During the last decade, several studies, particularly in USA and Europe [1–4] investigated the frequency, characteristics (e.g. seriousness, avoidance etc) and cost of adverse drug reactions (ADRs) leading to hospitalization. To our knowledge, no study was performed to determine the time devoted to the management of ADRs occurring in patients. Therefore, we performed a study in order to estimate the time taken by medical staff for detection of ADRs and identification of suspected drug(s) as well as the time necessary for pharmacovigilance staff to collect and validate the ADRs.
A member of our pharmacovigilance staff attended the weekly visit to the Department of Internal Medicine (University Hospital Purpan, Toulouse, South-west of France) for a period of 24 weeks (January to October 2003 excluding August). For each patient, four different ‘times’ were defined as: ‘visit time’ (h) was defined as the total duration of weekly visit, ‘drug time’ (h) as the time devoted during each visit to interrogate the patient about his drug intake and some complains possibly related to, ‘pharmacovigilance time’ as the time necessary to assess the causal relationship for all suspected ADRs and bibliographic research by pharmacovigilance staff and ‘ADR time’ as the sum of ‘drug time + pharmacovigilance time’. Time was measured using a chronometer, open as soon as the patient was asked about drug intake or ADRs.
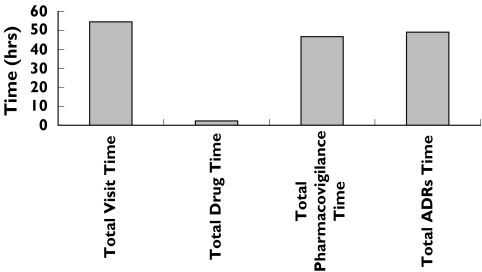
During this period, a total of 308 visits concerning 198 patients were recorded with the following values of different ‘times’: total ‘visit time’ was 54.5 h (16.5 min per patient), total ‘drug time’ was 2.3 h (i.e. 4.3% of total ‘visit time’). Among 198 patients, an ADRs was suspected in 51 patients. The total ‘pharmacovigilance time’ for these patients was 46.7 h. The causal relationship was confirmed in 26 patients about 30 ADRs. Finally, the total ‘ADR time’ was 49.0 h (i.e. 57.6 min per patient). Figure 1 shows the total duration of different times devoted to 308 visits and involving 198 patients.
Figure 1.
Repartition of different times devoted to 308 hospital visits in a department of internal medicine; for definition of the different times, see text
The incidence of ADRs was estimated to 15.2% with 19 ‘serious’ cases. In 13 cases, ADRs were the cause of hospitalization. ADRs were neurological (eight cases), metabolic (seven cases) and general (e.g. asthenia, anaphylactic reaction etc) (six cases) effects.
Our study estimates the time devoted to the management of ADRs in a hospital department of internal medicine where drug-related diseases are often discussed. Despite the relative inevitable imprecisions of time estimates, our work shows that the value of ‘drug time’ is low compared with ‘visit time’. In contrast, it is of note to underline that ‘pharmacovigilance time’ was quite similar to ‘visit time’. A similar study in ambulatory patients with general practitioners should be interesting as an increase of ‘drug time’ could improve the global management of patients and avoid the occurrence of some ADRs.
Acknowledgments
Competing interests: None declared.
References
- 1.Classen DC, Pestotnik SL, Evans RS, Lloyd LF. Adverse drug events in hospitalized patients: excess length of stay, extra-costs, and attibuable mortality. JAMA. 1997;277:301–306. [PubMed] [Google Scholar]
- 2.Goettler M, Schneeweiss S, Hasford J. Adverse drug reaction monitoring – Cost and benefit considerations. Part II. Cost and preventability of adverse drug reactions leading to hospital admission. Pharmacoepidemiol Drug Saf. 1997;6:S79–S90. doi: 10.1002/(sici)1099-1557(199710)6:3+<s79::aid-pds294>3.3.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Pouyanne P, Haramburu F, Imbs JL, Begaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French Pharmacovigilance Centres. BMJ. 2000;7241:1036. doi: 10.1136/bmj.320.7241.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apretna E, Haramburu F, Taboulet F, Begaud B. Medical and socio-economical impact of drug-induced adverse reactions. Presse Med. 2005;4:271–276. doi: 10.1016/s0755-4982(05)83904-1. [DOI] [PubMed] [Google Scholar]