Abstract
Collaboration between the medical school at Leicester and a local pharmaceutical company, AstraZeneca, led to the design and implementation of an optional third year special science skills module teaching medical students about drug discovery and development. The module includes didactic teaching about the complexities of the drug discovery process leading to development of candidate drugs for clinical investigation as well as practical experience of the processes involved in drug evaluation preclinically and clinically. It highlights the major ethical and regulatory issues concerned with the production and testing of novel therapies in industry and the NHS. In addition it helps to reinforce other areas of the medical school curriculum, particularly the understanding of clinical study design and critical appraisal. The module is assessed on the basis of a written dissertation and the critical appraisal of a drug advertisement. This paper describes the objectives of the module and its content. In addition we outline the results of an initial student evaluation of the module and an assessment of its impact on student knowledge and the opinion of the pharmaceutical industry partner. This module has proven to be popular with medical students, who acquire a greater understanding of the work required for drug development and therefore reflect more favourably on the role of pharmaceutical companies in the UK.
Keywords: medical education, clinical pharmacology and therapeutics, drug development, pharmaceutical industry
Introduction
There is little provision in the already overcrowded medical undergraduate curriculum to inform future prescribers about the complexity and cost of developing novel drug therapies. Although ignorance of this process may not influence prescribing practice, a knowledge of drug discovery and development is likely to better inform the prescriber of the safety, efficacy and economics of using new drugs and treatments. In addition, it may provide a better understanding of the importance of assessing new therapies including pharmacovigilance and encourage future collaboration with the pharmaceutical industry with respect to the development of new drugs.
There is comparatively little published about the teaching of drug discovery and development and most of this is directed at pharmacy, pharmacology or medical graduates [1–3]. A 1985 report from the Medico-Pharmaceutical Forum Working Party on Medical Education relates to the teaching of drug development to medical students [4]. The authors surveyed UK medical school clinical pharmacology and therapeutics (CPT) professors to identify the extent of teaching about drug development, safety and efficacy. They concluded that the specific GMC recommendation at the time regarding design and interpretation of clinical studies was met, but that some schools devoted less than 1 h to this topic. The report recommended more teaching about pharmaceutical medicine through greater interaction with the industry and further teaching about economics of drug therapy and how the industry markets and promotes novel therapies.
The GMC guidance on medical school curricula, Tomorrow's Doctors, which has recently been updated [5], indicates that the teaching content of specific subjects is the responsibility of organizations other than the medical schools. Maxwell and Walley on behalf of the Clinical Section Committee of the British Pharmacological Society (BPS) have identified the key elements of the core curricula for CPT [6], revising earlier recommendations [7]. Nevertheless, the recommendations regarding the core knowledge of drug development remain essentially unchanged from 1997. These include understanding of preclinical development and testing, Phase I–IV clinical trials and the requirements of a good clinical trial including the necessary ethical considerations, the role of the major regulatory bodies and pharmacovigilance. The greatest hurdle to full implementation of these recommendations has been timetabling constraints; in light of this, an optional module was planned for third year medical students at the Leicester Medical School. Students in both their second and third years are given the option of undertaking one of several special science skills modules that cover a wide range of topics allowing students to obtain greater in-depth knowledge about a subject of interest to them. The modules, which take place over 12 weeks, are designed to provide up to 70 h of teaching and include formal assessments. The ‘Molecules to Man’ Special Science Skills Module was developed in association with AstraZeneca (AZ), based at Loughborough and Nottingham. Eighteen students, representing 10% of the year, are accepted annually onto the module.
The course is now in its fifth year and has proven popular with the students. In addition, our industry partner has adapted it so that it can be delivered as a 1-day seminar to various departments within the industry, providing a useful introduction and overview to new staff within the organization. This paper describes the module objectives and content, the initial student evaluation and an assessment of its impact on student knowledge and their opinion of the pharmaceutical industry.
Objective of the course
The objective of the course was to convey the key features of drug development, from the discovery process to pharmacovigilance, using interactive theory and practical sessions. The order of the sessions was specifically designed to follow the sequence of a drug's development and the steps required for preclinical and clinical testing as well as the evaluation of safety, efficacy and clinical effectiveness.
Module content
The module teaching is held at three sites namely the Leicester Royal Infirmary, AZ Loughborough and the AZ Clinical Pharmacology Unit at the Queen's Medical Centre, Nottingham. A coach is provided for the students to travel from Leicester to the AZ sites.
The module is subdivided into 16 sessions and a more detailed description of each session is detailed in Appendix A.
In addition to the formal module sessions, students are required to spend 15 h of personal study time for the purposes of completing the teaching programme, which includes the time for preparation of the assessment tasks.
Staffing requirements and module organization
Defining the module structure and the development of the relationship between the Pharmacology Group at Leicester and the Department of Experimental Medicine at AZ required several planning meetings. Thereafter a cohort of 10–12 scientists and physicians at AZ offered teaching sessions in support of the module initially producing their own teaching materials (handouts/PowerPoint presentations), which are now reviewed at an annual meeting. Four university clinical pharmacologists, a senior pharmacist, an NHS Trust R&D manager and a nonclinical scientist provide the basis for the university/NHS clinical teaching. Additional support comes from an AZ head-office based marketing manager and university/NHS clinical research unit nurses.
The module leader is one of the university clinical pharmacologists who takes responsibility for coordinating the timetable, production of the handbook, teaching and assessments. He is specifically responsible for ensuring both the continuity and overall acceptability of the material used in all sessions. In addition, a senior scientist at AZ acted as a focus for supporting AZ-based tutors with the provision of the teaching at Loughborough and Nottingham. Both university and AZ secretarial staff provide additional administrative support.
Student assessments
Students are assessed during and at the end of the module. In addition to attendance requirements, the students’ grades are awarded on the basis of two components of the assessment namely the Drug Advert Project and a 2000-word dissertation. The Drug Advert Project, described in Appendix A, is evaluated by the session tutors. The dissertation is based on a choice between four questions, which are given to the students in week 4 of the module. Two questions are based on preclinical drug development proposed and marked by AZ tutors and two questions regarding clinical studies proposed and marked by medical school CPT physicians. The dissertation is also marked by an external examiner who oversees and ratifies the final assessment of the students’ grades.
Student evaluation of the module
An evaluation exercise was undertaken in which the students completed a standard feedback form at the end of each session, which enabled both tutors and module leader to modify the content and delivery of the sessions on subsequent occasions. Students scored each session (out of four) for content and presentation style. In addition, there was an opportunity to provide free text feedback.
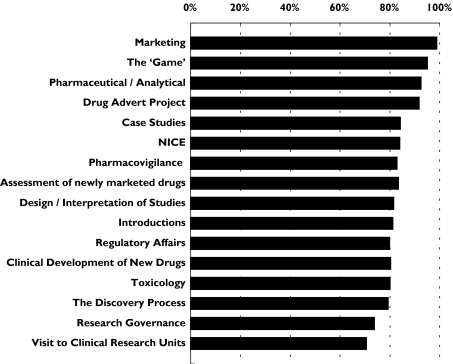
Overall attendance at the sessions has been good ranging from 67 to 100% with a module average of 85%. The scores from each of the student feedback forms were combined for each session giving a percentage score. The mean score from each session ranged from 71 to 99% with an overall module score of 83%. The most popular sessions were the most interactive, namely Marketing, The ‘Pharm’ Game, Pharmaceutical and Analytical R&D and the Drug Advert Project (see Figure 1). The popularity of the interactive sessions has been one of the key developmental aspects of the module, particularly for the AZ staff, who have now moved away from formal didactic lectures to using more problem-based learning techniques, requiring greater student participation. The least popular sessions were, surprisingly, the visits to the university/NHS clinical research units. This was principally because a number of the staff failed to interact with the students and consequently in following years, greater advice on what is required has been provided to the research nurses.
Figure 1.
Student evaluation of each session. The mean student score is presented as a percentage; all students completed feedback on content and presentation style (number of students providing feedback for each session ranged from 12 to 18 with mean 15.3)
The student feedback has also improved co-ordination of the sessions between AZ and the university. Although there is an advantage of appreciating both an industry and academic view of most of the module topics, the students perceived that this was occasionally repetitive.
Student knowledge and opinions
Acquiring definitive information about the long-term success of the module from a small cohort of students is difficult. However, a questionnaire was developed to test the students’ knowledge of certain aspects of drug development and their opinions of the pharmaceutical industry (Appendix B). We invited the last two cohorts of students to complete the questionnaire at the start and finish of the module. In addition, we identified a small group of students attending their final year lectures who had previously taken the module approximately 18 months previously. These findings were compared with newly qualified doctors in Leicester who had not attended the module – Pre-Registration House Officers (PRHO) attending a weekly teaching session and Senior House Officers (SHO) attending an annual CPT teaching module in preparation for the MRCP.
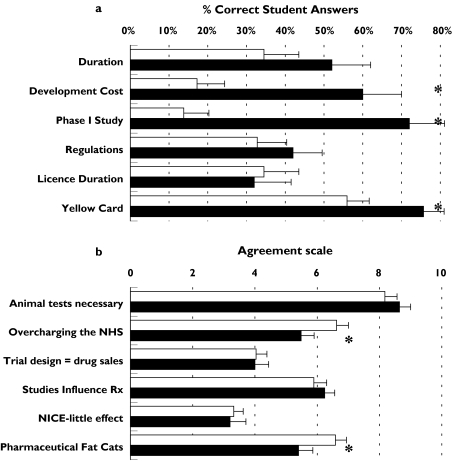
The mean overall score in the knowledge assessment for students undertaking the module increased from 33% at the start to 57% after completing the module. The most significant improvement occurred in the understanding Phase I trials, drug development costs and the yellow card scheme (Figure 2a). Student opinion of the pharmaceutical industry did not provide any unexpected findings. The majority were very supportive of animal testing. Initially, the students thought that the pharmaceutical company profited excessively from manufacturing drugs, but at the completion of the course, there was a significant change of view with less agreement with statements suggesting that the industry overcharges the NHS and that company bosses are ‘fat cats’ (Figure 2b).
Figure 2.
(a) Student knowledge of the pharmaceutical industry before and after module; 29 students completed answers before and 25 students following the module, pre-module (□), post-module (▪); (b) Student opinion of the pharmaceutical industry before and after module, pre-module (□), post-module (▪); A higher score represents greater agreement to the statement; 29 students completed answers before and 23 students following the module. Error bars represent SEM; *P < 0.05 (Mann–Whitney analysis)
The assessment of students’ knowledge and opinion of the pharmaceutical industry was compared with senior students who had completed the module 18 months previously and senior students and junior doctors who had not attended the teaching (Table 1). Only six final year students, who had completed the module 18 months previously, were available to complete the questionnaire. Six peers were chosen at random as a control group at the same time. This does not provide a robust group for analysis; however, there was a trend towards better knowledge in the module group with both groups scoring more highly on the more clinically relevant questions particularly the yellow card scheme. Student opinion of the industry reflected that seen in other groups; but those that had attended the module were still more favourably disposed towards the industry and in particular, they tended to agree less with the statement that the industry overcharges the NHS.
Table 1.
A comparison of mean scores of pharmaceutical industry knowledge between students and newly qualified doctors
| Module students | Final year students | ||||
|---|---|---|---|---|---|
| Group | Pre Course | Post course | Other | Drugs | Junior doctors |
| Number in group | 29 | 25 | 6 | 6 | 33 |
| Mean score (%) | 32.9 ± 3.7 | 56.8 ± 3.3 | 36.8 ± 9.6 | 44.8 ± 8.2 | 31.8 ± 2.5 |
The ‘Module Students’ were questioned directly before and after the module. ‘Final Year Students’ were questioned approximately 18 months after completing the module (Drugs) with a control cohort of final year students who had not attended the module (Other). Seventeen PRHOs and 16 SHOs accounted for the ‘Junior Doctors’.
The mean score represents averaged overall scores for each group represented as a percentage. One mark was available for each question giving a total raw score of six marks; in the case of two or three correct answers, the score for each answer was given as a fraction to a maximum value of one mark.
Error bars represent SEM.
The knowledge of drug development among qualified doctors was disappointingly poor with a mean score of only 32%. In comparison with students who had just completed the module, doctors were less knowledgeable in understanding Phase I trials and development costs, but their knowledge relating to the yellow card scheme was good. The opinions of the industry from doctors were similar to students who had not attended the module. They were generally in favour of animal testing, but not as supportive as those who had been attending the module. Doctors were also more likely to believe that the industry overcharges the NHS, that clinical trials are designed to promote sales and that company bosses are ‘fat cats’.
Qualitatively, the questionnaire feedback was reassuring that students were better informed about drug development at the end of the module. It was difficult to determine whether this was maintained long-term, but there was a trend in its favour. Additionally, knowledge of drug development amongst qualified doctors of 1–3 years standing was poor suggesting that drug development is not adequately taught at the undergraduate or early postgraduate stage and thus does not meet the BPS recommendations [7].
Attendance at a module with input from a pharmaceutical company appears to favourably influence the students’ opinion of the industry.
Conclusions
Most collaborative work between the pharmaceutical industry and medical schools is based on research; however, this paper describes an optional module for third year medical students at Leicester to study the processes of drug development in greater detail. This has required not only a commitment from AstraZeneca to resource the module, but to provide staff to prepare and teach a majority of the sessions, in addition to their usual work.
This has proven to be a popular course with the students and those sessions that involve the greatest student participation were highly rated. This has led to a change in emphasis towards a more open style of teaching in later modules. There is evidence that student knowledge at the completion of the module has improved, not unexpectedly, but it has been difficult to ascertain if this is retained long-term. However, students will have had greater opportunities than their peers to understand the complexities of drug development, the use of evidence-based practice and safe prescribing to meet the requirements of these components of the BPS core curriculum.
The industry partner has also benefited. The tutors have formed a better understanding of the teaching and learning requirements of medical undergraduates, which in turn has led to improvements in the course and teaching skills of individual tutors. In addition, this has prompted AstraZeneca to develop an abridged version of the module currently used as an induction aid for new recruits to the industry. Similarly, the students are more familiar with the benefits of working in the industry. We can only speculate if this will translate into increased recruitment, but good learning experiences at medical school can influence career decisions.
Particularly noteworthy was the effect of the module on the commonly held assumption that the industry profits excessively from sales reinforcing the fact that most students and doctors significantly underestimate the cost of drug development. The results of our albeit qualitative and simple evaluation of this module suggest that once the complex process of drug development and its limitations are explained, opinions regarding costs change significantly in favour of the industry. A recent British Medical Journal editorial highlighted the potentially unethical influences on drug prescribing due to the close relationship of doctors to the industry [8]. Additionally others have argued that ‘medical educators have a duty of care to protect students from influence by pharmaceutical companies’ based on concerns, rather than evidence, that students may be affected by gifts received from pharmaceutical company representatives [9]. However, this module was designed to provide a balance of opinion and other sessions, particularly the drug advert project, the NHS assessment of newly marketed drugs and the work of the National Institute for Clinical Effectiveness (NICE) provide a forum for the students to discuss these issues and not be solely influenced by the industry.
The questionnaire survey of junior doctor knowledge of drug development suggested this topic is not taught extensively. This is likely to reflect lack of teaching in the generic undergraduate CPT curriculum. Consequently a 1-h overview of the key issues of drug development is now provided as part of the SHO CPT training programme at Leicester. But could this module be taught to all undergraduate students? The module is very comprehensive and as most medical curricula are already time-constrained, it is not possible to directly insert an unmodified version of the module into the curriculum. However, the model of the SHO CPT training could be adapted for undergraduates, whereby several of the module sessions, particularly those that will support key BPS recommendations, can be included in the undergraduate programme.
Currently drug discovery and development continues to be offered to medical students at Leicester and demonstrates how industry and university can collaborate successfully in the delivery of a dedicated special science skills module.
Acknowledgments
The authors are grateful for the contribution of Dr Harsukh Parmar, Head of Experimental Medicine, AstraZeneca, Loughborough and his continued support throughout the development of the module. The following AstraZeneca and University colleagues have made substantial contributions to the teaching: AstraZeneca: Holger Adelmann, Dawn Adkin, Richard Arthur, Alison Ashmore, Carol Astbury, Darshan Bhatt, Graham Blakey, Kevin Cheeseman, David Cheshire, Nayna Govind, Salvatore Febbraro, Trish Hepburn, Chris Grime, Andrew Lockton, Tom McInally, Linda McMorland, Tony Quinn, Edwin Pearson, Linda Stephens, John Stevens, Clive Stringer, Dominic Smethurst, Mike Sullivan, John Talbot, Janet Watson and Tony Verco. University of Leicester: Diane Ketley, Iain Squire and Kent Woods. UHL NHS Trust: Susan Carr, and Nichola Seare. We thank Dr Nigel Baber for his support as the external examiner.
Appendix A – Module Content
1. Introduction sessions
Leicester Royal Infirmary and AstraZeneca (Loughborough) – lectures and site visit
The introductory sessions are dedicated to providing the students with an overview of the philosophy of course. The first is introduced by the university module leader in Leicester. Housekeeping issues are discussed, including attendance record keeping, travel arrangements and all necessary contact details. The students are made aware of what is expected of them, provided with a handbook (detailing timetables, a glossary of ‘pharmaceutical’ definitions and a handout for all the sessions) and the formal assessments are discussed.
The second session takes place at the pharmaceutical company and its key objectives are:
To introduce the students to AZ and the research site;
To explain the use of animals in the drug development process;
To introduce the regulations surrounding the production of a new medicine.
This is the first session at the pharmaceutical company's site and involves issues of security and the practicality of moving around a large site, unfamiliar to the students. The use of animals in research is always a contentious issue and a session on animal testing is therefore included in the first session. Generally, very few students know about drug development, hence this is a timely opportunity to highlight key aspects of the process. Knowledge of the regulatory process is introduced at this stage, as this is fundamental to the development process of pharmaceuticals.
2. The discovery process
AstraZeneca (Loughborough) – tutorial
This classroom session is based at the AZ research site and is taken by dedicated pharmaceutical scientists involved in the disciplines that contribute to the discovery of new therapies. The key objectives are to explain:
The drug development process with particular emphasis on the very early stages with appropriate examples;
The role of pharmacogenetics and to give details of its likely impact on the industry over the next decade;
The role of the medicinal chemistry department;
The role of molecular biology and High Throughput Screening (HTS) in the development process.
Due to time constraints, it is not possible to provide detailed teaching about the whole of the drug discovery process so an overview is given. Those areas, which have changed most significantly due to advances in technology over recent years, are discussed in detail. These include the use of pharmacogenetics, the use of HTS and the processes used to generate new structures in medicinal chemistry. The session is illustrated with examples from recent company drug developments.
3. Pharmaceutical/analytical and toxicology
AstraZeneca (Loughborough) – interactive tutorial
The next session was dedicated to two key elements of the early drug development process, namely the role of pharmaceutical and analytical research and development and the work of toxicologists.
The objectives of the session include:
To appreciate the contribution of the Pharmaceutical and Analytical Research and Development department to the drug development process;
To be familiar with the toxicology tests used in drug development to ensure its safety prior to use in man;
To understand the guidelines issued by the Medicines and Healthcare products Regulatory Agency (MHRA).
Pharmaceutical and analytical research and development includes a number of disciplines – pharmaceutical science, analytical chemistry, microbiology, product manufacture, quality control and quality assurance. At first, the product design of drug molecules is discussed in relation to the optimization of the clinical potency and the requirements of its formulation that provides drug chemical and physical stability. By using examples of novel drug delivery systems, particularly in the field of respiratory medicine, the methods for selective delivery of drugs to target sites is illustrated. Parcelsus (1493–1541) provides the prologue to the role of the toxicologist: ‘All things are poisons and there is nothing that is harmless, the dose alone decides that something is no poison’. The novel use of in vitro techniques providing early toxicology assessment is explained and examples are given of new potential compounds generated as the result of understanding drug toxicity mechanisms. Finally, the role of regulatory authorities such as the MHRA in setting and regulating product performance standards is discussed.
4. Pre-clinical practical
AstraZeneca (Loughborough) – practical
This 1-day session invites the students to get practical experience of some of the modern technology discussed in the classroom and is conducted by several of the senior experimental scientists. The main objectives are to demonstrate:
The process of HTS;
The use of molecular modelling;
The functions within a medicinal chemistry laboratory;
The functioning of a laboratory within biology;
The use of modern technology for information retrieval.
The students are divided into teams and rotate around the major disciplines as highlighted in the objectives, each lead by one of the dedicated scientists. This gives an insight into the scale of investment that is required to keep pace with the development of new methodologies for a pharmaceutical company to remain competitive.
5. Exploratory clinical development of new candidate drugs
AstraZeneca (Loughborough) – tutorial
This session discusses the initial investigation of drugs in healthy human volunteers and is led by the physicians based at AZ. This provides an effective introduction to the Clinical Practical Session. The main objectives are to understand:
How a compound can be safely given to man for the first time;
Basic pharmacokinetics during early studies;
The use of surrogate markers as a way of assessing compound activity;
How doses are selected for patient studies;
What other factors need to be defined prior to progression of the project;
The need to investigate compounds in special populations, e.g. paediatrics.
The teaching is supported by discussing the roles of Phase I and Phase IIa studies and how programmes are developed to test ‘first into man’ drug therapy. An explanation of the detailed investigations and assessments required in these studies is given with particular regard to pharmacokinetics.
6. Drug advert project
Leicester Royal Infirmary – tutorial and classroom activity
This project was adapted from teaching initially developed by Wilf Yeo (Senior Lecturer in Clinical Pharmacology at the University of Sheffield). One of the CPT clinical tutors and a senior NHS Pharmacist facilitate the sessions.
The objective of the project is to write a 1000-word critical review of a pharmaceutical advertisement that demonstrates:
Review of the published data for the drug;
Analysis of the claims made for the drug by the advertisement;
Discussion of the ‘sales pitch’ underscoring the advertisement;
Understanding of the ABPI (Association of the British Pharmaceutical Industry) code of practice.
In addition, the students are asked to give a 10-min PowerPoint presentation of their project to the class at the end of term. Both the written report (80%) and the presentation (20%) contribute half of the overall module assessment.
The first session introduces the requirements for the project and sets out the role of advertising in the promotion of treatments. Students have a choice of adverts specially selected (from a broad spectrum of companies) by the tutors for their promotional claims. The students not only find this enjoyable, but also learn to critically assess published data in the context of an advert.
7. Design, conduct and interpretation of clinical studies
Leicester Royal Infirmary – tutorial and classroom activity
University CPT clinical tutors involved in the conduct of investigator-led clinical studies lead this classroom session. The primary objectives are:
To understand the fundamental factors in clinical trial design;
To define the stages in the conduct of a clinical trial;
To critically appraise a clinical study and identify its relevance to routine practice.
The discussions are based on recently published clinical studies and provide a contrast to early phase studies outlined in the first part of the module. Using examples, the importance of eliminating bias and potential confounding factors, the definition of power and the need to use the most appropriate statistical analysis is highlighted. Each stage of the clinical study from hypothesis formulation through ethical approval and patient recruitment to study termination is described. In the second half of this session, the students lead the critical appraisal of several peer-reviewed papers from high quality journals and develop an understanding of how the research might be relevant to local routine practice.
8. Clinical practical
AstraZeneca (Nottingham) – practical
The practical session is based at the AZ Clinical Pharmacology Unit at The Queen's Medical Centre, but does not occur during a live drug evaluation programme. Nevertheless, the unit physicians support the teaching of the students during the day.
The key objective is to integrate the knowledge acquired in session 5 and teach the students, by observation, the process of giving a new drug to man. The students observe and use some of the equipment employed to evaluate the activity of a new drug in man. This allows the students to become familiar with the measurement and recording of lung function, ECG (QTc), CNS activity and other vital organ monitoring. The visit also allows the students to observe the electronic capture of data and the manner in which this is processed, analysed and reported.
9. Visit to university/NHS clinical research units
Leicester Royal Infirmary – site visits
There are approximately ten distinct university- or NHS-based clinical research teams across the spectrum of specialties at the three Leicester hospitals. The majority of these teams are conducting innovative investigator-led or commercial pharmaceutical Phase III/IV studies, but the oncology team often conducts Phase IIa studies. This provides a wealth of opportunities for small groups of students to spend time with research nurses learning how studies are conducted in this environment. This is organized with support of the senior nurse based in the R&D Department at the NHS Trust.
10. Research governance and regulatory affairs
Leicester Royal Infirmary – tutorial
An increasing part of the conduct of drug development and particularly clinical studies is the role of research governance and the regulatory environment. This session is led by the manager of the NHS Trust R&D and a senior scientist from the Department of Regulatory Affairs at AZ.
The objectives of the research governance tutorial are to understand:
The requirements placed on a NHS trusts that hosts Phase II and III clinical trials;
Local NHS Trust approvals processes for clinical studies;
The role of Local Research Ethics Committees in assessing proposed studies.
Students are given preparatory work to review a clinical trial protocol and its associated patient information leaflet and consent form. Therefore, as part of the teaching, the tutor leads a discussion with the students about the documentation in order that the important issues may be elucidated.
In support of this, the regulatory tutorial highlights the requirements of national and international regulatory agencies such as the MHRA and the US Food and Drug Administration. The processes that influence the industry at each stage of drug development are discussed with the interactive use of internet access to regulatory agencies’ websites. Following this, students should understand:
The role of modern industry regulatory affairs departments (regulatory intelligence, strategy and operations);
The nature of the regulatory framework of drug development (laws and guidelines);
The role of regulatory agencies and how to interact with them.
11. Marketing
Leicester Royal Infirmary – interactive tutorial
A senior product manager from AZ Head Office at Luton highlights the role of marketing departments in the promotion of novel therapies. This is the most enjoyable session for the students and it conveys the message that advertising is fundamental to the financial success of recently marketed drugs. Its main objectives are to understand:
The key commercial factors needed in the preliminary prioritization of early development projects;
The requirements when developing sales forecasts for projects in late development;
How the product is branded and how the promotional message is communicated;
The role of health economics.
The timing of this session provides an opportunity for students to learn how advertisements are created from an industry perspective and relate this to the critical appraisal of their specific advert as part of the Drug Advert Project.
12. Case studies
AstraZeneca (Loughborough) – classroom activity
The use of case studies or problem-based learning draws together, in an informal way, all the different components of the module, which have been taught over the previous 10 weeks. The main objective is to present the students with real examples of problems encountered in drug development in order for them to identify the problem and offer a solution.
This is has proven one of the more popular sessions of the course. There is a high tutor: student (1 : 4) ratio and at the end of the session the students agree that their knowledge of the drug development process has significantly increased particularly as they learn to grasp the concepts of the process as a whole.
13. NHS assessment of newly marketed drugs
Leicester Royal Infirmary – tutorial
This session is facilitated by a senior NHS pharmacist involved in the NHS Trust Therapeutics Advisory and Leicestershire Formulary committees.
By the end of this session the students should be able to:
Identify some of the difficulties for NHS Trusts associated with the entry of new drugs to the market;
List some benefits of a hospital formulary;
Explain how a new agent is approved for use within the local NHS Trust;
Identify some of the ways that hospital prescribing affects general practice;
Explain some basic terms used in health economics, e.g. cost effectiveness.
This session explores the roles of therapeutics committees and the development of local prescribing guides or formularies. It highlights the difficulties in the assessment of newly marketed drugs particularly in relation to relative efficacy, safety and cost-effectiveness.
14. National Institute for Clinical Effectiveness
Leicester Royal Infirmary – tutorial
The activity of local NHS Trust therapeutic advisory committees is increasingly influenced by guidance from the National Institute for Clinical Effectiveness (NICE) and therefore this session builds on the knowledge from Session 13. In addition, NICE will have a significant effect on the pharmaceutical companies within the UK, since to gain re-imbursement a new drug must show a cost-effective clear advantage over its competitors. Since this session is led by the chairman of the Appraisal Committee for NICE (DBB), this provides added interest for the students since they will get a first-hand account of the workings of this organization.
15. Pharmacovigilance
Leicester Royal Infirmary – tutorial
All doctors and other healthcare professionals should be mindful of the monitoring and reporting of unexpected side-effects as this is an important part of patient care. This has particular value for new-to-market therapies and this session. Therefore, the main objectives are to understand
The need for drug safety-monitoring systems of licensed drugs;
The limitations of the ‘yellow card’ system;
The methods available for postmarketing surveillance;
The role and responsibilities of doctors and other parties in the monitoring of drug safety.
AZ and NHS staff lead this session jointly. The limitations of premarketing clinical trials and issues of postmarketing surveillance are discussed with the use of case studies. The responsibilities of healthcare professionals in the reporting of adverse events are highlighted.
15. The ‘game’
AstraZeneca (Loughborough) – classroom activity
This is the final session of the module and organized by the AZ staff. The Pharm Game (The Learning Key Inc: http://www.thelearningkey.com/games/pharmgame.htm) is played like ‘snakes and ladders’ and involves answering questions on different aspects of drug development. This is run on a competitive basis and prizes are given to the winning team. Not surprisingly, this session is very popular with the students, but it does reveal how much, or how little, they have learnt.
Appendix B – Questionnaire
The 6 knowledge questions were multiple choice, a number accepting more than one answer.
1 Development of a novel drug takes up to:
5 years
10 years
15 years
20 years
2 The average cost of developing a drug:
£50 million
£100 million
£200 million
£300 million
3 Phase I clinical trials involve:
Analysis of drug activity in animals or cultured human cells
Analysis of drug pharmacology in normal volunteers
Analysis of drug pharmacology in patients
Comparison of drug pharmacology between novel and current therapies in patients
4 Prior to human clinical trials:
The drug needs to be safe in animals
The drug needs to be safe in human volunteers
The Medicine Control Agency must provide a licence
The drug can be marketed
5 Once a drug is licensed for marketing, the length of its patent, allowing the pharmaceutical company to be sole supplier, is usually:
5 years
10 years
15 years
20 years
6 Adverse drug reactions can be reported using the Yellow Card Scheme by:
Patients
Doctors
Pharmacists
Coroners
A 10-point Likert-style scale was used to ascertain opinions on several contentious issues relating to the pharmaceutical industry – a higher score reflected greater agreement with the statements:
Animal testing is necessary for the development of novel medicines.
The pharmaceutical industry overcharges the National Health Service.
Clinical trials are designed by pharmaceutical companies to promote drug sales.
Results of clinical studies rather than marketing influence doctor prescribing.
NICE has little bearing on doctors’ prescribing.
Pharmaceutical company bosses are ‘fat cats’.
References
- 1.Harman RJ. The drug development process – 1. Introduction, Overview. Pharmaceut J. 1999;262:2–5. [Google Scholar]
- 2.Heath G, Colburn WA. An evolution of drug development and clinical pharmacology during the 20th century. J Clin Pharmacol. 2000;40:918–29. doi: 10.1177/00912700022009657. [DOI] [PubMed] [Google Scholar]
- 3.Smith JA. An introduction to clinical research and drug development for pharmacy and pharmacology graduate students. J Clin Pharmacol. 2002;42:867–9. doi: 10.1177/009127002401102777. [DOI] [PubMed] [Google Scholar]
- 4.George CF, Burley DM, Brant HA, Hicks B, Rees WL, Mitchell GM. Education of medical students in relation to drug development, safety and efficacy. Med Educ. 1985;19:320–5. doi: 10.1111/j.1365-2923.1985.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 5.GMC. Tomorrow's Doctors. London: GMC; 2002. http://www.gmc-uk.org/med_ed/tomdoc.htm. [Google Scholar]
- 6.Maxwell S, Walley T. Teaching Safe and Effective Prescribing in UK Medical Schools: A Core Curriculum for Tomorrow's Doctors. doi: 10.1046/j.1365-2125.2003.01878.x. http://www.bps.ac.uk/bps.html. [DOI] [PMC free article] [PubMed]
- 7.Walley T, Webb DJ. Core content of a course in clinical pharmacology. Br J Clin Pharmacol. 1997;44:171–4. doi: 10.1046/j.1365-2125.1997.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith R. Medical journals and pharmaceutical companies: uneasy bedfellows. BMJ. 2003;326:1202–5. doi: 10.1136/bmj.326.7400.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers WA, Mansfield PR, Braunack-Mayer AJ, Jureidini JN. The ethics of pharmaceutical industry relationships with medical students. Med J Aust. 2004;180:411–4. doi: 10.5694/j.1326-5377.2004.tb05995.x. [DOI] [PubMed] [Google Scholar]