Abstract
Aims
We investigated whether the insertion/deletion (I/D) polymorphism of the ACE gene modified the adherence to ACE inhibitors as measured by the discontinuation of an ACE inhibitor, or addition of another antihypertensive drug.
Methods
This was a cohort study among 239 subjects who started ACE inhibitor therapy. A Cox proportional hazard model was used to calculate relative risk (RR).
Results
During follow-up there was no significant difference between subjects with the DD, ID or II genotype (DD vs II; RR = 1.17, 95%CI: 0.78, 1.77 and ID vs II; RR = 1.06, 95%CI: 0.73, 1.55) in adherence.
Conclusions
The I/D polymorphism of the ACE gene does not influence the adherence to ACE inhibitors.
Keywords: ACE gene, ACE inhibitors, polymorphism
Introduction
Hypertension is a major public health hazard and despite the availability of a variety of effective antihypertensive drugs inadequate control of blood pressure is common in hypertensive patients. One of the factors in interpreting the variability in outcome of drug therapy includes the genetic profile of the patient [1].
A candidate gene for the control of blood pressure is the angiotensin converting enzyme (ACE) gene. Individuals with the DD genotype display twice as high serum ACE concentrations as individuals with the II genotype [2], but without clear correlation to blood pressure [2]. The I/D polymorphism of the ACE gene has been associated with differential blood pressure responses to ACE inhibitors. However, the results have been controversial [3–6].
Given the controversial results, we investigated whether the I/D polymorphism of the ACE gene was associated with the response to ACE inhibitor therapy as measured by the discontinuation of an ACE inhibitor, or addition of another antihypertensive drug class.
Methods
Setting
The Rotterdam study started in 1990 as a population-based prospective follow-up study. In total, 7983 residents of the suburb Ommoord in Rotterdam, aged 55 years or over participated. The baseline measurements took place until 1993. The design of this population-based study has been described elsewhere [7]. Pharmacy records were available for approximately 99% of the cohort as of January 1st, 1991.
Cohort and outcome definition
For the analysis, we included patients who had at least 6 months of medication history at the pharmacy before starting with an ACE inhibitor and who did not use antihypertensive drugs during that period. We excluded persons with only one ACE inhibitor prescription.
To study the potential interaction between I/D polymorphism and response to ACE inhibitors, we used two proxy outcomes. The first outcome was defined as the discontinuation of ACE inhibitors for ≥180 days. The second outcome was addition of another antihypertensive drug to the ACE inhibitor therapy. Subjects were followed until the outcome of interest, death, moving outside of the study area, or the end of the study period, whichever came first.
Genotype
The I and D allele of the ACE genotype were identified on the basis of polymerase chain reaction (PCR) amplification of the respective fragments from intron 16 of the ACE gene and size fractionation and visualization by electrophoresis as described before [8].
Analysis
We used anova (continuous variables) and Chi-square testing (categorical variables) to compare baseline characteristics of people with different genotypes. For the outcome of interest, a Cox proportional hazard model was used to calculate the relative risk (RR) and 95% confidence interval (95% CI) of discontinuation of the ACE inhibitor or addition of another antihypertensive drug.
Results
Between January 1st, 1991 and December 31st, 1999, 1488 subjects were identified as ACE inhibitor users and 239 subjects had not used antihypertensive medication between January 1st and July 1st, 1991 prior to the start of ACE inhibitor medication.
Cohort study among starters of ACE inhibitors
In total, 65, 117 and 57 had the DD, ID and II genotypes, respectively. Different ACE inhibitors were used as a first prescription including: enalapril (48.3%), lisinopril (16.8%), captopril (9.7%), quinapril (8.8%), perindopril (6.7%), fosinopril (6.3%), ramipril (3.0%) and cilazapril (0.4%). Age, gender, diastolic blood pressure, smoking, body mass index and alcohol use were similar in the three genotype groups (Table 1).
Table 1.
Baseline characteristics. Values are presented as means (± SD), or number (%)
| Variable | DD (n = 65) | ID (n = 117) | II (n = 57) | P value |
|---|---|---|---|---|
| Gender, F | 36 (55.4%) | 67 (57.3%) | 32 (56.1%) | 0.969 |
| Age (years) | 68.7 ± 9.7 | 69.2 ± 7.4 | 69.3 ± 8.2 | 0.901 |
| SBP1 (mmHg) | 148.6 ± 20.9 | 154.7 ± 22.4 | 150.4 ± 22.0 | 0.205 |
| DBP1 (mmHg) | 78.7 ± 10.6 | 80.3 ± 12.9 | 79.1 ± 9.8 | 0.663 |
| Smoking | ||||
| ″current | 12 (18.5%) | 33 (29.2%) | 13 (22.8%) | 0.532 |
| ″past | 31 (47.7%) | 46 (40.7%) | 23 (40.4%) | 0.964 |
| ″never | 22 (33.8%) | 34 (30.1%) | 21 (36.8%) | 0.760 |
| BMI (kg m−2) | 26.5 ± 3.2 | 26.4 ± 3.7 | 26.8 ± 4.0 | 0.108 |
| Diabetes mellitus, yes | 11 (16.9%) | 21 (17.9%) | 7 (12.3%) | |
| MI, yes | 6 (9.2%) | 16 (13.7%) | 2 (3.5%) |
Only persons with a blood pressure measurement before they started an ACE inhibitor therapy were included (n = 211).
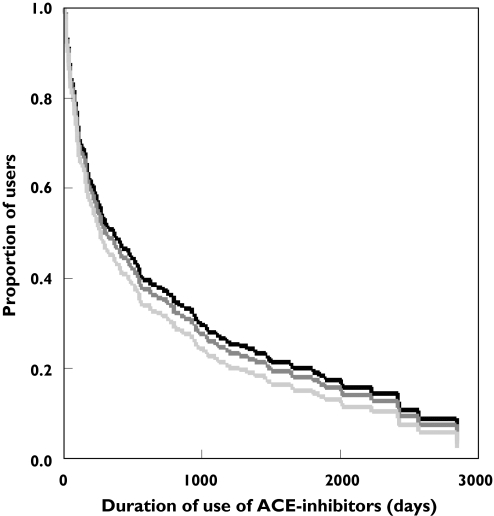
The Kaplan-Meier function showed that there were no significant differences in the rate of discontinuation of ACE inhibitors or addition of other antihypertensive medication (DD vs II, RR = 1.17, 95% CI: 0.78, 1.77; ID vs II, RR = 1.06, 95% CI: 0.73, 1.55) (Figure 1). During the entire follow-up there was no significant difference between the three genotypes. The results were similar when the outcomes of discontinuation and addition of other antihypertensive drugs were analyzed separately (data not shown). Excluding of stoppers (n = 38) did not effect our findings. The effect was not caused by a difference in the average last prescribed daily dose before a censoring event (0.76 ± 0.38, 0.83 ± 0.64 and 0.78 ± 0.37 for the DD, ID and II genotype; overall P value = 0.63).
Figure 1.
Kaplan-Meier function of addition of other antihypertensive medication and/or stopping of an ACE inhibitor by ACE genotyping stratified by ACE genotype (adjusted for gender, BMI, SBP, DBP, MI, diabetes mellitus, smoking and death). ACE genotype: II ( ), ID (
), ID ( ), DD (
), DD ( )
)
Discussion
Our findings suggest that the ACE I/D genotype in starters of ACE inhibitors does not influence the response when evaluated by discontinuation of ACE inhibitors and/or addition of other antihypertensive drugs.
Previously, four other studies investigated the role of the ACE gene in the response of blood pressure to ACE inhibitors, but the results were inconclusive [3–6]. Our study corroborates the results of two of four studies [5, 6]. However, we used a proxy for blood pressure response instead of actual blood pressure measurements. Although, insufficient blood pressure control and side-effects account for most of the treatment switching [9], our proxy might not be good for measuring the nonsatisfactory response of blood pressure to ACE inhibitors. For example, our results could be influenced by adverse reactions to ACE inhibitors, like dry cough. The role of the ACE gene in the occurrence of cough is, however, still unclear [10, 11]. It is also possible, that due to satisfactory blood pressure response a physician advises a patient to stop using antihypertensive medication. However, this is a rare occurrence and we saw no difference between switchers and stoppers.
This study suggests that the ACE I/D polymorphism of the ACE gene does not influence the adherence to ACE inhibitors.
Acknowledgments
This study was financially supported by the Netherlands Heart Foundation, grant number 2001.064. The Rotterdam study is funded by the Netherlands Organization for Scientific Research (NWO) and the Municipality of Rotterdam.
References
- 1.Sander C. Genomic medicine and the future of health care. Science. 2000;287:1977–8. doi: 10.1126/science.287.5460.1977. [DOI] [PubMed] [Google Scholar]
- 2.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohmichi N, Iwai N, Uchida Y, Shichiri G, Nakamura Y, Kinoshita M. Relationship between the response to the angiotensin converting enzyme inhibitor imidapril and the angiotensin converting enzyme genotype. Am J Hypertens. 1997;10:951–5. doi: 10.1016/s0895-7061(97)00121-0. [DOI] [PubMed] [Google Scholar]
- 4.Stavroulakis GA, Makris TK, Hatzizacharias AN, Anastasiadis G, Triposkoadis P, Kyriakidis MK. Predicting response to chronic antihypertensive treatment with fosinopril: the role of the angiotensin-converting enzyme gene polymorphism. Cardiovascular Drugs Ther. 2000;14:427–32. doi: 10.1023/a:1007820401377. [DOI] [PubMed] [Google Scholar]
- 5.Dudley C, Keavney B, Casadei B, Conway J, Bird R, Ratcliffe P. Prediction of patient responses to antihypertensive drugs using genetic polymorphisms: investigation of renin-angiotensin system genes. J Hypertens. 1996;14:259–62. doi: 10.1097/00004872-199602000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani AD, Jia H, Stevens PA, Hopper R, Dickerson JE, Brown MJ. Renin-angiotensin system gene polymorphisms influence blood pressure and the response to angiotensin converting enzyme inhibition. J Hypertens. 1995;13:1602–9. [PubMed] [Google Scholar]
- 7.Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–22. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 8.Lindpaintner K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, Buring J, Hennekens CH. A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332:706–11. doi: 10.1056/NEJM199503163321103. [DOI] [PubMed] [Google Scholar]
- 9.Ambrosioni E, Leonetti G, Pessina AC, Rappelli A, Trimarco B, Zanchetti A. Patterns of hypertension management in Italy: results of a pharmacoepidemiological survey on antihypertensive therapy. Scientific Committee of the Italian Pharmacoepidemiological Survey on Antihypertensive Therapy. J Hypertens. 2000;18:1691–9. doi: 10.1097/00004872-200018110-00023. [DOI] [PubMed] [Google Scholar]
- 10.McGarvey LP, Savage DA, Feeney SA, Heaney LG, Ennis M, MacMahon J, Maxwell AP. Is there an association between angiotensin-converting enzyme gene variants and chronic nonproductive cough? Chest. 2000;118:1091–4. doi: 10.1378/chest.118.4.1091. [DOI] [PubMed] [Google Scholar]
- 11.Furuya K, Yamaguchi E, Hirabayashi T, Itoh A, Hizawa N, Ohnuma N, Kawakami Y. Angiotensin-I-converting enzyme gene polymorphism and susceptibility to cough. Lancet. 1994;343:354. doi: 10.1016/s0140-6736(94)91190-8. [DOI] [PubMed] [Google Scholar]