Abstract
Background
Evidence confirms the positive effects of lipid-lowering agents on the risk of cardiovascular disease. Local guidelines in France (AFSSAPS) have defined therapeutic objectives for LDL-cholesterol. These objectives vary with the number of cardiovascular risk factors in addition to dyslipidaemia. We determined the proportions of patients at therapeutic objective in different classes of cardiovascular risk to test the hypothesis that compliance with guidelines varies across the levels of risk. Comparison with international guidelines (ANDEM) was also performed.
Methods
A group of 3173 dyslipidaemic patients treated with lipid-lowering agents and managed by general practitioners was randomly selected from BKL-Thales panel, a French computerized database. For each patient, history of coronary heart disease and the number of cardiovascular risk factors were documented. Compliance with guidelines was assessed from achievement of therapeutic objective.
Results
The study population included 79% primary prevention patients (1.6, 25.5, 31.7 and 20.1%, with 1, 2, 3, and >3 risk factors, respectively) and 21.0% secondary prevention patients. Applying AFSSAPS guidelines, the proportions of primary prevention patients not at LDL-cholesterol objectives varied across risk categories (P < 0.0001), from 3.9% for patients with one risk factor to 46.5% for patients with >3 risk factors, and therapeutic failure reached 39.9% in secondary prevention. Only 26% of patients who were at high cardiovascular risk (>3 risk factors or prior coronary heart disease) and not at therapeutic objective received high doses (>standard recommended doses) of lipid-lowering agents in monotherapy. Applying ANDEM guidelines, 74% of secondary prevention patients were not at treatment goal.
Conclusion
Compliance with guidelines varied inversely with the level of cardiovascular risk. Besides, most patients not at therapeutic objective were not up-titrated. The use of lipid-lowering agents is inadequate, depriving many patients of an effective protection against cardiovascular diseases.
Keywords: Dyslipidaemia, coronary heart disease, lipid-lowering agents, effectiveness
Introduction
Cardiovascular disease is a common condition in western countries. In France, it accounts for almost one third of overall deaths, i.e. 173 000 deaths in 1996 [1]. Dyslipidaemia is a major modifiable risk factor for coronary heart disease (CHD) in large segments of the population, particularly when it occurs in association with other risk factors such as hypertension, smoking or diabetes [2]. Many countries have national guidelines that recommend treatment goals for dyslipidaemia. As a rule, this treatment should start earlier or be applied more intensively when patients have several risk factors combined [3]. Many studies in recent years reported that patients treated with lipid lowering agents (LLAs) in clinical settings fail to reach the target recommended lipid levels [4, 5].
The lack of goal attainment in clinical setting deprives the patient of an effective protection against CHD, as well as generating unnecessary costs for patients and the community [2].
This study was performed to evaluate the distribution of risk factors for CHD in a sample of the French population treated by general practitioners (GPs) for dyslipidaemia, and to estimate the proportions of patients that attained the recommended lipid level based on their individual level of risk of CHD. We used data from BKL-Thales, a French computerized GPs’ database, and an instrument already used in outcome research [6].
Materials and methods
Study population
A random sample of BKL-Thales practices was used to identify at least 3000 patients treated for dyslipidaemia. Out of the 1698 GPs in the database, 348 (20.5%) were randomly selected.
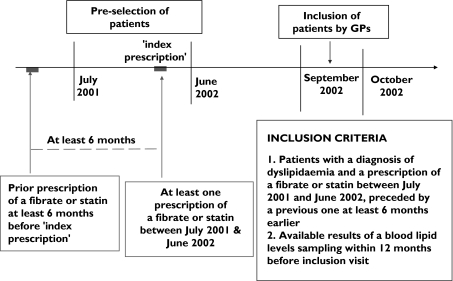
Selection process of patients is illustrated in Figure 1. Patients were to have a diagnosis of dyslipidaemia recorded in the database and to be prescribed a statin or fibrate between July 2001 and June 2002 (‘index prescription’). Only patients for whom a prescription of statin or fibrate was recorded at least 6 months before the ‘index prescription’ were included in the eligible population. GPs started inclusion of patients in September 2002. Pre-selected patients were included at their first visit to their GP after study initiation. They were informed about the study by their GP. Recent blood lipid levels (performed within the past 12 months before inclusion visit) had to be available to participate the study. When several lipid levels were available during this period, only the most recent one was retained for the analyses. GPs included patients until the requested number of patients was reached. No ethics approval was required, since the study was based only on anonymized records from the Thales database. The protocol was sent for information, as requested by local regulations, to the CNIL (National Committee of Informatics and Liberty), a French Regulatory Body.
Figure 1.
Patients selection process
Data collected
Patient demographic, baseline and prescription information was collected from the BKL-Thales database and a specific questionnaire completed by the GP during patients’ visits was used to capture laboratory data and the CHD risk factors.
The following information was obtained from the BKL-Thales database: gender, age, number of visits to GP and prescribed medication.
The primary exposure of interest was the prescription of LLA identified from BKL-Thales prescription records. LLA were classified as statins, fibrates, and others (cholestyramine and tiadenol). The LLA medications recorded in the analyses were the most recent ones prescribed.
For each available LLA, a dose level (standard vs. high) was defined. After review of Physician Desk Reference, a board of experts defined for each product a threshold between ‘standard’ doses and ‘high’ doses (Table 1). For patients treated with a single LLA, these thresholds allowed distribution into two groups: standard doses vs. high doses of LLA.
Table 1.
Standard and high doses for available lipid-lowering agents based on expert panel
| Standard dosages | High dosages | |
|---|---|---|
| Atorvastatin | 10 mg | >10 mg |
| Fluvastatin | 20–40 mg | >40 mg |
| Pravastatin | 10–20 mg | >20 mg |
| Simvastatin | 10–20 mg | >20 mg |
| Bezafibrate | 600 mg | >600 mg |
| Ciprofibrate | 100 mg | >100 mg |
| Fenofibrate | 300 mg | >300 mg |
| Fenofibrate micronized | 2 capsules of 67 mg | >2 caps of 67 mg |
| Gemfibrozil | 900 mg | >900 mg |
Non-LLA medicines (cotherapies) were coded according to the EPhMRA classification (European Pharmaceutical Market Research Association).
On each visit of a patient recruited for the study, the GP recorded the following data on ad hoc study screens: results of the last sampling of LDL-cholesterol (LDL-C) available. This variable was mandatory. If no LDL-C-value was available, it was computed based on the last sampling of total-cholesterol, HDL-cholesterol and triglycerides, using the Friedwald formula [7].
GPs also reported other CHD risk factors: patient family history of premature CHD; recent smoking status; hypertension, diabetes, patient history of CHD (medically treated angina or myocardial infarction). Hypertension was defined by any prescription of anti hypertensive therapy between July 2001 and June 2002, or by a mean systolic/diastolic blood pressure exceeding 140/90 mmHg during the same period. Diabetes was defined by the record of a specific diagnosis in the database or by the prescription of insuline, sulfamides or biguanides during the 12 months before inclusion.
Risk levels of chd, therapeutic objectives
Risk level of CHD and distribution of patients into groups of risk
In addition to dyslipidaemia, risk factors in our analysis included age (males >45, females >55 or postmenopausal); family history of premature CHD (myocardial infarction or sudden death in male first-degree relatives before the age of 55 years or in female first-degree relatives before the age of 65 years); current smoking; hypertension; HDL-cholesterol level <0.9 mmol l−1; diabetes; history of CHD (medically treated angina pectoris, history of myocardial infarction).
According to these criteria, all study patients were assigned to one of the five following groups:
one risk factor for CHD (no risk factor except dyslipidaemia)
two risk factors for CHD (one risk factor in addition to dyslipidaemia)
three risk factors for CHD (two risk factors in addition to dyslipidaemia)
more than three risk factors for CHD (more than two risk factors in addition to dyslipidaemia)
known CHD.
Diabetic patients known to be at increased risk of CHD were classified into the risk subgroups according to the AFSAPPS Guidelines [8].
Patients were considered to be at high risk of CHD when more than three risk factors were identified, or when they had a history of CHD.
Distribution of patients not at therapeutic objective
In addition to defining risk factors for CHD, the guidelines have defined for each level of risk a target LDL-cholesterol level for therapy the therapeutic objective (TO). We used first the most recent national guidelines from AFSSAPS which define LDL-cholesterol intervention threshold according to the number of CHD risk factors in primary prevention and to prior CHD. The therapeutic objective is defined as being lower than the intervention threshold (e.g. intervention threshold for CHD is LDL-C >3.4 mmol l−1 and the therapeutic objective is <3.4 mmol l−1). These levels were used to identify the proportions of patients not at therapeutic objective and to evaluate compliance with guidelines as outlined in Table 2. As a second basis, we applied the ANDEM (‘Agence Nationale pour le Développement de l’Evaluation Médicale’) guidelines, that recommend therapeutic objective levels identical to those defined in the USA by the NCEP (‘National Cholesterol Education Program’) [9, 10].
Table 2.
CHD risk factors and recommended target levels of LDL-cholesterol
| One RF* | Two RFs | Three RFs | >Three RFs | History of coronary heart disease | |
|---|---|---|---|---|---|
| LDL-C Therapeutic Objective | <2.2 g l−1 | <1.9 g l−1 | <1.6 g l−1 | <1.3 g l−1 | <1.3 g l−1 |
| ″″″″according to AFSSAPS | (5.7 mmol l−1) | (4.9 mmol l−1) | (4.1 mmol l−1) | (3.4 mmol l−1) | (3.4 mmol l−1) |
| LDL-C Therapeutic Objective | <1.6 g l−1 | <1.6 g l−1 | <1.3 g l−1 | <1.3 g l−1 | <1.0 g l−1 |
| ″″″″according to ANDEM | (4.1 mmol l−1) | (4.1 mmol l−1) | (3.4 mmol l−1) | (3.4 mmol l−1) | (2.6 mmol l−1) |
RF: risk factors.
Data analysis
Analyses were conducted only for patients who had complete data (Thales records and completed GP questionnaire). The analyses were descriptive and inferential.
Descriptive analyses
Risk stratification based on the number of risk factors (RFs) for CHD and a description of socio-demographic data, treatment characteristics, lipid levels and patient management in groups of patients with homogeneous risk of CHD was carried out. Quantitative data were described with mean values and standard deviation. Percentages and 95% confidence intervals (95% CI) were used for qualitative data.
Inferential analysis
The relationship between the level of risk of CHD and the proportion of patients at therapeutic objective was studied with a Cochran-Armitage trend test using SAS 8.02 software (Freq procedure). To compute these statistics, the groups ‘1 RF’, ‘2 RFs’, ‘3 RFs’, ‘>3 RFs’ and ‘known CHD’ were assigned the levels 1, 2, 3, 4 and 5, respectively.
Finally, among patients with single LLA and not at therapeutic objective, the comparison of the number of patients who received high doses vs. standard doses of LLA was performed according to CHD risk level (low and medium vs. high) with a chi-square test.
Results
Patients characteristics, CHD risk and LLA
Out of the 1693 GPs recorded in the database, a sample of 348 GPs was randomly selected (20.6%). Altogether, 136 agreed to participate in the study and 123 included patients in the study. Inclusion visits started in September 2002 until October 2002, allowing the inclusion of 3173 patients (26 patients per GP on average).
Socio-demographic and medical characteristics of these patients are detailed in Table 3. Mean age was 65.8 years, and 50.7% of the patients were males. Most common CHD risk factors besides age included hypertension (61.9%) and diabetes (16.7%). In our sample, 72.9% of patients treated for dyslipidaemia had at least three risk factors or a history of CHD, including 41.1% at high risk of CHD (>3 risk factors or history of CHD).
Table 3.
Patient characteristics, CHD risk factor distribution and LLA therapy
| Demographic and medical data | n | % | 95% CI |
|---|---|---|---|
| Patients (n) | 3173 | 100 | |
| males (n) | 1609 | 50.7 | 48.9–54.2 |
| Mean | (SD) | ||
| Age, years | 65.8 | (10.8) | |
| Number of visits to the GP* | 8.7 | (4.8) | |
| Frequency of distinct risk factors | n | % | 95% CI |
| Age (men: >45 years; female: >55 years) | 3009 | 94.8 | 94.0–95.6 |
| Family history of premature CHD | 513 | 16.2 | 14.9–17.5 |
| Current smoking | 373 | 11.8 | 10.7–12.9 |
| Hypertension | 1965 | 61.9 | 60.2–63.6 |
| HDL-C < 0.35 g l−1† | 62 | 5.2 | 4.0–6.7 |
| Diabetes | 529 | 16.7 | 15.4–18.0 |
| History of CHD | 666 | 21.0 | 19.6–22.4 |
| Patient distribution in groups at increasing risk of CHD | |||
| One Risk Factor | 51 | 1.6 | 1.2–2.1 |
| Two Risk Factors | 810 | 25.5 | 24.0–27.1 |
| Three Risk Factors | 1007 | 31.7 | 30.1–33.4 |
| >Three Risk Factors | 639 | 20.1 | 18.8–21.6 |
| Known coronary disease | 666 | 21.0 | 19.6–22.4 |
| LLA therapy used at inclusion | |||
| Patients treated with single LLA | 3109 | 98.0 | 97.4–98.4 |
| Statin alone | 1892 | 59.6 | 57.9–61.3 |
| Fibrate alone | 1217 | 38.4 | 36.7–40.1 |
| Patients with combined therapy‡ | 64 | 2.0 | 1.6–2.6 |
| Including colestyramine§ | 27 | 0.9 | 0.6–1.2 |
| Including tiadenol§ | 19 | 0.6 | 0.4–0.9 |
| Including statin and fibrate | 20 | 0.6 | 0.4–1.0 |
During the past 12 months before inclusion visit.
This information is available for 1185 patients only.
The detailed count does not add to 64 since 2 patients were under tritherapy (Statin, fibrate, Tiadenol and cholestiramin, Fibrate, Tiadenol.
With statine or fibrate.
Mean LDL-cholesterol value was 1.28 g l−1 (SD = 0.36). For CHD risk groups, mean LDL-C-values were 1.35 g l−1 (SD = 0.35), 1.30 g l−1 (SD = 0.34), 1.31 g l−1 (SD = 0.37), 1.27 g l−1 (SD = 0.39), 1.22 g l−1 (SD = 0.37) for one, two three, >3 RFs and known CHD, respectively.
Ninety-eight per cent (98%) of patients were treated with a single LLA (59.6% with statins alone and 38.4% with fibrates alone).
Of the patients treated with statins or fibrates, 80.4% received a ‘standard dose’ (81.9% of patients at therapeutic objective, and 75.6% of those who were not at therapeutic objective).
Proportions of patients not at therapeutic objective
Applying AFSSAPS guidelines, 800 patients (25.2%) were not at therapeutic objective. These proportions were 25.1% and 31.3% in patients with single LLA and with combined LLA, respectively. The proportions of patients not at therapeutic objective varied with the risk level of CHD. It ranged from 3.9% for patients with one risk factor, to 46.5% and 39.9% for patients with more than three risk factors and a history of CHD, respectively (Table 4). A significant trend appeared across CHD risk groups (Cochran-Armitage trend test = −18.8, P < 0.0001). When ANDEM guidelines were applied, the proportions of patients not at therapeutic objective varied from 18.0% for patients with two risk factors, to 74.3% for patients with a history of CHD (Table 4).
Table 4.
Number of patients not at therapeutic objective in different CHD risk groups
| Total | One RF | Two RFs | Three RFs | >Three RFs | Known coronary disease | |
|---|---|---|---|---|---|---|
| (n = 3173) | n = 51 | n = 810 | n = 1007 | n = 639 | n = 666 | |
| Patients not at therapeutic | n | 2 | 45 | 190 | 297 | 266 |
| objective applying AFSSAPS | % | 3.9 | 5.6 | 18.9 | 46.5 | 39.9 |
| guidelines | 95% CI | 0.5–13.5 | 4.1–7.4 | 16.5–21.4 | 42.6–50.4 | 36.2–43.8 |
| Patients not at therapeutic | n | 10 | 146 | 484 | 297 | 495 |
| objective applying ANDEM | % | 19.6 | 18.0 | 48.1 | 46.5 | 74.3 |
| guidelines | 95% CI | 9.8–33.1 | 15.4–20.8 | 44.9–51.2 | 42.6–50.4 | 70.8–77.6 |
Cochran-Armitage trend test using AFSSAPS guidelines = −18.8, P < 0.0001. Cochran-Armitage trend test using ANDEM guidelines =−17.6, P < 0.0001.
Intensity of LLA therapy in patients treated with single LLA and not at therapeutic objective
Applying AFSAPPS guidelines, 780 patients were treated with a single LLA, and not at therapeutic objective. About a quarter of them (24.4%) received ‘high’ doses of lipid-lowering agents. This proportion did not significantly vary between patients at low-medium CHD risk (= 3 RFs) and those at high risk (>3 RFs or CHD): 20.6%vs. 26.0%, P = 0.13.
Discussion
The study illustrates the management of dyslipidaemia by GPs in France and evaluates the proportions of patients not at therapeutic objective in groups at different CHD risk levels, in primary and secondary prevention. Data shows that the management of lipid disorders in primary care is inadequate, since compliance with treatment guidelines decreases with the level of CHD risk and since patients not at the objective are not up titrated.
Our study used computerized records from general practitioners (BKL-Thales database) to identify patients treated with lipid-lowering therapy, and to check their lipid levels under therapy. BKL-Thales has been demonstrated as a reliable source of data, based on previous investigations and on the ability to link recorded data with ad hoc questionnaire [6]. Comparison of the data routinely recorded with the data collected with the specific questionnaire showed a good consistency for most variables, except for family history of premature coronary artery disease (1.7% of patients in the records vs. 16.2% in the survey) and for current smoking (8.3% in the records vs. 11.8 in the survey), as it could be expected [11].
Several precautions were used to maximize the validity of our findings, and we took care to include only patients with documented evidence of risk factors for CHD. In particular, the diagnosis of angina pectoris was only accepted when records of specific antianginal therapy were available.
The results can be extrapolated to some extent to the general population of patients treated with lipid-lowering therapy in primary care, since the comparison of the sample of 3173 patients with all patients treated for dyslipidemia and with documented LDL cholesterol in the BKL Thales database (n = 37 682) showed similar proportion of patients not at therapeutic objective.
The application of ANDEM guidelines with lower objectives showed that 45% of patients were not at therapeutic objective (37% and 74% in primary and secondary prevention, respectively), i.e. nearly twice the 25% obtained after application of AFSSAPS guidelines (21% and 34% in primary and secondary prevention, respectively). A study conducted with ANDEM guidelines in 1999–2001 by the largest French health care insurance showed very similar results with, respectively, 40% and 68% of patients treated with statins for primary and secondary prevention not at LDL-C objective [12].
The recent European Guidelines on cardiovascular disease prevention [13] which recommend LDL-C objective = 1.0 g l−1 in patients with established CHD and in patients with diabetes emphasize even more the relevance of applying treatment goals similar to those of ANDEM.
Since ANDEM therapeutic objectives are identical to those of NCEP and have also similar treatment goals for CHD patients as the new European Guidelines, this computation makes comparisons possible. The difference between both computations also illustrates the sensitivity of all descriptions to local guidelines, and more importantly, the potential impact of these guidelines on local management of these populations at high cardiovascular risk.
A US study that investigated the success rates of therapy, as defined by the proportions of patients reaching LDL-cholesterol levels <1.0 g l−1[10], showed even more worrying data, i.e. 63% and 82% of patients with high risk of CHD or known CHD, respectively, not reaching the recommended LDL-cholesterol levels [4].
Other studies [14, 15] confirmed that LDL-C therapeutic objective are far from being reached by all treated patients.
Despite the complexity of a comparison, e.g. as a result of different populations, different therapeutic objective, and absence of documentation about the CHD risk level in the some studies, these findings are in line with our data, and support our finding that dyslipidaemia is poorly managed, particularly in patients at high risk of CHD, despite considerable evidence that these patients would benefit more from appropriate lipid lowering therapy [16–20].
Although it was not the objective of the present study to determine the reasons of this poor management, various reasons can be foreseen: low awareness of the LDL-C goal, limited efficacy of current therapies by inhibiting only one of the two main sources of cholesterol (liver synthesis, intestinal absorption), fear of adverse events related to higher doses of LLA and lack of adherence of patients to therapy.
This study has some limitations. First limitations originate from the use of a computerized database of medical records [21]. In this study, there were no data on patients’ socioeconomic status or location, and we could not study the geographical variations of risk factors that have been described in France [14]. As GPs who participated in the Thales database were distributed throughout all French regions, it can nevertheless be assumed that their patients were similarly distributed, and that our results apply to the national population.
Access to recent lipid levels (last 12 months before inclusion visit) was required for including patients in the study, to compute the level of risk of CHD, and to verify patients’ levels compared with their individual therapeutic objective. This requirement may have biased the selection of our patient sample towards better-managed patients or better-trained GPs, with more adequate management of dyslipidaemia. This is supported in part by the comparison between our sample and the general population of LLA-treated patients in Thales, suggesting that our sample had on average more frequent contacts with general practitioners (8.7 vs. 6.5 visits year−1), probably as a result of more numerous comorbid conditions. However, if this was the case, it would result in an underestimation of inadequate therapy, and our assessment may be viewed as conservative. Some risk factors of CHD may be more difficult to identify, e.g. a family history of premature CHD, but we applied all accepted criteria for prescribing LLA in France. Also, efforts were made to identify existing CHD from patient information in addition to recorded GP diagnoses. Indeed, in France, patients may visit specialists at their own initiative, with the result that specialists may neglect to inform GPs of new diagnoses. It is, however, unlikely that this information gap would occur for major, life-threatening diagnoses like angina pectoris or myocardial infarction. This is supported by our findings showing comparable prevalence of CHD in our sample and in the general population of patients treated with lipid-lowering therapy in BKL-Thales (17.9%vs. 21.0% in our data).
In addition, we had no information on lipid levels prior to initiation of LLA. It is therefore not excluded that some high risk patients had higher lipid levels before the initiation of therapy, with a greater percentage reduction of LDL-cholesterol.
Finally, as always the case in databases of GP records, we used prescribed doses, and not the doses delivered at the pharmacy or the doses actually used by the patient. Nonetheless, using prescribed doses is the best approach to the study of disease management, since it provides direct access to physicians’ decisions [6].
Our finding that many patients treated for dyslipidaemia do not reach the target lipid level corresponding to their risk of CHD, has significant implications. The relationship between LDL-cholesterol levels and CHD risk is well recognized, even for levels as low as 80 mg dl−1. Likewise, the efficiency of LLA therapy is well established from experimental studies. However, our data suggest that, in actual medical practice, many patients at high risk of CHD are exposed to a high risk of cardiovascular morbidity or mortality as a result of inadequate therapy, and this is worrying [22, 23].
In summary, this study has provided information on the management of dyslipidaemia by French GPs in 2002. Our study delivers some alarming information, with high proportions of patients not at therapeutic objective. More aggressive approaches to the management of cholesterol, particularly in high risk patients, are needed.
Acknowledgments
The study was supported by MSD-Chibret, Paris, France.
References
- 1.Selke B, Laurent P. Rapport au Syndicat National de L’Industrie Pharmaceutique. France: (CRESGE) Lille; 1999. Maladies Cardio-Vasculaires. Impact Medico-Economique de la Prise en Charge Médicamenteuse par Hypolipemiants a partie d’une Revue de la Littérature. [Google Scholar]
- 2.Stein EA. Managing dyslipidaemia in the high-risk patient. Am J Cardiol. 2002;89(Suppl):50C–57C. doi: 10.1016/s0002-9149(02)02229-4. [DOI] [PubMed] [Google Scholar]
- 3.Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341:498–511. doi: 10.1056/NEJM199908123410707. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L-TAP) Arch Intern Med. 2000;160:459–67. doi: 10.1001/archinte.160.4.459. [DOI] [PubMed] [Google Scholar]
- 5.EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries. Principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J. 2001;22:554–72. doi: 10.1053/euhj.2001.2610. [DOI] [PubMed] [Google Scholar]
- 6.Van Ganse E. Use of computerized data in pharmacoepidemiology. Therapie. 2000;55:123–6. [PubMed] [Google Scholar]
- 7.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 8.Agence Française de Sécurité Sanitaire des Produits de Santé. Saint Denis, France: Afsapps; 2000. La prise en charge thérapeutique du patient dyslipidémique – Recommandations. September. [Google Scholar]
- 9.Agence Nationale pour le Développement et l’Evaluation Médicale (ANDEM) Hypolipidémiants Concours Méd. 1996;41:58–72. [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;17(106):3143–421. [PubMed] [Google Scholar]
- 11.Hemmelgarn B, Blais L, Collet JP, et al. Automated databases and the need for fieldwork in pharmacoepidemiology. Pharmacoepidemiol Drug Safety. 1994;3:275–82. [Google Scholar]
- 12.Chevalier C, Giral P, Chinaud F, Carzon M, Gartenlaub D, Blanchon B, et al. Adherence to national guidelines for lipid management and use of statins. Rev Epidemiol Santé Publique. 2002;50:463–73. [PubMed] [Google Scholar]
- 13.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines in cardiovascular disease prevention in clinical practice. Eur Heart J. 2003;24:1601–10. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 14.Marques-Vidal P, Bongard V, Arveiler D, Amouyel P, Ducimetiere P, Ferrieres J. Cost of cardiovascular risk factor prevention in France. Arch Mal Cœur. 2002;95:275–81. [PubMed] [Google Scholar]
- 15.Hippisley-Cox J, Cater R, Pringle M, Coupland C. Cross-sectional survey of effectiveness of lipid lowering drugs in reducing cholesterol concentration in patients in 17 general practices. BMJ. 2003;326:689–93. doi: 10.1136/bmj.326.7391.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heart Protection Study Heart Protection Study Collaborative Group. MRC / BHF Heart Protection Study of Cholesterol lowering simvastatin in 20 536 high-risk individuals: a randomized placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 17.Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Survival Study (4S) Lancet. 1994;334:1383–9. [PubMed] [Google Scholar]
- 18.The long-term intervention with pravastatin in ischemic disease (LIPID) Study Group. Prevention of cardiovascular events and deaths with pravastatin in patients with coronary heart disease and broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 19.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. For the Cholesterol and Recurrent Events Trial (CARE) investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. For the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro S. The role of automated record linkage in the post-marketing surveillance of drug safety: a critique. Clin Pharmacol Ther. 1989;46:371–86. doi: 10.1038/clpt.1989.154. [DOI] [PubMed] [Google Scholar]
- 22.Cullen P, Assmann G. Treatment goals for low-density lipoprotein cholesterol in the secondary prevention of coronary heart disease. absolute levels or extent of lowering? Am J Cardiol. 1997;80:1287–94. doi: 10.1016/s0002-9149(97)00667-x. [DOI] [PubMed] [Google Scholar]
- 23.Ito MK, Delucca GM, Aldridge MA. The relationship between low-density lipoprotein cholesterol goal attainment and prevention of coronary heart disease-related events. J Cardiovasc Pharmacol Therapeut. 2001;6:129–35. doi: 10.1177/107424840100600204. [DOI] [PubMed] [Google Scholar]