/ritonavir once daily was generally well tolerated. Four subjects reported adverse events that were likely to have been related to the study drug regimen. These were mild gastrointestinal discomfort starting shortly after switching to once daily dosing (diarrhoea/loose stool (n = 3), abdominal gas (n = 1), and abdominal cramps (n = 1)), but they did not require treatment or discontinuation of once daily dosing. The symptoms resolved after 2–14 days. No changes were observed for alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase or total bilirubin after 4 weeks of lopinavir/ritonavir once daily compared with twice daily dosing (P > 0.15).
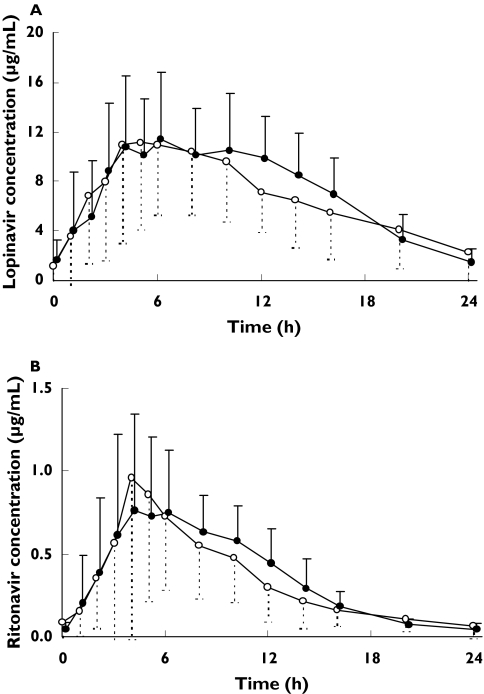
The mean plasma lopinavir and ritonavir concentration vs time profiles are presented in Figure 1, and pharmacokinetic parameters are summarized in Table 1. There was a strong positive correlation between the exposure to ritonavir and lopinavir with respect to AUC(0,24 h), Cmax, and C24 h (rs > 0.90, P < 0.01).
Figure 1.
Mean (± SD) steady-state plasma lopinavir (A) and ritonavir (B) concentration vs time profile after administration of lopinavir/ritonavir 800/200 mg once daily with a standardized meal in the morning (open circles) and in the evening (closed circles) in 14 HIV-1-infected male individuals
Table 1.
Steady-state pharmacokinetic parameters for lopinavir and ritonavir after the administration of lopinavir/ritonavir 800/200 mg once daily in 14 HIV-1-infected mena
| Morning dosing | Evening dosing | |||||
|---|---|---|---|---|---|---|
| Measurement | Median (IQRb) | Range | Median (IQR) | Range | GMR (95% CI)c | P valued |
| Lopinavir | ||||||
| ″″″″AUC(0,24 h) (µg ml−1 h) | 143 (116–214) | 60.5–298 | 172 (120–232) | 61.6–293 | 0.91 (0.79–1.06) | 0.36 |
| ″″″″Cmax (µg ml−1) | 12.8 (10.3–17.2) | 6.23–28.9 | 12.9 (8.22–16.3) | 5.29–22.4 | 1.11 (0.94–1.32) | 0.15 |
| ″″″″C24h (µg ml−1) | 1.34 (0.58–3.25) | 0.13–9.14 | 1.15 (0.59–1.98) | 0.18–3.53 | 1.19 (0.72–1.96) | 0.59 |
| ″″″″tmax (h) | 4.5 (3.8–5.3) | 4.5 (4.0–6.0) | 0.38 | |||
| ″″″″t1/2 (h) | 5.8 (4.9–12.9) | 5.9 (4.8–7.8) | 0.59 | |||
| ″″″″CL/F (l h−1) | 5.61 (3.77–7.01) | 4.71 (3.45–6.66) | 0.40 | |||
| ″″″″V/F (l) | 51.1 (39.0–121) | 46.9 (27.8–64.2) | 0.36 | |||
| Ritonavir | ||||||
| ″″″″AUC(0,24 h) (µg ml−1 h) | 8.30 (4.98–9.94) | 2.80–17.7 | 8.32 (5.42–1.91) | 2.79–15.8 | 0.93 (0.80–1.08) | 0.59 |
| ″″″″Cmax (µg ml−1) | 1.05 (0.58–1.98) | 0.43–3.58 | 0.90 (0.62–1.36) | 0.32–2.42 | 1.27 (1.00–1.63) | 0.10 |
| ″″″″C24h (µg ml−1) | 0.04 (0.03–0.08) | 0.02–0.32 | 0.05 (0.04–0.07) | 0.02–0.11 | 1.04 (0.68–1.60) | 0.84 |
| ″″″″tmax (h) | 4.5 (3.8–5.3) | 4.0 (3.0–6.5) | 0.93 | |||
| ″″″″t1/2 (h) | 4.2 (3.7–6.4) | 4.6 (3.8–5.3) | 0.55 | |||
| ″″″″CL/F (l h−1) | 24.1 (20.1–40.7) | 24.2 (16.8–36.5) | 0.68 | |||
| ″″″″V/F (l) | 160 (104–427) | 165 (106–258) | 0.18 | |||
Lopinavir/ritonavir was ingested with a standardized meal (800 KCal, 25% from fat) on pharmacokinetic sampling days
IQR is Interquartile range
GMR Geometric Mean Ratio calculated as ratio of the pharmacokinetic measurement after am dosing to the measurement after pm dosing
Wilcoxon Signed-Rank Test. AUC(0,24 h), Area under the plasma concentration vs time curve over the 24 h dosing interval; Cmax,maximal plasma concentration; C24 h; concentration at 24 h postdose; tmax, time of Cmax; t1/2, plasma elimination half-life; CL/F, apparent oral plasma clearance; V/F, apparent volume of distribution.
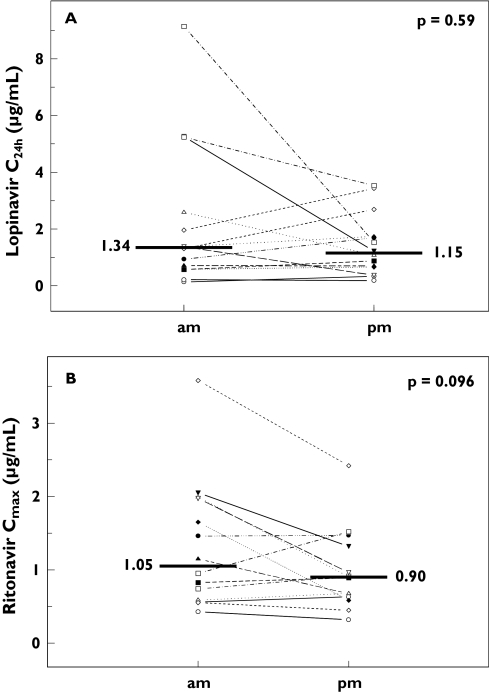
No statistically significant differences were observed between the pharmacokinetic parameters for lopinavir or ritonavir after morning vs evening dosing (Table 1 and Figure 2). However, there was a trend towards a higher (27%) Cmax for ritonavir after morning dosing, (95% CI 0, 63%, P = 0.096; Figure 2B). tmax was not affected (P = 0.93).
Figure 2.
Individual changes in lopinavir concentration at 24 h postdose (C24h; Figure 2A) and maximum ritonavir concentration (Cmax; Figure 2B) after steady-state administration of lopinavir/ritonavir 800/200 mg once daily with food in the morning (am) or in the evening (pm) in 14 HIV-1-infected male individuals. The horizontal lines indicate the median
Considerable interindividual variability in the pharmacokinetics of lopinavir and ritonavir was observed, which seemed higher after morning compared with evening administration (Figure 1). The coefficients of variation (%CV) for the lopinavir AUC(0,24 h) and C24h after morning dosing were 46% and 115%, respectively, compared to 38% & 77%, respectively, after evening dosing. Variability in the ritonavir AUC(0,24 h) and C24h after morning and evening administration was comparable with lopinavir (data not shown).
Discussion
In the current study no differences in pharmacokinetics after morning vs evening dosing of lopinavir/ritonavir 800/200 mg once daily with a standardized meal were observed. The 27% higher Cmax of ritonavir after morning dosing (P = 0.096) is in close agreement with previously reported lower absorption of ritonavir after evening administration [5, 18, 19]. However, the total exposure to either drug (measured as AUC(0,24 h)) was not significantly different between morning and evening dosing. The pharmacokinetic parameter for HIV protease inhibitors most closely related to long-term suppression of viral replication seems to be the trough concentration [20], which was not different between administration times in the current study. For the treatment of antiretroviral naïve patients a minimum lopinavir trough concentration of 0.7 µg ml−1 has been proposed. For the treatment of pretreated patients with drug-resistant strains, the viral susceptibility should be considered in the determination of a minimum effective concentration [21, 22]. During both morning and evening dosing of lopinavir/ritonavir 800/200 mg four out of 14 patients (29%) had trough lopinavir concentrations (C24 h) below 0.7 µg ml−1 (three subjects on both occasions). Thus, our results suggest that lopinavir/ritonavir once daily can be administered with food either in the morning or evening. Flexibility with regards to the choice of dosing time may further facilitate optimal adherence to once-daily dosing regimens, which is of paramount importance to achieve a sustained benefit of therapy [11].
Three studies have reported a circadian phase dependency in the pharmacokinetics of ritonavir, resulting in higher plasma concentrations after the morning dose as compared with subsequent doses. Hsu et al. observed a 40% higher Cmax, 32% higher AUC(0,12 h), and 32% lower concentrations at the end of the dosing interval (C12h) after morning administration of ritonavir in doses ranging from 200 to 500 mg twice daily compared with evening dosing [5]. It was estimated that the absorption rate constant for the evening dose was 68% lower than for the morning dose. Similarly, other studies of ritonavir pharmacokinetics reported 20–30% higher AUCs and Cmax values after administration of ritonavir in the morning vs later doses [18, 19].
These observations may be explained by circadian variation in hepatic blood flow, hepatic enzyme activity, or gastrointestinal motility and gastric emptying [1–4]. Lemmer et al. showed that hepatic blood flow in healthy volunteers was about 30% higher during the night (measured at 02.00 h) than during the day (14.00 h) [3]. However, as lopinavir clearance is low in the presence of ritonavir, changes in hepatic blood flow are not expected to significantly influence its pharmacokinetics. A possible circadian rhythm in human hepatic CYP3A4 activity has been inferred from a 2.8-fold change in the 6β-hydroxycortisol to cortisol ratio (an established marker of hepatic CYP3A4 activity), with the lower ratio being in the morning [4]. As the half-life of human CYP3A4 is more than 24 h, circadian changes in enzyme activity are most likely to be caused by variation in circulating concentrations of inhibitory endogenous compounds [4]. Significant circadian variation in gastroduodenal motility leads to greater contractions but at a lower propagation rate during sleep [2].
We hypothesized that the previously reported circadian pharmacokinetics of ritonavir would translate into differences in lopinavir pharmacokinetics after morning vs evening dosing. Although exposure to ritonavir and lopinavir showed a strong and positive correlation (rs > 0.90), no circadian differences in pharmacokinetics were observed. A possible explanation might be saturation of circadian dependent physiological processes by the high lopinavir dose (800 mg) in the current once daily regimen. Hsu et al. reported less diurnal difference in Cmax at higher doses of ritonavir (400–500 mg twice daily) than for the lower dose groups (200–300 mg twice daily) [5].
Contrary to the previous observations with ritonavir, circadian variation in the pharmacokinetics of nelfinavir results in higher exposure after evening compared with morning administration. A recent study reported a relative bioavailability of 220% for evening dosing compared with morning dosing of nelfinavir 1250 mg twice daily, which is in agreement with several other studies [23]. As food intake in this study was not controlled, food effects may partly explain these observations. A positive correlation has been reported between nelfinavir absorption and food intake. Nelfinavir AUC(0,8 h) increased 3–5 fold after ingestion of a single 1250 mg dose with meals containing 500–1000 KCal and 20–50% fat compared with ingestion on an empty stomach [24]. However, intake of nelfinavir with a 800 KCal meal (35 g fat) increased the steady-state AUC(0,12 h) by only 13%compared with ingestion with a 350 KCal meal (13 g fat), suggesting only a moderate effect of food composition on nelfinavir absorption, which cannot explain the reported two-fold difference in bioavailability after evening dosing [25]. Indeed, a recent population pharmacokinetic analysis showed that inclusion of both a diurnal and a food-effect factor in the model provided a better prediction of nelfinavir pharmacokinetics than either factor alone [26]. However, a formal pharmacokinetic study to determine whether nelfinavir exhibits true diurnal variation has not yet been reported.
To minimize the effect of food, meals on the study days were strictly controlled and comparable between the morning and evening doses with regards to calories and fat content. It is unlikely that previously reported circadian effects on the pharmacokinetics of ritonavir can be explained by an effect of food on drug absorption, even if meals were not standardized between doses. Oral absorption of ritonavir from the original oral formulation was not affected by food [27], and the extent of absorption of ritonavir from the soft gelatin capsules is only 13% higher after a meal (615 KCal, 14.5% fat) relative to fasting conditions [28]. However, it is recommended that patients should take lopinavir/ritonavir with food to enhance the bioavailability of lopinavir and minimize pharmacokinetic variability [29, 30].
In conclusion, we did not observe a difference in the pharmacokinetics of lopinavir/ritonavir 800/200 mg once daily after morning compared with evening administration with food. As some recent prospective randomized trials of lopinavir/ritonavir once daily vs twice daily in 190 antiretroviral naïve HIV-1-infected patients reported comparable virological and immunological response over the 48 week study period, once daily dosing of lopinavir/ritonavir may be an attractive and more practical treatment option for certain patients [15, 16]. Our results suggest that lopinavir/ritonavir 800/200 mg once daily can be administered together with a meal either in the morning or evening, which may facilitate incorporation of antiretroviral therapy into the daily routine of patients and improve adherence.
Acknowledgments
The authors would like to thank the patients who participated in this study. Diane Côté is kindly acknowledged for her help with the clinical conduct of the study. We thank Sonya Huneault for her assistance with the analysis of lopinavir and ritonavir concentrations. Abbott Laboratories is acknowledged for financial support of the study. RvH is supported by a Scholarship Award from the Ontario HIV Treatment Network (OHTN; SCH 108) and has received research and travel grants from Abbott Laboratories, DWC is supported by a Career Scientist Award from the OHTN and has received research grants from Abbott Laboratories.
References
- 1.Bruguerolle B. Chronopharmacokinetics. Current status. Clin Pharmacokinet. 1998;35:83–94. doi: 10.2165/00003088-199835020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bortolotti Annese V, Coccia G. Twenty-four hour ambulatory antroduodenal manometry in normal subjects (co-operative study) Neurogastroenterol Mot. 2000;12:231–8. doi: 10.1046/j.1365-2982.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- 3.Lemmer B, Nold G. Circadian effects in estimated hepatic blood flow in healthy subjects. Br J Clin Pharmacol. 1991;32:627–9. doi: 10.1111/j.1365-2125.1991.tb03964.x. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno M, Yamaguchi I, Ito T, Saiki K, Yammamoto I, Zuma J. Circadian variation of the urinary 6β-hydroxycortisol to cortisol ratio that would reflect hepatic CYP3A4 activity. Eur J Clin Pharmacokinet. 2000;55:861–5. doi: 10.1007/s002280050708. [DOI] [PubMed] [Google Scholar]
- 5.Hsu A, Granneman R, Witt G, et al. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justesen US, Pedersen C. Diurnal variation of plasma protease inhibitor concentrations. AIDS. 2002;16:2487–9. doi: 10.1097/00002030-200212060-00019. [DOI] [PubMed] [Google Scholar]
- 7.Erol K, Kilic FS, Batu OS, Yildirim E. Morning-evening administration time differences in digoxin kinetics in healthy young subjects. Chronobiol Int. 2001;18:841–9. doi: 10.1081/cbi-100107519. [DOI] [PubMed] [Google Scholar]
- 8.Lemmer B, Nold G, Behne S, Kaiser R. Chronopharmacokinetics and cardiovascular effects of nifedipine. Chronobiol Int. 1991;8:485–94. doi: 10.3109/07420529109059184. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Chong-Kook K, Lee BJ. Administration-time differences in the pharmacokinetics of gentamicin intraveneously delivered to human beings. Chronobiol Int. 1999;16:821–9. doi: 10.3109/07420529909016948. [DOI] [PubMed] [Google Scholar]
- 10.Canal P, Sqalli A, de Forni M, et al. Chronopharmacokinetics of doxorubicin in patients with breast cancer. Eur J Clin Pharmacol. 1991;40:287–91. doi: 10.1007/BF00315211. [DOI] [PubMed] [Google Scholar]
- 11.Le Moing V, Chene G, Carrieri MP, et al. Predictors of virological rebound in HIV-1-infected patients initiating a protease inhibitor-containing regimen. AIDS. 2002;16:21–9. doi: 10.1097/00002030-200201040-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sham HL, Kempf DJ, Molla A, et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob Agents Chemother. 1998;42:3218–24. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–46. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 14.Benson CA, Deeks SG, Brun SC, et al. Safety and antiviral activity at 48 weeks of lopinavir/ritonavir plus nevirapine and 2 nucleoside reverse-transcriptase inhibitors in human immunodeficiency virus Type 1-infected protease inhibitor experienced patients. J Infect Dis. 2002;185:599–607. doi: 10.1086/339014. [DOI] [PubMed] [Google Scholar]
- 15.Eron JJ, Feinberg JH, Kessler HA, et al. Once-daily versus twice-daily lopinavir/ritonavir in antiretroviral naïve HIV-positive patients: a 48 week randomized clinical trial. J Infect Dis. 2004;189:265–72. doi: 10.1086/380799. [DOI] [PubMed] [Google Scholar]
- 16.Gathe J, Podzamczer D, Johnson M, et al. Once-daily vs. twice-daily lopinavir/ritonavir in antiretroviral-naïve patients, 48-week results. 11th Conference on Retrovirus and Opportunistic Infections; San Francisco, CA. 2004. February 8–11, (abstract 570) [Google Scholar]
- 17.Droste JA, Aarnoutse RE, Koopmans PP, Hekster YA, Burger DM. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. JAIDS. 2003;32:287–91. doi: 10.1097/00126334-200303010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Cato A, III, Cao G, Hsu A, Cavanaugh J, Leonard J, Granneman R. Evaluation of the effect of fluconazole on the pharmacokinetics of ritonavir. Drug Metab Dispos. 1997;25:1104–6. [PubMed] [Google Scholar]
- 19.Ouellet D, Hus A, Granneman GR, et al. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin Pharmacol Ther. 1998;64:355–62. doi: 10.1016/S0009-9236(98)90065-0. [DOI] [PubMed] [Google Scholar]
- 20.Back DJ, Gatti G, Fletcher C, et al. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS. 2002;16:S5–S37. doi: 10.1097/00002030-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 21.Acosta E, Gerber JG. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res Hum Retrovirus. 2002;18:825–34. doi: 10.1089/08892220260190290. [DOI] [PubMed] [Google Scholar]
- 22.Hsu A, Isaacson J, Brun S, et al. Pharmacokinetic-pharmacodynamic analysis of lopinavir/ritonavir in combination with efavirenz and two nucleoside reverse-transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrobial Agents Chemother. 2003;47:350–9. doi: 10.1128/AAC.47.1.350-359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capparelli E, Goebel FD, William I, et al. Nelfinavir (NFV) population pharmacokinetics (PK) in long-term suppressors compared with the PK of the new 625 mg formulation in healthy volunteers. Clin Pharmacol Ther. 2004;75:P32. (abstract PI-111) [Google Scholar]
- 24.Petersen C, Pun E, Strada R, et al. Pharmacokinetics of nelfinavir (Viracept 250 mg tablet): effect of food intake on single-dose PK parameters. 10th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2003. February 10–14, [abstract 544] [Google Scholar]
- 25.Kurowski M, Kaeser B, Sawyer A, et al. Limited effect of food composition on the pharmacokinetics of nelfinavir administered twice-daily. Eur J Med Res. 2002;7:453–6. [PubMed] [Google Scholar]
- 26.Le Guellec C, Rodon P, Bastides F, et al. Bayesian estimation of nelfinavir exposure based on a model integrating diurnal and food-effects. 5th International Workshop on Clinical Pharmacology in HIV Therapy; Rome, Italy. 2004. April 1–3, (abstract 6.13) [Google Scholar]
- 27.Chicago, IL: Abbott Laboratories; 2003. Product information Norvir (ritonavir) Revised october. [Google Scholar]
- 28.Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–91. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 29.Chicago, IL: Abbott Laboratories; 2003. Product information Kaletra (lopinavir/ritonavir) Revised January. [Google Scholar]
- 30.Bertz R, Renz C, Foit C, et al. Steady-state pharmacokinetics of Kaletra (lopinavir/ritonavir 400/100 mg bid) in HIV-infected subjects when taken with food. 2nd International Workshop on Clinical Pharmacology in HIV Therapy; Noordwijk, The Netherlands. 2001. April 2–4, (abstract 3.10) [Google Scholar]