Abstract
Aims
Case reports suggest that gemfobrozil can increase the anticoagulant effect of warfarin. Because gemfibrozil inhibits CYP2C9 in vitro, we studied its effects on the pharmacokinetics and pharmacodynamics of racemic warfarin.
Methods
In a randomized cross-over study, 10 healthy subjects ingested 600 mg gemfibrozil or placebo twice daily for 8 days. On day 3, they were administered a single dose of 10 mg racemic R-S-warfarin orally. The concentrations of R- and S-warfarin in plasma and thromboplastin time were monitored up to 168 h.
Results
Gemfibrozil decreased the mean (±SD) area under the plasma concentration-time curve [AUC(0–∞)] of S-warfarin by 11%, from 19.9 ± 5.2 mg l−1 h to 17.6 ± 4.7 mg l−1 h (95% CI on the difference −3.7, −0.78; P < 0.01) and that of R-warfarin by 6% from 31.3 ± 7.5 mg l−1 h during the gemfibrozil phase to 29.5 ± 6.9 mg l−1 h during the placebo phase (95% CI −3.3, −0.33; P < 0.05). There were no significant differences in the elimination half-lives of S- or R-warfarin between the phases. Gemfibrozil did not alter the anticoagulant effect of warfarin.
Conclusion
Unexpectedly, gemfibrozil slightly decreased the plasma concentrations of R- and S-warfarin. Displacement of warfarin from plasma albumin by gemfibrozil or its interference with the absorption of warfarin could explain the present findings. Usual therapeutic doses of gemfibrozil seem to have limited effects on the pharmacokinetics and pharmacodynamics of single dose warfarin in healthy subjects.
Keywords: warfarin, gemfibrozil, CYP2C9, drug–drug interaction
Introduction
Warfarin is a widely used orally administered anticoagulant. Its therapeutic effect is based on inhibition of the synthesis of the vitamin K-dependent clotting factors II, VII, IX, and X [1]. Warfarin is administered as a racemic mixture of two enantiomers, R-warfarin and S-warfarin. The S-enantiomer is about 5–7-fold more potent as an anticoagulant than R-enantiomer. Warfarin is well absorbed, has a bioavailability of over 95% and is highly (>97%) bound to plasma albumin. Warfarin enantiomers are eliminated by extensive biotransformation in the liver. The S-isomer is metabolized into its main metabolite, 7-OH-warfarin, primarily by CYP2C9 [2, 3], whereas R-warfarin is metabolized by several CYP enzymes [2].
Gemfibrozil is a fibric acid derivative that is used for the treatment of certain dyslipidemias. It markedly increases the concentrations of some statins (e.g. cerivastatin) and antidiabetic drugs (e.g. repaglinide) which are substrates for CYP2C8 [4, 5]. Gemfibrozil inhibits CYP2C9 in vitro even more potently than it inhibits CYP2C8 [6, 7]. Some case reports have indicated an excessive anticoagulation during warfarin therapy when administered concomitantly with gemfibrozil [8, 9]. The aim of this study was to investigate possible effects of gemfibrozil on the pharmacokinetics and pharmacodynamics of warfarin.
Methods
Subjects
Ten healthy subjects (nine men and one woman; age range, 19–27 years; weight range, 54–90 kg) participated in the study. Each subject was deemed to be in good health as assessed by a medical history, clinical examination, and routine laboratory testing. Only subjects with normal thromboplastin time (international normalized ratio (INR) <1.3) and without known bleeding or haemostatic disorders were recruited. The subjects were not taking any continuous medication and all of them were nonsmokers. Consumption of grapefruit products from 1 week before the first study day was forbidden. The study protocol was approved by the Ethics Committee for Studies in Healthy Subjects of the Hospital District of Helsinki and Uusimaa and by the National Agency for Medicines. The subjects gave their written informed consent before entering the study.
Study design
A randomized cross-over study design with two phases was used with an interval of 4 weeks. The subjects ingested 600 mg gemfibrozil (one Lopid 600 mg tablet, Parke Davis, Gödecke, Freiburg, Germany) or placebo twice daily, at 08:00 h and 20:00 h, for 8 days. On day 3, after an overnight fast, a single oral dose of 10 mg racemic R-S-warfarin (one Marevan 10 mg tablet, Orion Pharma, Espoo, Finland) was administered with 200 ml water at 09:00 h, i.e. 1 h after gemfibrozil or placebo. Three hours after the intake of warfarin, the subjects were served a warm standard meal, and 7 h after warfarin intake, they were given a light meal.
Sampling
On day 3, timed blood samples from a forearm vein and for drug determinations were drawn into siliconized plastic tubes containing ethylenediaminetetraacetic acid (EDTA) before the administration of warfarin and 1, 2, 3, 4, 6, 9, 12, 24, 33, 48, 72, 96, 120, and 168 h later. Plasma was separated and stored at −70 °C until analysed. In addition, blood samples were drawn into citrate containing tubes before administration of warfarin and 9, 12, 24, 33, 48, 72, 96, 120, and 168 h later for determination of plasma thromboplastin time.
Drug analysis
Plasma concentrations of R- and S-warfarin were determined by HPLC [10]. After acidification of plasma, warfarin and internal standard, p-chlorowarfarin, were extracted into an organic phase. Warfarin R- and S-enantiomers were separated using a CHIRAL-AGP column (150 × 4 mm (ChromTech AB, Hägersten, Sweden)) and detected by fluorescence [11]. The limit of quantification was 0.02 mg l−1 for both enantiomers. The between-day coefficients of variation (CV) for the S-isomer were 3.0–5.1% (n = 10) and for the R-isomer 2.8–8.0% (n = 10) over the range of concentrations used.
Plasma gemfibrozil concentrations were determined by a reversed phase HPLC with UV-detection [12]. Ibuprofen was used as an internal standard. The limit of quantification was 0.1 mg l−1. The between-day CVs were <11% (n = 6).
Pharmacokinetic analysis
The peak concentration in plasma (Cmax) and time to Cmax (tmax) were obtained directly from the data. The elimination rate constant (ke) was determined by linear regression analysis of the terminal log-linear part of the plasma warfarin enantiomer concentration-time curve. The area under the plasma concentration-time curve up to the last time point (AUC(0−168)) for R- and S-warfarin was calculated by the linear trapezoidal rule, and the AUC(0–∞) with extrapolation to infinity by dividing the last measured concentration by ke. The elimination half-life (t1/2) was determined from the equation t1/2 = ln2/ke. The apparent oral clearance (Cl/F) of warfarin enantiomers was obtained from the equation Cl/F = D/AUC(0–∞)/weight, where D (dose) is 5 mg. The apparent volume of distribution (Vd/F) was calculated from the equation Vd/F = Cl/F/ke.
Pharmacodynamic analysis
Plasma thromboplastin time (PT) was monitored using a BCS Coagulation System (Dade Behring, Marburg, Germany) with Nycotest Pt reagent. The pharmacodymamics of warfarin were characterized by the maximum effect (Emax) obtained directly from the raw data, and area under the effect time-curve (AUE(0−168)) calculated by the linear trapezoidal rule.
Statistical analysis
The data are expressed as mean values ± SD, except for tmax, which is presented as a median with range. The differences between the placebo and gemfibrozil phases were analysed by the Student t-test for paired values. In addition, 95% confidence intervals (CI) were calculated on the mean difference between the phases. tmax was compared using the Wilcoxon test. The statistical program Systat for Windows, version 6.0.1 (Systat, Evanston, IL) was used for the analysis. Differences were considered to be statistically significant when P < 0.05.
Results
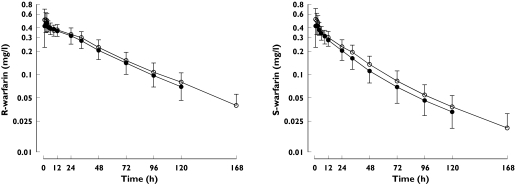
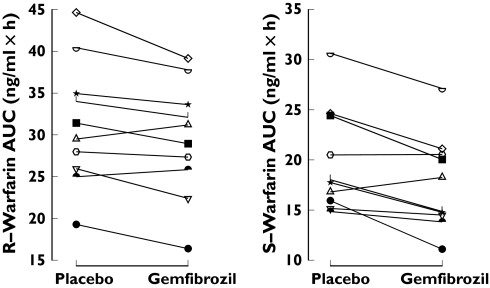
Gemfibrozil decreased the plasma concentrations of both warfarin enantiomers (Figure 1). During the gemfibrozil phase, the AUC(0–∞) of S-warfarin was 89% (P < 0.01) and of R-warfarin 94% (P < 0.01) of the corresponding values during the placebo phase (Table 1). The AUC of R- and S-warfarin was decreased in 8 of 10 subjects by gemfibrozil (Figure 2). The elimination half-lives of S- and R-warfarin did not differ significantly between the phases. Gemfibrozil significantly increased the apparent clearances of S- and R-warfarin and the apparent volume of distribution of S-warfarin (Table 1). During the gemfibrozil phase, the mean plasma concentration of gemfibrozil was 17.8 mg l−1 2 h after administration of warfarin on day 3. Morning trough concentrations of gemfibrozil averaged 1.6 mg l−1, 2.3 mg l−1 and 1.9 mg l−1 on days 4, 5, and 6, respectively.
Figure 1.
The effect of gemfibrozil on the plasma concentrations (mean ± SD) of R- and S-warfarin in 10 healthy subjects. A single oral dose of 10 mg racemic warfarin was ingested on day 3 of pretreatment with gemfibrozil 600 mg or placebo twice daily for 8 days. Concentrations during placebo phase (○), concentrations during gemfibrozil phase (•)
Table 1.
The effect of gemfibrozil on the pharmacokinetics of R-warfarin and S-warfarin in 10 healthy subjects. A single oral dose of 10 mg racemic warfarin was ingested on day 3 of pretreatment with gemfibrozil 600 mg or placebo twice daily for 8 days
| Variable | Placebo phase | Gemfibrozil phase | Mean difference between placebo and gemfibrozil phase (95% CI) |
|---|---|---|---|
| R-Warfarin | |||
| Cmax (mg l−1) | 0.55 ± 0.15 | 0.52 ± 0.09 | −0.03 (−0.10, 0.04) |
| Percentage of placebo (range) | 100 | 94 (77–136) | |
| tmax (h) | 1.5 (1–3) | 1.5 (1–4) | |
| t1/2 (h) | 47.2 ± 8.3 | 48.4 ± 6.4 | 1.2 (−2.2, 4.7) |
| AUC(0–168) (mg l−1 h) | 28.5 ± 6.5 | 26.5 ± 5.9† | −1.9 (−3.5, −0.37) |
| Percentage of placebo (range) | 100 | 93 (82–104) | |
| AUC(0–∞) (mg l−1 h) | 31.3 ± 7.5 | 29.5 ± 6.9† | −1.8 (−3.3, −0.33) |
| Percentage of placebo (range) | 100 | 94 (85–106) | |
| Vd/F (l kg−1) | 0.15 ± 0.02 | 0.17 ± 0.03 | 0.02 (−0.00, 0.04) |
| Cl/F (ml h−1 kg−1) | 2.3 ± 0.5 | 2.5 ± 0.6† | 0.17 (0.01, 0.33) |
| S-Warfarin | |||
| Cmax (mg l−1) | 0.55 ± 0.16 | 0.51 ± 0.10 | −0.05 (−0.12, 0.03) |
| Percentage of placebo | 100 | 92 (74–134) | |
| tmax (h) | 1.0 (1–3) | 1.0 (1–4) | |
| t1/2 (h) | 40.0 ± 7.6 | 41.4 ± 6.9 | 1.4 (−4.0, 6.7) |
| AUC(0−168) (mg l−1 h) | 18.6 ± 4.5 | 16.4 ± 4.2* | −2.3 (−3.6, −0.94) |
| Percentage of placebo (range) | 100 | 88 (72–105) | |
| AUC(0–∞) (mg l−1 h) | 19.9 ± 5.2 | 17.6 ± 4.7* | −2.3 (−3.7, −0.78) |
| Percentage of placebo (range) | 100 | 89 (70–109) | |
| Vd/F (l kg−1) | 0.20 ± 0.02 | 0.24 ± 0.05† | 0.04 (0.01, 0.07) |
| Cl/F (ml h−1 kg−1) | 3.6 ± 0.7 | 4.1 ± 0.9† | 0.50 (0.09, 0.91) |
Data are mean values (± SD); tmax data are given as median and range.
Cmax, Peak plasma concentration; tmax, time to reach Cmax; t1/2, elimination half-life; AUC(0–168), area under the plasma concentration-time curve from 0 to 168 h; AUC(0-∞), area under the plasma concentration-time curve from 0 to infinity; Vd/F, apparent volume of distribution; Cl/F, apparent oral clearance.
P < 0.01 vs. placebo phase.
P < 0.05 vs. placebo phase.
Figure 2.
Individual area under the concentration-time curve (AUC(0–∞)) values of R- and S-warfarin in 10 subjects after ingestion of 10 mg racemic warfarin during the placebo and gemfibrozil phases. Cross-over treatment sequences: placebo-gemfibrozil (open symbols), gemfibrozil-placebo (solid symbols)
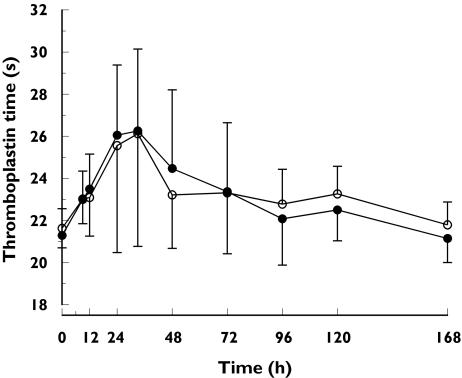
The median time to reach the Emax of warfarin was 33 h during both phases. Gemfibrozil did not alter the anticoagulant effect of warfarin measured as the AUE(0−168) or the Emax of plasma thromboplastin time (Figure 3, Table 2).
Figure 3.
Plasma thromboplastin time profiles (mean ± SD) in 10 healthy subjects. A single oral dose of 10 mg racemic warfarin was ingested on day 3 of pretreatment with gemfibrozil 600 mg or placebo twice daily for 8 days. PT during placebo phase (○), PT during gemfibrozil phase (•)
Table 2.
The effect of gemfibrozil on the pharmacodynamics of warfarin in 10 healthy subjects. A single oral dose of 10 mg racemic warfarin was ingested on day 3 of pretreatment with gemfibrozil 600 mg or placebo twice daily for 8 days
| Variable | Placebo phase | Gemfibrozil phase | Mean difference between placeboand gemfibrozil phase (95% CI) |
|---|---|---|---|
| PT baseline (s) | 21.6 ± 0.9 | 21.3 ± 0.6 | −0.34 (−0.94, 0.27) |
| AUE(0−168) (s · h) | 3941 ± 382 | 3884 ± 349 | −57.2 (−128, 13.8) |
| Emax (s) | 26.5 ± 5.3 | 26.6 ± 3.9 | 0.08 (−1.14, 1.31) |
Data are mean values (±SD).
PT, Thromboplastin time; AUE(0−168), area under the effect-time curve from 0 to 168 h; Emax, maximum value of PT.
Discussion
In the present study, gemfibrozil did not have a major effect on the plasma concentrations of warfarin enantiomers nor did it alter the anticoagulant action of the drug. Unexpectedly, plasma concentrations of both warfarin isomers were slightly lower during treatment with gemfibrozil than during the placebo phase.
Warfarin was administered as a single dose for safety reasons, but gemfibrozil was given at the usual therapeutic dose for 8 consecutive days in order to achieve and maintain clinically relevant plasma concentrations. Two case reports indicate that this 1200 mg daily dose of gemfibrozil is associated with increased anticoagulation with warfarin [8, 9]. In our previous studies, it was found that a 1200 mg daily dose of gemfibrozil increased the mean AUC of cerivastatin by about 6-fold and that of repaglinide by 8-fold [4, 5]. In contrast to warfarin, cerivastatin and repaglinide undergo significant first pass metabolism, have a short half-life of about 1–3 h, and are both metabolized mainly by CYP2C8.
The metabolism of warfarin is stereo- and regioselective. The pharmacologically more active S-warfarin is biotransformed mainly by 7-hydroxylation catalysed by CYP2C9 [3]. The formation of 6-OH-warfarin from S-warfarin is also mediated by CYP2C9. These two pathways account for 75–85% of the clearance of S-warfarin [13]. Several CYP isoforms contribute to the metabolism of R-warfarin [14]. Thus, the 6-, 7-, and 8-hydroxylations are mediated by CYP1A2, CYP2C8, CYP2C18, and CYP2C19, and CYP3A4 catalyses the 10-hydroxylation of R-warfarin. In addition, both enantiomers of warfarin are reduced to alcohols by a ketoreductase.
Warfarin has a narrow therapeutic index and is susceptible to several drug–drug interactions, many of which are caused by an altered metabolic clearance of the drug [13, 15–20]. For example, metronidazole, sulfinpyrazone, amiodarone, miconazole, and fluconazole increase plasma concentrations of S-warfarin, with a smaller effect on those of R-warfarin, and augment the anticoagulant effect [13, 15–18]. A likely mechanism for these interactions is inhibition of the CYP2C9-mediated metabolism of S-warfarin.
Concomitant administration of gemfibrozil with a number of the statins, e.g. cerivastatin, lovastatin, simvastatin, pravastatin, and rosuvastatin, has been shown to increase plasma [4, 21–24]. In addition, gemfibrozil markedly increases the plasma concentrations of some antidiabetic agents, e.g. repaglinide and rosiglitazone [5, 25]. Many of these interactions can be explained, at least partly, by inhibition of CYP2C8-mediated metabolism by gemfibrozil [4–6].
In an in vitro study, gemfibrozil inhibited the hydroxylation of tolbutamide, a marker reaction for CYP2C9, with a Ki (apparent inhibitory constant) of 5.8 µm[7]. Assuming 95% protein binding of gemfibrozil in plasma [26], the free plasma concentration of gemfibrozil would have been about 3.6 µm (0.9 mg l−1) at 2 h after warfarin with morning free trough concentrations of about 0.4 µm (0.1 mg l−1) on days 4–6. Using these free concentrations and the Ki for CYP2C9 inhibition in an in vitro–in vivo scaling model [27], gemfibrozil would be predicted to inhibit the clearance of CYP2C9 substrates by about 38% (3.6 µm) and 6.1% (0.4 µm), respectively. Thus, our results suggest that the concentrations of gemfibrozil at the CYP2C9 active site are even lower than its free concentrations in plasma, leading to no appreciable CYP2C9 inhibition in vivo. In previous studies, gemfibrozil has been shown not to alter plasma concentrations of fluvastatin, which is primarily metabolized by CYP2C9 [28], and only modestly increased the concentrations of glimepiride, also metabolized by CYP2C9 [29]. Gemfibrozil-1-O-glucuronide has been shown to inhibit CYP2C8 which may explain the interaction of gemfibrozil with substrates of CYP2C8 [4, 5, 25, 30]. The active site of CYP2C9 is much smaller than that of CYP2C8 [31, 32] and may not be able to accommodate this metabolite of gemfibrozil. Thus the difference in the active topologies may explain the diverging in vivo effects of gemfibrozil on the metabolism of substrates of CYP2C8 and CYP2C9.
Another potential explanation for our findings is that inhibition of CYP2C9 is counteracted by other mechanisms. The concentrations of both the S-isomer, which is more dependent on CYP2C9, and R-isomer were slightly decreased by gemfibrozil. This could be explained by the displacement of warfarin from albumin by gemfibrozil resulting in increased hepatic extraction and enhanced clearance of the enantiomers [19]. The increased apparent volume of distribution of the warfarin enantiomers is also consistent with this explanation. However, previous studies of the displacement of warfarin from its protein binding by gemfibrozil are contradictory, one demonstrating an effect, the other not [33, 34]. Another theoretical explanation for our findings would be decreased absorption of warfarin by gemfibrozil [20]. However, this is not a known property of gemfibrozil.
The present study did not reveal a clear pharmacokinetic mechanism for the possible interaction between gemfibrozil and warfarin [8, 9]. Increased anticoagulation and bleeding has been reported in two patients stabilized on warfarin, 2–4 weeks after addition of gemfibrozil. Gemfibrozil has been shown to increase the fibrinolytic effect of plasma and decrease concentration of clotting factor VII-phospholipid complex in plasma, whereas in other studies no effect was seen [35–37]. Furthermore, the effects of gemfibrozil on platelet activity have been discrepant [38, 39]. Thus, a pharmacodynamic interaction between gemfibrozil and warfarin cannot be excluded, although in our study gemfibrozil did not increase thromboplastin time measured before or after administration of warfarin.
In conclusion, daily administration of gemfibrozil had minor effects on the pharmacokinetics of a single dose of warfarin and did not alter its anticoagulant action. The slight decrease in plasma concentrations of R- and S-warfarin may have resulted from displacement of warfarin from plasma albumin by gemfibrozil. However, definitive conclusions about the possible influence of gemfibrozil on the anticoagulant effect during long-term use of warfarin may not be drawn on the basis of this single-dose warfarin study in healthy subjects.
Acknowledgments
We thank Mr Jouko Laitila, Mrs Eija Mäkinen-Pulli and Mrs Lisbet Partanen for skilful technical assistance.
This study was supported by grants from the Helsinki University Central Hospital Research Fund and the Sigrid Juselius Foundation, Finland.
References
- 1.Stenflo J, Suttie JW. Vitamin K-dependent formation of γ-carboxyglutamic acid. Annu Rev Biochem. 1977;46:157–72. doi: 10.1146/annurev.bi.46.070177.001105. [DOI] [PubMed] [Google Scholar]
- 2.Hignite C, Uetrecht J, Tschanz C, Azarnoff D. Kinetics of R and S warfarin enantiomers. Clin Pharmacol Ther. 1980;28:99–105. doi: 10.1038/clpt.1980.137. [DOI] [PubMed] [Google Scholar]
- 3.Rettie AE, Korzekwa KR, Kunze KL, et al. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin drug interactions. Chem Res Toxicol. 1992;5:54–9. doi: 10.1021/tx00025a009. [DOI] [PubMed] [Google Scholar]
- 4.Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–91. doi: 10.1067/mcp.2002.128469. [DOI] [PubMed] [Google Scholar]
- 5.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46:347–51. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang J-S, Neuvonen M, Wen X, Backman JT, Neuvonen PJ. Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos. 2002;30:1352–6. doi: 10.1124/dmd.30.12.1352. [DOI] [PubMed] [Google Scholar]
- 7.Wen X, Wang J-S, Backman JT, Kivistö KT, Neuvonen PJ. Gemfibrozil is a potent inhibitor of human cytochrome P450 2C9. Drug Metab Dispos. 2001;29:1359–61. [PubMed] [Google Scholar]
- 8.Ahmad S. Gemfibrozil interaction with warfarin sodium (coumadin) Chest. 1990;98:1041–2. doi: 10.1378/chest.98.4.1041b. [DOI] [PubMed] [Google Scholar]
- 9.Rindone JP, Keng HC. Gemfibrozil–warfarin drug interaction resulting in profound hypoprothrombinemia. Chest. 1998;114:641–2. doi: 10.1378/chest.114.2.641. [DOI] [PubMed] [Google Scholar]
- 10.Unge P, Svedberg LE, Nordgren A, et al. A study of the interaction of omeprazole and warfarin in anticoagulated patients. Br J Clin Pharmacol. 1992;34:509–12. doi: 10.1111/j.1365-2125.1992.tb05656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAleer SD, Chrystyn H, Foondun AS. Measurement of the (R)- and (S)-isomers of warfarin in patients undergoing anticoagulant therapy. Chirality. 1992;4:488–93. doi: 10.1002/chir.530040806. [DOI] [PubMed] [Google Scholar]
- 12.Hengy H, Kölle EU. Determination of gemfibrozil in plasma by high performance liquid chromatography. Arzneimittelforschung. 1985;35:1637–9. [PubMed] [Google Scholar]
- 13.Black DJ, Kunze KL, Wienkers LC, et al. Warfarin-fluconazole. II. A metabolically based drug interaction: in vivo studies. Drug Metab Dispos. 1996;24:422–8. [PubMed] [Google Scholar]
- 14.Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. doi: 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly RA. The stereoselective interaction of warfarin and metronidazole in man. New Eng J Med. 1976;295:354–7. doi: 10.1056/NEJM197608122950702. [DOI] [PubMed] [Google Scholar]
- 16.Toon S, Low LK, Gibaldi M, et al. The warfarin–sulfinpyrazone interaction: stereochemical considerations. Clin Pharmacol Ther. 1986;39:15–24. doi: 10.1038/clpt.1986.3. [DOI] [PubMed] [Google Scholar]
- 17.Heimark LD, Wienkers L, Kunze K, et al. The mechanism of the interaction between amiodarone and warfarin in humans. Clin Pharmacol Ther. 1992;51:398–407. doi: 10.1038/clpt.1992.39. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly RA, Goulart DA, Kunze KL, et al. Mechanisms of the stereoselective interaction between miconazole and racemic warfarin in human subjects. Clin Pharmacol Ther. 1992;51:656–67. doi: 10.1038/clpt.1992.78. [DOI] [PubMed] [Google Scholar]
- 19.Banfield C, O'Reilly R, Chan E, Rowland M. Phenylbutazone–warfarin interaction in man: further stereochemical and metabolic considerations. Br J Clin Pharmacol. 1983;16:669–75. doi: 10.1111/j.1365-2125.1983.tb02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuentzel WP, Brunk SF. Cholestyramine–warfarin interaction in man. Clin Res. 1970;18:594. [Google Scholar]
- 21.Kyrklund C, Backman JT, Kivistö KT, Neuvonen M, Laitila J, Neuvonen PJ. Plasma concentrations of active lovastatin acid are markedly increased by gemfibrozil but not by bezafibrate. Clin Pharmacol Ther. 2001;69:340–5. doi: 10.1067/mcp.2001.115542. [DOI] [PubMed] [Google Scholar]
- 22.Backman JT, Kyrklund C, Kivistö KT, Wang J-S, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–9. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 23.Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther. 2003;73:538–44. doi: 10.1016/S0009-9236(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 24.Schneck DW, Birmingham BK, Zalikowski JA, et al. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2004;75:455–63. doi: 10.1016/j.clpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Niemi M, Backman JT, Granfors M, Laitila J, Neuvonen M, Neuvonen PJ. Gemfibrozil considerably increases the plasma concentrations of rosiglitazone. Diabetologia. 2003;46:1319–23. doi: 10.1007/s00125-003-1181-x. [DOI] [PubMed] [Google Scholar]
- 26.Dollery C, editor. Theraputic Drugs. 2. Edinburgh, UK: Churchill Livingstone; 1999. pp. G34–G37. [Google Scholar]
- 27.von Moltke LL, Greenblatt DJ, Schmider J, Wright CE, Harmatz JS, Shader RI. In vitro approaches to predicting drug interactions in vivo. Biochem Pharmacol. 1998;55:113–22. doi: 10.1016/s0006-2952(97)00239-6. [DOI] [PubMed] [Google Scholar]
- 28.Spence JD, Munoz CE, Hendricks L, Latchinian L, Khouri HE. Pharmacokinetics of the combination of fluvastatin and gemfibrozil. Am J Cardiol. 1995;76:80A–83A. doi: 10.1016/s0002-9149(05)80024-4. [DOI] [PubMed] [Google Scholar]
- 29.Niemi M, Neuvonen PJ, Kivistö KT. Effect of gemfibrozil on the pharmacokinetics and pharmacodynamics of glimepiride. Clin Pharmacol Ther. 2001;70:439–45. doi: 10.1067/mcp.2001.119723. [DOI] [PubMed] [Google Scholar]
- 30.Shitara Y, Hirano M, Sato H, Sugiyama Y. Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/OATP1B1: SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin – Analysis of the mechanism of the clinically relevant drug–drug interaction between cerivastatin and gemfibrozil. J Pharmacol Exp Ther. 2004;311:228–36. doi: 10.1124/jpet.104.068536. [DOI] [PubMed] [Google Scholar]
- 31.Williams PA, Cosme J, Ward A, Angove HC, Matak Vinkovi D, Jhoti H. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature. 2003;424:464–8. doi: 10.1038/nature01862. [DOI] [PubMed] [Google Scholar]
- 32.Schoch GA, Yano JK, Wester MR, Griffin KJ, Stout CD, Johnson EF. Structure of human microsomal cytochrome P450 2C8. Evidence for a peripheral fatty acid binding site. J Biol Chem. 2004;279:9497–503. doi: 10.1074/jbc.M312516200. [DOI] [PubMed] [Google Scholar]
- 33.Todd PA, Ward A. Gemfibrozil. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in dyslipidaemia. Drugs. 1988;36:314–39. doi: 10.2165/00003495-198836030-00004. [DOI] [PubMed] [Google Scholar]
- 34.Hamberger C, Barre J, Zini R, Taiclet A, Houin G, Tillement JP. In vitro binding study of gemfibrozil to human serum proteins and erythrocytes: interactions with other drugs. Int J Clin Pharmacol Res. 1986;6:441–9. [PubMed] [Google Scholar]
- 35.Andersen P, Smith P, Seljeflot I, Brataker S, Arnesen H. Effects of gemfibrozil on lipids and haemostasis after myocardial infarction. Thromb Haemost. 1990;63:174–7. [PubMed] [Google Scholar]
- 36.Bröijersén A, Hamsten A, Silveira A, et al. Gemfibrozil reduces thrombin generation in patients with combined hyperlipidaemia, without influencing plasma fibrinogen, fibrin gel structure or coagulation factor VII. Thromb Haemost. 1996;76:171–6. [PubMed] [Google Scholar]
- 37.Kockx M, de Maat MPM, Knipscheer HC, et al. Effects of gemfibrozil and ciprofibrate on plasma levels of tissue-type plasminogen activator, plasminogen activator inhibitor-1 and fibrinogen in hyperlipidaemic patients. Thromb Haemost. 1997;78:1167–72. [PubMed] [Google Scholar]
- 38.Laustiola K, Lassila R, Koskinen P, Pellinen T, Manninen V. Gemfibrozil decreases platelet reactivity in patients with hypercholesterolemia during physical stress. Clin Pharmacol Ther. 1988;43:302–7. doi: 10.1038/clpt.1988.36. [DOI] [PubMed] [Google Scholar]
- 39.Bröijersén A, Eriksson M, Angelin B, Hjemdahl P. Gemfibrozil enhances platelet activity in patients with combined hyperlipoproteinemia. Arterioscler Thromb Vasc Biol. 1995;15:121–7. doi: 10.1161/01.atv.15.1.121. [DOI] [PubMed] [Google Scholar]