Abstract
Aims
To report a pharmacokinetic interaction between valproic acid (VPA) and anticancer agents observed in an epileptic patient.
Methods
A 34-year old male epileptic patient receiving VPA underwent cisplatin-based chemotherapy for the treatment of a testicular tumour. The first chemotherapeutic cycle decreased the serum VPA concentration and caused severe generalized tonic-clonic seizures. Thus, thereafter, the serum VPA concentration was monitored along with the chemotherapy.
Results
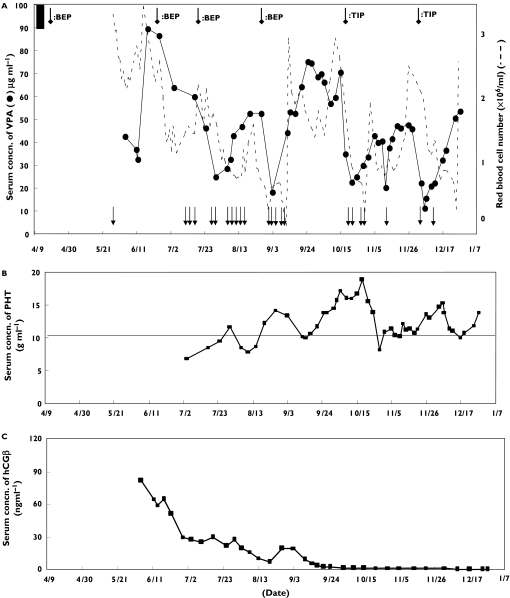
In a patient receiving VPA daily, severe seizures were observed 7 weeks after the first chemotherapeutic cycle, at which the serum VPA concentration was found to be reduced by approximately 50% of the initial level (90–100 µg ml−1). The following cycles (six cycles over a 7-month period) also caused seizures in association with decreased serum VPA concentrations. In contrast, the serum concentration of phenytoin, which was given daily after the second chemotherapeutic cycle, remained at a therapeutic concentration (10–20 µg ml−1). After the completion of chemotherapy, the serum concentration of a tumour marker, hCGβ, decreased to 1.2 ng ml−1 from more than 120 ng ml−1 prior to the chemotherapy in this patient.
Conclusions
Careful monitoring of VPA concentrations are necessary during cisplatin-based chemotherapy because anticancer agents can reduce the serum concentration and antiepileptic activity of VPA.
Keywords: anticancer agents, chemotherapy, cisplatin, epileptic patient, pharmacokinetic interaction, valproic acid
Introduction
Antiepileptic and chemotherapeutic drugs are used together in the treatment of cancer in epileptic patients [1–3]. However, antiepileptic drugs such as carbamazepine, phenobarbital, and phenytoin (PHT) interact with chemotherapeutic drugs, inducing various cytochrome P450 (CYP) enzymes, thereby inhibiting the activity of the chemotherapeutic drugs [2, 3]. Chemotherapeutic drugs also affect the pharmacokinetics of antiepileptic drugs such as PHT [4–7]. Valproic acid (VPA) has been recommended as the drug of choice in the treatment of epilepsy, particularly in association with chemotherapy [3]. However, VPA also interacts with chemotherapeutic drugs. For example, VPA has reportedly increased the toxicity of such chemotherapeutic drugs as nitrosoureas, cisplatin, and etoposide by inhibiting various CYP isoenzymes, mainly CYP2C9 [2, 3]. VPA treatment has also resulted in a three-fold higher incidence of reversible haematological side-effects when prescribed with a fotemustine-cisplatin regimen in patients with high-grade gliomas [8]. Reduced VPA activity caused by chemotherapeutic drugs has been reported, with serum (plasma) VPA concentrations decreased after chemotherapy with adriamycin and cisplatin [9] and high-dose methotrexate infusion [10].
In this article, we report a case of a severe interaction between VPA and bleomycin, etoposide, and cisplatin (BEP) or paclitaxel, ifosfamide, and cisplatin (TIP) in a patient with epilepsy and a testicular tumour.
Methods
Chemotherapy in a patient with epilepsy and testicular tumour
A 34-year old male patient (weight 53 kg; height 167 cm) with symptomatic epilepsy was admitted to hospital on April 9 2002 for the treatment of a testicular tumour. He had been treated with VPA (Selenica-R (Mitsubishi Pharma Cooperation, Osaka, Japan, 1200 mg daily, once at the bed time) and PHT (Aleviatin (Dainippon Pharmaceutical Co., Ltd, Osaka, Japan, 100 mg each, three times a day) orally. Serum concentrations of VPA and PHT in this patient had been steady at 90–100 and 10–12 µg ml−1, respectively, for about 1 year. Blood samples were taken at approximately 07.00 h in the morning (just before the administration of PHT). Epileptic seizures were well controlled with VPA and PHT. The use of PHT was terminated on April 10, because PHT is reported to induce CYP isoenzymes and thereby may reduce the effectiveness of chemotherapeutic drugs [3]. The schedule of chemotherapeutic cycles with BEP (four cycles) and TIP (two cycles) and the dosage for each chemotherapeutic drug are shown in Table 1. Informed consent was obtained from the patient for the chemotherapeutic treatment and for taking blood to monitor serum VPA concentrations. The chemotherapy was conducted in the morning throughout the treatment period, and the first cycle with BEP was started on April 12. To evaluate the efficiency of the chemotherapy, the concentration of the free β-subunit of human chorionic gonadotropin (hCGβ), a tumour marker, was monitored periodically, in addition to many other serological parameters. Blood samples (approximately 4 ml each) were taken in the morning, before the administration of drugs, for the monitoring of serological parameters. The monitoring of serum VPA and PHT concentrations was also performed as part of the clinical care, in which the plasma left over was used (the serum volume necessary for analysis was 50 µl for VPA and 50 µl for PHT).
Table 1.
Chemotherapy with BEP and TIP regimens used for the treatment of a testicular tumour in an epileptic patient
| BEP regimen | TIP regimen | |
|---|---|---|
| Drugs (treatment) | Bleomycin (30 mg day−1 on day 1) | Paclitaxel (240 mg day−1 on day 1) |
| Etopocide (124 mg day−1 on days 2–6) | Ifosfamide (1800 mg day−1 on days 2–5) | |
| Cisplatin (33 mg day−1 on days 2–6) | Cisplatin (30 mg day−1 on days 2–5) | |
| Trial number | 4 | 4 |
| Starting date | April 12, June 25. July 26, August 7 | October 17, November 30 |
Analysis
Concentrations of VPA and PHT in serum were analyzed by fluorescent polarization immunoassay using the TDX FLX system (Abbott Japan Diagnostics division, Tokyo, Japan). Concentrations of hCGβ were analyzed by immuno- enzymometric assay using a TOSOH AIA system analyzer (TOSOH Co., Tokyo, Japan). The limits of sensitivity and coefficient of variation in the analysis for VPA were 0.7 µg ml−1 and 5% and those for PHT 0.5 µg ml−1 and 5%, respectively. The detection limit of hCGβ was 0.5 ng ml−1 and the coefficient of variation was 15%.
Results
Time courses for serum VPA and PHT concentrations and red blood cell counts
The patient received the first cycle of the BEP regimen on April 12. Approximately 7 weeks later, he had severe generalized tonic-clonic seizures. He was treated with a 5 min intravenous infusion of PHT (250 mg) and diazepam (10 mg) with oxygen inhalation (2–3 l min−1 for about 10 min). During this event, serum VPA was found to be lower than it had been prior to chemotherapy (90–100 µg ml−1) (Figure 1). Subsequently, serum VPA was monitored frequently, together with such important serological parameters as the number of red blood cells (RBC) and hCGβ concentrations. The second cycle with the BEP regimen was conducted after the VPA concentrations recovered to the initial therapeutic concentration (more than 90 µg ml−1). Generalized tonic-clonic seizures were again observed several days later, though the seizures were less severe than those initially observed. Treatment with intravenous infusion of PHT (125 mg) and diazepam (5 mg) was effective against these seizures. Oral administration of PHT (100 mg each, three times daily) was resumed after the second set of seizures, because VPA alone was not deemed sufficient to prevent them. During the following two cycles of chemotherapy with the BEP regimen, tonic-clonic seizures were frequently observed in association with a marked reduction of serum VPA. RBC also decreased after each chemotherapeutic treatment. The serum concentrations of VPA and RBC recovered with time, and the time required to reach maximum reduction appeared to become shorter with each chemotherapeutic treatment. Chemotherapy was not discontinued because the concentration of hCGβ gradually decreased (Figure 1C). In addition, the patient could feel the tonic-clonic seizures coming in advance. He was treated safely each time by an intravenous infusion of PHT (125 mg) and diazepam (5 mg). After the completion of four cycles with BEP, the anticancer drugs were switched to a TIP regimen. This TIP regimen also markedly decreased serum VPA and the number of RBC, and seizures were observed each time, as with the BEP regimen. In contrast, the chemotherapy appeared not to affect the serum concentrations of PHT, because PHT concentrations remained in the therapeutic range (10–20 µg ml−1), as they were was prior to the chemotherapy (Figure 1B). VPA was deemed not to affect the chemotherapeutic drugs because hCGβ was gradually reduced to 1.2 ng ml−1 from more than 120 ng ml−1 in this patient (Figure 1C). The patient was able to leave hospital on January 15 2003.
Figure 1.
Time courses for serum concentration of valproic acid (VPA), red blood cells (RBC) and episodes of epileptic seizures (A), serum concentration of phenytoin (PHT) (B), and serum concentration of hCGβ (C) during chemotherapy in an epileptic patient. The daily dose of VPA was 1200 mg and that of PHT was 300 mg. Seizwes ( ); chemotherapy (
); chemotherapy ( ); serum concn. of VPA before chemotherapy (▪)
); serum concn. of VPA before chemotherapy (▪)
Discussion
As described in the introduction, antiepileptic drugs such as VPA and PHT are commonly used together with chemotherapeutic drugs in treating seizures in patients with brain tumours or brain metastases [2, 3]. Antiepileptic drugs are known to cause clinically relevant interactions mainly by induction or inhibition of enzymes in shared metabolic pathways. PHT, carbamazepine, primidone and phenobarbital are enzyme-inducing agents and VPA is an enzyme-inhibiting agent. When these antiepileptic drugs are prescribed with chemotherapeutic drugs, the enzyme inducers decrease and inhibitors increase serum concentrations of chemotherapeutic drugs [2,3]. Such metabolic interactions are also common between VPA and other antiepileptic drugs including PHT, carbamazepine, primidone and phenobarbital, in which the serum concentration and half-life of VPA decrease. In the present case, we encountered a severe pharmacokinetic interaction between VPA and the chemotherapeutic drugs bleomycin, etoposide, and cisplatin (BEP) or paclitaxel, ifosfamide, and cisplatin (TIP). The serum VPA was dramatically reduced by each chemotherapeutic cycle, and convulsive seizures were observed. The reduction of serum VPA was not ascribed to the administration of PHT, since the reduced VPA concentration was also observed prior to PHT administration. Also, scattered serum concentrations of VAP were observed with almost constant concentrations of serum PHT (Figure 1A). Some studies report an interaction between PHT and chemotherapeutic agents such as cisplatin, bleomycin, and vinblastin [4–8]. However, serum PHT concentrations remained almost constant in the present study at the same concentration as that prior to the initiation of chemotherapy (Figure 1B). It has also been reported that antiepileptic drugs such as PHT may reduce the activity of chemotherapeutic drugs by inducing CYP enzymes [3]. However, in this patient, the serum concentration of hCGβ was very low (1.2 ng ml−1) by the time the chemotherapy was completed (Figure 1C).
In the present clinical study, cisplatin was considered to be the main drug interacting with VPA because the pharmacokinetic interaction with VPA was observed for both BEP and TIP regimens. Very few studies have reported an interaction between VPA and cisplatin. Neef & der Straaten reported that a young woman with epilepsy receiving PHT, carbamazepine and VPA had tonic-clonic seizures during antineoplastic therapy with adriamycin and cisplatin, in which lower plasma concentrations of carbamazepine and VPA were observed after 2 days of chemotherapy [9]. In that study, PHT, which was given intravenously, was also reduced by chemotherapy. The authors suggested that the decreased plasma concentrations of carbamazepine and VPA could be due to impaired intestinal absorption, and the mechanism could be the decreased plasma PHT due to the increased metabolism and distribution volume. In the present study, the concentration of albumin in serum was almost constant, in a range from 3.8 to 4.1 g dl−1 and the protein binding of VPA, which was determined by ultrafiltration using a Microcon Centrifugal Filter Device (Microcon YM-3, Millipore Corporation), was also constant (85–92%) throughout the chemotherapy. Thus the decreased VPA concentration was not ascribed to protein binding displacement and/or to the change in its distribution volume. Also, VPA has been reported to increase the toxicity of cisplatin by inhibiting various CYP isoenzymes, mainly of CYP2C9 [2, 3]. Interestingly, the change in RBC appeared to be associated with the change in serum VPA, though there was some time lag. The correlation coefficient between serum VPA and RBC was 0.522. At this moment, however, it is not clear whether such a correlation has a significant meaning or not, and further study is needed to clarify the relationship. A severe pharmacokinetic interaction between VPA and carbapenem antibiotics is widely recognized, and the concomitant use of these drugs has been prohibited. In this interaction, serum VPA concentrations are reduced to 0–40% of the original levels in patients [11–13]. Several mechanisms for the interaction between VPA and carbapenem antibiotics have been proposed, including the suppression of enterohepatic recirculation of VPA by a decrease in the number of enteric bacteria [14], enhancement of glucuronidation of VPA as well as the suppression of VPA glucuronide hydrolysis in the liver [15–18], and direct suppression of the intestinal absorption of VPA at the basolateral membrane by carbapenem antibiotics [18, 19]. Investigations using experimental animals will be necessary to analyze the mechanism of the interaction between VPA and anticancer agents, taking the above-proposed mechanisms into consideration.
In conclusion, cisplatin-based chemotherapy decreased serum VPA concentrations and caused convulsive seizures in a patient with epilepsy. Careful monitoring of VPA is necessary when VPA is used with cisplatin-based chemotherapy.
Acknowledgments
Competing interests: None declared.
References
- 1.Villemure JG, de Tribolet N. Epilepsy in patients with central nervous system tumors. Curr Opin Neural. 1996;9:424–8. doi: 10.1097/00019052-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Vecht CJ, Wagner GL, Wilms EB. Treating seizures in patients with brain tumors: Drug interactions between antiwpileptic and chemotherapeutic agents. Semin Oncol. 2003;30:49–52. doi: 10.1053/j.seminoncol.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Vecht CJ, Wagner GL, Wilms EB. Interactions between antiepileptic and chemotherapeutic drugs. Lancet Neurol. 2003;2:404–9. doi: 10.1016/s1474-4422(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 4.Fincham RW, Schottelius DD. Decreased phenytoin levels in antineoplastic therapy. Ther Drug Monit. 1979;1:277–83. doi: 10.1097/00007691-197901020-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bollini P, Riva R, Albani F, Ida N, Cacciari L, Bollini C, Baruzzi A. Decreased phenytoin level during antineoplastic therapy: a case report. Epilepsia. 1983;24:75–8. doi: 10.1111/j.1528-1157.1983.tb04868.x. [DOI] [PubMed] [Google Scholar]
- 6.Dofferhoff AS, Berendsen HH, vd Naalt J, Haaxma-Reiche H, Smit EF, Postmus PE. Decreased phenytoin level after carboplatin treatment. Am J Med. 1990;89:247–8. doi: 10.1016/0002-9343(90)90308-z. [DOI] [PubMed] [Google Scholar]
- 7.Gattis WA, May DB. Possible interaction involving phenytoin, dexamethasone, and antineoplastic agents. a case report and review. Ann Pharmacother. 1996;30:520–6. doi: 10.1177/106002809603000516. [DOI] [PubMed] [Google Scholar]
- 8.Bourg V, Lebrun C, Chichmanian RM, Thomas P, Frenay M. Nitroso-urea-cisplatin-based chemotherapy associated with valproate: increase of haematologic toxicity. Ann Oncol. 2001;12:217–9. doi: 10.1023/a:1008331708395. [DOI] [PubMed] [Google Scholar]
- 9.Neef C, de Voogd-van der Straaten I. An interaction between cytostatic and anticonvulsant drug. Clin Pharmacol Ther. 1988;43:372–5. doi: 10.1038/clpt.1988.45. [DOI] [PubMed] [Google Scholar]
- 10.Schroder H, Ostergaard JR. Interference of high-dose methotrexate in the metabolism of valproate? Pediatr Hematol Oncol. 1994;11:445–9. doi: 10.3109/08880019409140546. [DOI] [PubMed] [Google Scholar]
- 11.Nagai K, Shimizu T, Togo A, Takeya M, Yokomizo Y, Sakata Y, Matsuishi T, Kato H. Decrease in serum levels of valproic acid during treatment with a new carbapenem, panipenem/betamipron. J Antimicrob Chemother. 1997;39:295–6. doi: 10.1093/jac/39.2.295. [DOI] [PubMed] [Google Scholar]
- 12.Yamagata T, Momoi MY, Murai K, Ikematsu K, Suwa K, Sakamoto K, Fujimura A. Panipenem-betamipron and decreases in serum valproic acid concentration. Ther Drug Monit. 1998;20:396–400. doi: 10.1097/00007691-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Llinares Tello F, Bosacoma Ros N, Hernandez Prats C, Climent Grana E, Selva Otaolaurruchi J, Ordovas Baines JP. Pharmacokinetic interaction between valproic acid and carbapenem-like antibiotics: a discussion of three cases. Farm Hosp. 2003;27:258–63. [PubMed] [Google Scholar]
- 14.Kojima S, Nadai M, Kitaichi K, Wang L, Nabeshima T, Hasegawa T. Possible mechanism by which the carbapenem antibiotic panipenem decreases the concentration of valproic acid in plasma in rats. Antimicrob Agents Chemother. 1998;42:3136–40. doi: 10.1128/aac.42.12.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamura N, Imura K, Naganuma H, Nishimura K. Panipenem, a carbapenem antibiotic, enhances the glucuronidation of intravenously administered valproic acid in rats. Drug Metab Dispos. 1999;27:724–30. [PubMed] [Google Scholar]
- 16.Yamamura N, Imura-Miyoshi K, Naganuma H. Panipenum, a carbapenem antibiotic, increases the level of hepatic UDP-glucuronic acid in rats. Drug Metab Dispos. 2000;28:1484–6. [PubMed] [Google Scholar]
- 17.Yokogawa K, Iwashita S, Kubota A, Sasaki Y, Ishizaki J, Kawahara M, Matsushita R, Kimura K, Ichimura F, Miyamoto K. Effect of meropenem on disposition kinetics of valproate and its metabolites in rabbits. Pharm Res. 2001;18:1320–6. doi: 10.1023/a:1013046229699. [DOI] [PubMed] [Google Scholar]
- 18.Torii M, Takiguchi Y, Saito F, Izumi M, Yokota M. Inhibition by carbapenem antibiotic imipenem of intestinal absorption of valproic acid in rats. J Pharm Pharmacol. 2001;53:823–9. doi: 10.1211/0022357011776171. [DOI] [PubMed] [Google Scholar]
- 19.Torii M, Takiguchi Y, Izumi M, Fukushima T, Yokota M. Carbapenem antibiotics inhibit valproic acid transport in Caco-2 cell monolayers. Int J Pharm. 2002;233:253–6. doi: 10.1016/s0378-5173(01)00916-4. [DOI] [PubMed] [Google Scholar]