Abstract
Aims
Studies of novel centrally acting drugs in healthy volunteers are traditionally concerned with kinetics and tolerability, but useful information may also be obtained from biomarkers of clinical endpoints. This paper provides a systematic overview of CNS-tests used with SSRIs in healthy subjects. A useful biomarker should meet the following requirements: a consistent response across studies and drugs; a clear response of the biomarker to a therapeutic dose; a dose–response relationship; a plausible relationship between biomarker, pharmacology and pathogenesis.
Methods
These criteria were applied to all individual tests found in studies of selective serotonin reuptake inhibitors (SSRIs), performed in healthy subjects since 1966, identified with a systematic MedLine search. Separate databases were created to evaluate the effects of single or multiple dose SSRI-studies, and for amitriptyline whenever the original report included this antidepressant as a positive control. Doses of the antidepressant were divided into high- and low-dose ranges, relative to a medium range of therapeutic doses. For each test, the drug effects were scored as statistically significant impairment/decrease (−), improvement/increase (+) or no change (=) relative to placebo.
Results
56 single dose studies and 22 multiple dose studies were identified, investigating the effects of 13 different SSRIs on 171 variants of neuropsychological tests, which could be clustered into seven neuropsychological domains. Low single doses of SSRIs generally stimulated tests of attention and memory. High doses tended to impair visual/auditory and visuomotor systems and subjective performance, while showing an acceleration in motor function. The most pronounced effects were observed using tests that measure flicker discrimination (improvement at low doses: 75%, medium doses: 40%, high doses: 43% of studies); REM sleep (inconsistent decrease after medium doses, decrease in 83% of studies after high doses); and EEG recordings, predominantly in alpha (decrease in 60% and 43% of studies after low and medium doses, respectively) and in theta activity (increase in 43% and 33% of studies after medium and high doses, respectively). Amitriptyline generally impaired central nervous system (CNS) functions, which increased with doses. Multiple doses caused less pronounced effects on the reported tests. The most responsive tests to amitriptyline appeared to be EEG alpha and theta, and REM sleep duration.
Conclusions
SSRIs in healthy subjects appear to cause slight stimulating effects after low doses, which tend to diminish with dose. The most consistent effects were observed with flicker discrimination tests, EEG (alpha and beta bands), REM sleep duration, and subjective effects at higher doses. These effects are small compared with amitriptyline and other CNS-active drugs. Multiple dosing with SSRIs caused even fewer measurable differences from placebo, probably due to adaptive processes. SSRI-effects are best detected with a test battery that is sensitive to general CNS-stimulation, but such tests only comprise a very small portion of the close to 200 different methods that were found in current review.
Keywords: Biomarker, SSRIs, Phase I
Introduction
Traditionally, Phase I studies are mainly concerned with the pharmacokinetics and tolerability of a new drug in healthy subjects. However, increasing efforts are made to include measures for efficacy as early as in Phase I studies. This may be especially useful for neuropsychiatric disorders, where Phase II studies in patients can be difficult to realize due to ethical or practical issues, such as concomitant or previous treatment, adaptation of dose, and the wide variety of types and severity of psychopathology. Studies in healthy subjects evade most of the methodological and logistical problems of patient studies. However, the effects of central nervous system (CNS)-active drugs in healthy subjects may not be comparable to the results in patients. A validated biomarker in early Phase I studies would be very useful for the development of new therapeutic psychoactive drug. Currently, no such validated biomarker exists for selective serotonin reuptake inhibitors (SSRIs) and other antidepressants, but a systematic review of studies with such drugs in healthy subjects may reveal tests that are intricately related to the drug class. In general, such a useful biomarker should meet the following criteria:
a clear, consistent response across studies (from different research groups) and drugs from the same class
a clear response of the biomarker to therapeutic doses
a dose (concentration)–response relationship
a plausible relationship between the biomarker, the pharmacology of the drug class and the pathogenesis of the therapeutic area.
Previously, these criteria were used to evaluate the usefulness of biomarkers for the effects of antipsychotic drugs [1] and benzodiazepines [2] in healthy subjects. These reviews revealed clear relationships between neuroleptics and prolactin responses, and benzodiazepine anxiolytics and saccadic peak velocity. In the current review, the effects of SSRIs in healthy subjects were evaluated using the same methodology.
Methods
Structured literature evaluation
A broad MedLine search (keywords: (SSRI or Citalopram or Fluvoxamine or Fluoxetine or Paroxetine or Sertraline or Venlafaxine or Trazodone or Nefazodon) and healthy and (subjects or volunteers)) revealed a large number of individual tests, with no apparent standardization between the studies even for the same tests. First, all studies were divided into either single dose or multiple dose (drug administration for more than 13 consecutive days to ensure that steady-state has been reached). The results of these studies for each individual test, drug and dosage were put into separate single- and multiple dose Microsoft Access® databases. Studies using a positive control mostly included amitriptyline as positive control, therefore another database was created to evaluate all amitriptyline results found in the single-dose studies' SSRI database.
Grouping of individual test results
A structured procedure described previously was adopted in order to obtain an overview [1, 2]. This method includes progressive evaluation of all the reported tests on the basis of the mentioned criteria. The purpose of this review was to identify generally applicable biomarkers of SSRI action. Results from tests which were used only once or by one research group could not be generalized, and were therefore not individually analysed. Tests that could be regarded as variants from a basic form were grouped (e.g. all tests determining the ability to discriminate flash- or flicker frequencies grouped as the test cluster ‘flicker discrimination’). Subsequently, a catalogue of psychological tests was used to group these test clusters further, according to their predominant neuropsychological domain [3]. The results of the effects on these domains were also reviewed.
Individual test results could not be recorded quantitatively, considering the large diversity of methods, parameters and treatments. Instead, the ability of a test to show a statistically significant difference from placebo or baseline was scored as +(improvement/increase), =(no significant effect) or – (impairment/decrease). Whether a difference from placebo was scored as improvement (+) or impairment (–) depended on social desirability. Although statistical significance is not only determined by the test variance but also by other factors like group size, this approach at least allowed an evaluation of the applicability of a test as a biomarker in typical early drug development studies, with limited numbers of participants. No efforts were made to further quantify the level of statistical significance at this stage.
Consistency of responses
Responses of a test can be positive (increase or improvement) or negative (decrease or impairment) or both, depending on the test. Tests where considered to respond consistently when results were significant and of the same outcome (either positive or negative), in at least 20% of the reviewed studies in a particular group. Test results were considered to be inconsistent when fewer than 20% of the tests showed statistically significant results, or when the directions of the responses were variable (i.e. more than 20% were negative and more than 20% were positive).
Dose normalization
The chance that a test will detect a difference from placebo is expected to increase with dose. To investigate this possibility, for each individual SSRI and test it was determined whether the number of statistically significant results increased with dose. In this way, only the most frequently used tests and drug dosages could be compared for dose-dependency. In all but a few cases, however, the number of tests or doses was too small to determine a relationship. To obtain an overview of dose-effects across SSRIs, drug dosages were pooled into ‘lower’, ‘medium’ and ‘higher’ dosages. For the single dose database the ‘medium’ dose was determined as the lowest recommended therapeutic starting dose (or dose range), as shown in Table 1. The ‘lower’ and ‘higher’ doses were all dosages below or above this range. For the multiple dose database, the ‘medium’ dose was determined as the lowest to highest recommended therapeutic maintenance dose range.
Table 1.
Reviewed drugs reviewed with ‘medium’ ranges
| Drug | Route | Single dose Medium low | Medium high | Multiple dose Medium low | Medium high | References |
|---|---|---|---|---|---|---|
| Amitriptyline | po | 25 | 50 | [15–18] | ||
| Citalopram | po | 20 | 20 | 20 | 60 | [19–27] |
| Citalopram | iv | 20 | 20 | |||
| Fluoxetine | po | 20 | 20 | 20 | 80 | [28–38] |
| Fluvoxamine | po | 50 | 100 | 100 | 300 | [26, 39–52] |
| Nefazodon | po | 100 | 200 | [6, 11, 53, 54] | ||
| Paroxetine | po | 10 | 20 | 20 | 50 | [6, 13, 35, 55–66] |
| Sertraline | po | 50 | 50 | 50 | 100 | [15–18,66–68] |
| Trazodone | po | 50 | 150 | [69] | ||
| Trazodone controlled release | po | 50 | 150 | [69] | ||
| Venlafaxine | po | 37.5 | 75 | 75 | 150 | [21, 59, 70–74] |
| Venlafaxine | iv | 15 | 30 | |||
| Venlafaxine extended release | po | 37.5 | 75 | [73] | ||
| Zimelidine | po | 50 | 100 | [9, 17, 75–78] |
This approach allowed the identification of tests that showed a consistent response across studies and SSRIs, and those with a clear response to a therapeutic dose of the antidepressant (requirements 1 and 2 from the introduction). All measurements fulfilling these criteria were further tested for compliance with requirements 3 and 4: the existence of a dose–response relationship and the plausibility of a mechanistic relationship, by reference to the original publications and the neuropharmacological literature. In this case, the original test-results were used if possible, rather than statistical significance and effect direction.
Measure of group size
Results were depicted graphically as average responses, ranging from −1 (all studies show impairment/decrease) through 0 (on average, studies show no change) to +1 (all studies show improvement/increase). In order to give an indication of group size in the graphical display of test and group results, error bars were constructed using confidence intervals for binomial distribution.
Tests
Neuropsychological/motor skill tests
Although many different methods are used to evaluate the functional effects of SSRIs, most actually measure a limited number of core features. Neuropsychological and motor-skills tests can be categorized according to a catalogue of neurocognitive tests (attention, executive, etc.) [1, 2]. This catalogue divides tests according to different neuropsychological domains, assuming that the results of each test are mainly (although not exclusively) determined by one of these domains (Tables 2 and 3).
Table 2.
Reported tests, clusters and domains
| Cluster | Tests |
|---|---|
| Executive | |
| Complex information processing | Car following test [30] |
| Driving test | Driving test [30, 53, 61, 71], Driving speed [53, 61], Driving simulator task [34], Highway driving [68] |
| Inhibition task | Pre-pulse inhibition of startle response [25] |
| Language | Verbal fluency [66] |
| Time estimation | Time estimation test [47], Duration discrimination [28] |
| Attention | |
| Divided attention | Divided attention task [61, 71] |
| DSST-like | DSST [21, 24, 54, 69] |
| Flicker discrimination | Visual discrimination [61], Blurred point [9], Auditory flutter fusion [28], Critical flicker fusion [9, 16, 17, 21, 28, 30, 47, 48, 54, 58, 61, 69, 71, 72] |
| Other vigilance | Continuous performance task [42], Sustained attention test [30], Mackworth clock test [74] |
| P300 | P300 potential [29], N1 latency [70], P2 latency [70], P300 latency [70] |
| Memory | |
| Delayed recall | Delayed recall [45], Free recall [54], Words delayed recall [66], Memory scanning [66] |
| Delayed recognition | Words delayed recognition [66] |
| Immediate recall | Visual working memory [54], Continuous recall [61], Verbal memory [42], Sternberg numerical memory [17, 48, 53, 58, 61, 72], Words immediate recall [66] |
| Learning | Non-sense syllable learning test [47] |
| Span tests | Digit span test [24, 54], Pauli test [17, 48, 72], Sustained Attention test [37] |
| Visual, visuomotor & auditory | |
| Hand-eye coordination | Tracking task [42, 58, 61, 71, 78], Wire-maze tracing [69], Archimedean spiral [17], Gibson spiral maze [54], Pursuit rotor [54], Trail making [21] |
| Reaction time | Sternberg numerical memory reaction time [53], Choice reaction time [16, 46], Visual reaction time [24], Sternberg additive factor method [40], Sternberg additive factor method – reaction time [2], Alphabetical reaction test [17, 72], Letter/digit differentiation reaction time [53], Choice reaction time angles [42, 78], Reaction time [17, 48, 54, 58, 72], Choice reaction time decision time [21], Viennese determination apparatus [17, 72], Viennese reaction apparatus [48], Words reaction time [61], Choice reaction time words [42, 78], Letter matching reaction time [53], Divided Attention task reaction time [61] |
| Visual acuity | Visual attention (oddball) task [34] |
| Search | Alphabetic crossout test percentage errors [17, 47, 48], Alphabetic crossout test [17, 47, 48], Digit cancellation time [54, 69], Letter/digit differentiation [53], Letter matching [53] |
| Motor | |
| Motor control | Symbol copying test [24, 54, 69], Tapping rate [24, 47, 54, 61, 69], Body sway [9, 54, 78], Motor reaction time [58], Choice reaction time movement time [21], Feinmotorik test [17, 47, 48, 72], High intensity exercise [33] |
| Subjective | |
| Scale alertness | VAS arousal [32], VAS stimulated [62], VAS concentration [61], VAS wakefulness [48], Polarity profile concentration [48], Polarity profile wakefulness [48], VAS sedation [72], VAS stimulated [62], VAS alertness [15, 19, 21, 23, 24, 28, 41, 53, 54, 58, 62, 69, 71], VAS drowsiness [28, 58, 61], VAS mental activation [53], POMS concentration [68] |
| Scale calmness | VAS restlessness [19], VAS distress [19], VAS anxiety [19, 41, 62], VAS calmness [24, 54, 69, 71], State Trait Inventory Scale [27], POMS Anxiety [68] |
| Scale craving | VAS hungry [62], VAS hunger [32, 62], VAS thirst [32], VAS Satiety [32] |
| Scale dizziness | VAS dizziness [19] |
| Scale drug effect | DEQ like drug [62], DEQ feel high [62], VAS high (drug high) [62], DEQ want more [62], DEQ feel drug [62] |
| Scale fatigue | POMS fatigue [68], VAS fatigue [61], VAS Weakness [37] |
| Scale memory | VAS memory [61] |
| Scale mood | VAS affectivity [48], VAS drive [48, 72], POMS friendliness [62], POMS vigour [62], VAS down [62], VAS well-being [19, 72], POMS depression [62], POMS confusion [62], POMS anxiety [62], POMS anger [62], VAS satiety [62], POMS elation [62], Polarity profile mood [48], Von Zerrsen befindlichkeitsscala [17, 47, 48], Semantic differential polarity profile [47], Guilt scale [42], Suspicion scale [42], Irritability scale [42], Buss-Durkee scale of assaultiveness [42], Mood scale [42], Spielberger state anxiety scale [42], VAS contendedness [24, 41, 54, 69, 71], VAS mood[19, 32, 48, 60, 70], Polarity profile extraversion [48], Cloninger's temperament and character inventory (TCI) novelty seeking [27], TCI cooperativeness [27], TCI selfdirectiveness [27], TCI reward dependence [27], TCI self-transcendence [27], TCI harm avoidance [27], Enjoyment and satisfaction questionnaire [35], Comray personality scale [35], Quality of life (SASS) [68], General well-being schedule [35], Hamilton depression scale [35], Hamilton anxiety scale [35], PANAS mood [68] |
| Scale nausea | VAS nausea [19, 32, 61] |
| Scale performance | VAS effort drive [53, 61], VAS driving quality [53, 61], VAS co-ordination [37], Perceptual motor skills [60] |
| Scale sleep | VAS tiredness [58], Leeds sleep evaluation questionnaire [30, 58], VAS sleep [60], VAS sleep duration [61], VAS sleep quality [61], VAS feeling refreshed after awaking [50], VAS difficulty opening eyes in the morning [50], VAS depth of sleep [50] |
| Scale symptoms | Side-effects [30, 77], VAS gastric discomfort [32], VAS head ache [19], Hopkins symptoms checklist [42], Side-effects questionnaire [42], VAS dryness of mouth [19, 23, 77], VAS sweating [19], VAS bodily symptoms [54], VAS tremor [19], VAS shakiness [19] |
| Neurophysiological | |
| EEG | EEG [17], EEG total power [47, 48, 72], Transcranial magnetic cortical stimulation [18] |
| EEG alpha | EEG alpha [19, 23, 77] |
| EEG beta | EEG beta [19, 23, 77] |
| EEG delta | EEG delta [19, 23, 77] |
| EEG theta | EEG theta [19, 23, 77] |
| Evoked potentials | Auditory vortex potential [54] |
| Eye movements blink | Palpebral fissure [44], Blink rate [44, 70] |
| Eye movements no-/anti-saccade | No-saccade distractibility [15], Antisaccadic velocity [15], Antisaccadic latency [15], Antisaccadic distractability [15] |
| Eye movements pursuit | Smooth pursuit position error [15], Smooth pursuit velocity error [15] |
| Eye movements saccadic | Visually guided saccade latency [15], Visually guided saccade velocity [15], Saccadic intrusions [15], Saccade error [15], Acceleration/deceleration ratio [34], Peak acceleration [34], Peak deceleration [34] |
| REM | Delta REM [6] |
| Sleep latency | Sleep latency [53] |
| Neuroendocrine | |
| Corticotrophin | Serum corticotrophin [63] |
| Catecholamine | Norepinefrine [42, 75] |
| Cortisol | Cortisol [19] |
| Growth hormone | HGH [19] |
| LH | Serum LH [63] |
| Melatonin | Nocturnal melatonin secretion [57], Melatonin [26, 31, 39] |
| Prolactin | Prolactin [19] |
| Testosterone | Serum testosterone [63] |
| Physiological | |
| Exercise | Exercise time [55, 56] |
| Blood pressure/heart rate | Blood pressure/heart rate [9, 17, 19, 42–44,47, 54] |
| Pupillometry | Pupillary unrest index [42], Power of pupil fluctuations [41], Pupil diameter [17, 32, 41, 43, 44, 48, 60, 69, 72], Pupillary light reflex [59] |
| Salivation | Salivation [9, 22, 23, 43, 44, 69, 76, 77], Saliva composition [76] |
| Skin conductance | Skin conductance [17, 48, 72] |
| Sodium | Serum sodium [52] |
| Startle reflex | Startle response [25] |
| Platelet 5-HT | Intraplatelet 5 HT concentration [65], Platelet plug formation [65], Platelet 5HT2a receptor Bmax[51], Platelet 5HT2a receptor Kd [51] |
| Cerebral blood flow | Cerebral blood flow [38] |
| Temperature | Sublingual temperature [54] |
| Visual accommodation | Accommodation range of the eye [77] |
Table 3.
Single and multiple dose studies; responsive and frequently (>5 times) performed clusters and tests
| Cluster | Test | Overall | Low dose | Medium dose | High dose | n |
|---|---|---|---|---|---|---|
| Single dose | ||||||
| Executive | ||||||
| Driving ability | +25 | 10 | ||||
| Driving test | +33 | +40 | 6 | |||
| Attention | ||||||
| Flicker discrimination | +40 | +75 | +35 | +33 | 30 | |
| Critical flicker fusion | +46 | +75 | +40 | +43 | 26 | |
| Memory | ||||||
| Immediate recall | +33 | +18 | −20 | 14 | ||
| −9 | ||||||
| Sternberg numerical memory | +23 | +50 | +29 | −25 | 13 | |
| −14 | ||||||
| Span tests | +67 | −20 | 12 | |||
| Pauli test | +25 | +100 | −33 | 8 | ||
| Visual, visuomotor & auditory | ||||||
| Reaction time | +10 | +10 | +20 | 55 | ||
| −10 | −27 | |||||
| Alphabetical reaction test | −43 | −50 | −67 | 7 | ||
| Choice reaction time | +60 | +50 | +100 | 5 | ||
| Search | −25 | +8 | −50 | 20 | ||
| −15 | ||||||
| Viennese determination apparatus | −29 | +50 | 0 | −67 | 7 | |
| Alphabetic cross-out test percentage errors | −50 | −50 | −67 | 6 | ||
| Motor | ||||||
| Motor control | +21 | +29 | 31 | |||
| −5 | −29 | |||||
| Symbol copying test | +33 | +25 | +100 | 6 | ||
| Tapping rate | +22 | +17 | +50 | 9 | ||
| Feinmotorik test | −22 | +25 | −33 | 9 | ||
| −25 | ||||||
| Subjective | ||||||
| Scale mood | +4 | 23 | ||||
| −9 | ||||||
| VAS affectivity | −43 | −50 | −67 | 7 | ||
| VAS contendedness | −25 | −33 | 8 | |||
| Von Zerssen befindlichkeits scala | −33 | −67 | 6 | |||
| Neurophysiological | ||||||
| EEG | +40 | −100 | −50 | +75 | 10 | |
| −30 | ||||||
| EEG total power | −80 | −100 | −67 | 5 | ||
| EEG alpha | EEG alpha | −40 | −60 | −43 | 15 | |
| EEG beta | EEG beta | +47 | +60 | +57 | 15 | |
| −29 | ||||||
| EEG delta | EEG delta | +20 | −20 | +29 | +33 | 15 |
| −14 | ||||||
| EEG theta | EEG theta | +27 | +43 | +33 | 15 | |
| REM | Delta REM | −69 | −60 | −83 | 16 | |
| +20 | ||||||
| Neuroendocrine | ||||||
| Melatonin | +25 | +25 | +33 | 8 | ||
| Melatonin | +29 | +33 | +33 | 7 | ||
| Physiological | ||||||
| Pupillometry | +35 | +100 | +18 | +57 | 26 | |
| −23 | −24 | −29 | ||||
| Pupil diameter | +43 | +100 | +23 | +67 | 21 | |
| −23 | −17 | |||||
| Skin conductance | Skin conductance | +50 | +100 | +66 | 8 | |
| Salivation | −36 | −33 | −50 | 13 | ||
| Multiple dose | ||||||
| Subjective | ||||||
| Scale sleep | −50 | −25 | 8 | |||
| Neurophysiological | ||||||
| Eye movements saccadic | +50 | +50 | 6 | |||
Overall: overall scores; percentage results that differ statistically significant from placebo; – decrease/impairment, + increase/improvement (unchanged results not indicated).n : total times performed.
Subjective assessments
For the subjective assessments, most individual scales corresponded to (the individual VAS lines of) the subscales ‘alertness’, ‘mood’ and ‘calmness’ proposed by Norris and applied to CNS-drug evaluation by Bond and Lader [4]. Other subjective scales could be grouped under ‘craving’, ‘dizziness’, ‘drug effect’, ‘psychomimetic’, ‘sleep’ or ‘symptoms’.
Neurophysiological assessments
Electroencephalography (EEG) and sleep analysis
EEG is sensitive to a wide range of centrally active substances, although the exact mechanism is hardly ever known. EEG-studies differ in numbers of leads, technical settings and EEG-quantification methods, but they usually report effects per EEG-frequency band, which are divided into delta (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–11.5 Hz) and beta (>11.5 Hz; subdivided into beta 1 (11.5–30Hz) and beta 2 (>30 Hz) if possible) [5]. REM sleep effects after psychoactive drugs have been reported previously [6] and are scored as total duration of REM sleep after drug administration subtracted from REM sleep duration under unmedicated conditions. Sleep latency is used as a measure for onset of sleep.
Eye movements
Smooth pursuit and saccadic eye movements have been frequently used to assess CNS drug (side)-effects [3, 7]. Saccadic eye movements provide information on the sedative properties of benzodiazepines. Although there are different techniques to measure eye movements, most studies report peak velocity for visually guided saccades or sometimes antisaccades (where subjects are instructed to look away from the target). No- and antisaccadic movements involve more complex cognitive processing than stimulus-evoked saccades and are considered as a separate cluster. Smooth pursuit eye movements are also treated separately. They are often reported as deviations from the time that the eyes closely followed the target. Eye blinks were clustered as spontaneous eye blinks or as blinks elicited by a startle response.
Neuroendocrine
Hormonal reactions to psychoactive drugs are common. Frequently assessed hormones included catecholamines, cortisol, growth hormone, melatonin and prolactin. All the reported neuroendocrine responses to SSRIs were analysed individually.
Other physiological tests
Physiological parameters are often assessed to monitor peripheral reactions and/or systemic reactions. Physiological tests were not considered relevant for the purpose of this review if the objective of the measurement was solely to monitor the subjects’ safety. Relevant (psycho) physiological tests included pupillary responses, salivation and saliva composition, and skin conductance.
Results
Single dose
The literature search yielded 56 different single dose studies using 13 different SSRIs, published since 1983. 171 different tests were used, on average three tests per study. On average 13 subjects participated in each study (ranging from six to 30 subjects). 136 tests were used fewer then five times. Only a limited number of tests (35 out of the 171, 20%) were used at least five times or more. Twenty-two of these 35 tests showed a consistent response across different SSRIs. Thirty-two out of 63 test-clusters were used at least five times. Of these 32 test-clusters, 23 did not show a consistent response and nine test-clusters showed a consistent response across different SSRIs (Table 3). Tests that were used more than five times but were nonresponsive (as defined above) are listed in Table 4. All reported tests are represented in Table 2.
Table 4.
Single dose studies; unresponsive but frequently (> 5 times) performed tests
| Test | Total times used |
|---|---|
| DSST | 8 |
| Alphabetical cross-out test | 6 |
| Archimedean spiral | 5 |
| Tracking task | 7 |
| Reaction time | 11 |
| Viennese reaction apparatus | 5 |
| VAS alertness | 20 |
| VAS anxiety | 5 |
| VAS calmness | 7 |
| VAS mood | 7 |
| Blood pressure | 16 |
| Heart rate | 16 |
Executive
Six different tests of executive function were used a total of 14 times. Most of these were related to driving-ability. Driving tests were used six times and showed an improvement in 33% of the medium SSRI doses.
Attention
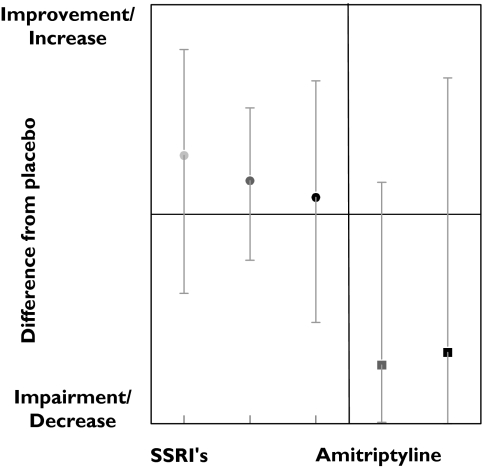
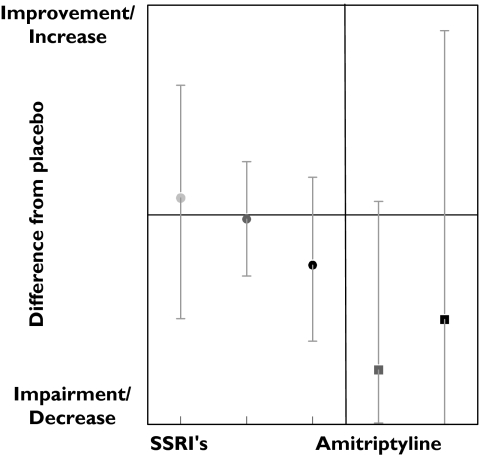
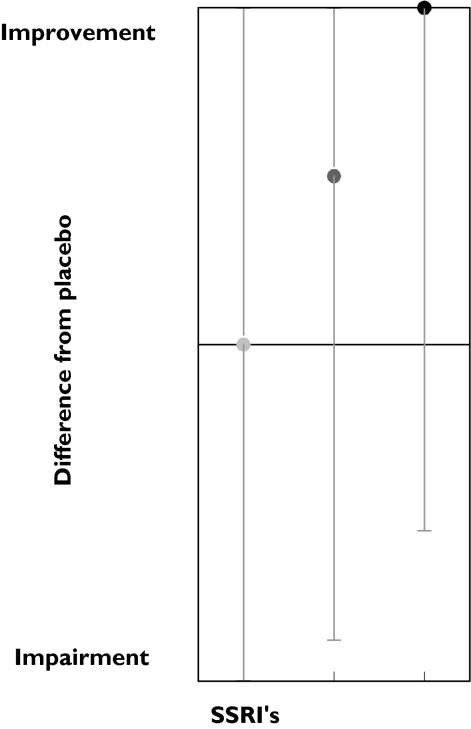
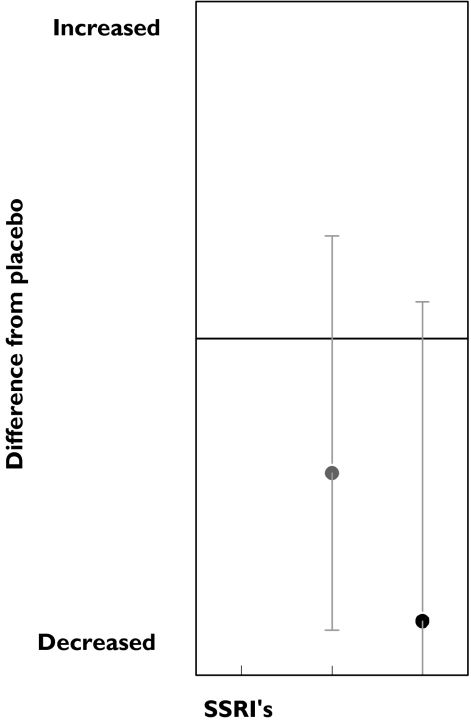
Tests measuring attention on average improved after SSRIs administration. The improvement was small but was observed in all dose ranges (Figure 1). This improvement decreased slightly with dose, suggesting an inverted dose–response relation. Amitriptyline caused strong impairment at both medium and high doses. A total of 12 tests measuring attention were performed 54 times. Most tests were measured only occasionally, except the critical flicker fusion (CFF) test, which was performed 26 times. In the CFF test, the lowest frequency of a flickering light that is perceived by the subject as a stable light is determined. CFF showed an improvement in 46% of the studies. Divided according to dose ranges, results again suggest an inverted dose related response; the low doses showed 75% improvement while the medium and high doses showed an improvement in 40% and 43% of the studies, respectively (Table 3, Figure 2). Amitriptyline administration showed an opposite response; medium and high doses caused impairment in a large percentage of CFF results.
Figure 1.
SSRI and amitriptyline effects onattention.Low dose ( ), med dose (
), med dose ( ) high dose (•), med dose (
) high dose (•), med dose ( ), high dose (▪)
), high dose (▪)
Figure 2.
SSRI and amitriptyline effects on Critical Flicker Fusion. Low dose ( ), med dose (
), med dose ( ), high dose (•), med dose (
), high dose (•), med dose ( ), high dose (▪)
), high dose (▪)
Memory
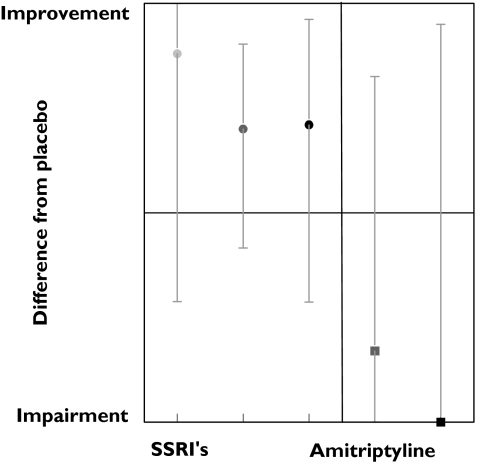
Figure 3 displays the average results for tests measuring memory. Similar to the other cognitive tests, these results suggest an inverted dose–response relation. Of the nine tests, only two responded to SSRI treatment.The Sternberg numerical memory test and the Pauli test showed an improvement in the results after SSRI administration in 23% and 25%, respectively. This improvement decreased slightly with increasing doses. In contrast, an impairment was found only in the amitriptyline study that used the Sternberg numerical memory test.
Figure 3.
SSRI and amitriptyline effects on memory. Low dose ( ), meddose (
), meddose ( ), high dose (•), med dose (
), high dose (•), med dose ( ), high dose (▪)
), high dose (▪)
Visual, visuomotor and auditory
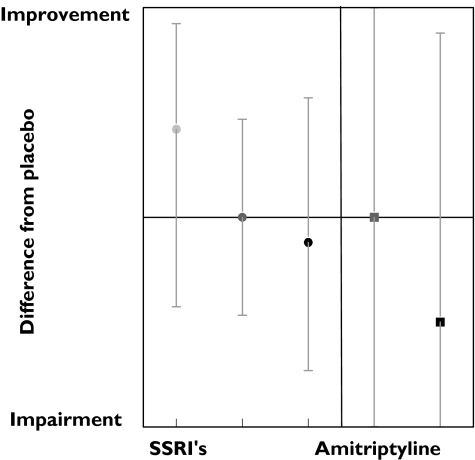
Twenty-eight tests measured visual, visuomotor or auditory responses, of which 17 measured a variation of reaction time. Overall only high doses caused some impairment, as depicted in Figure 4. Of the 17 variants of reaction time tests, only the alphabetical reaction test was consistently impaired in 43% of the studies. On average, choice reaction time also showed a dose-related impairment (Figure 5), but the individual results were often inconsistent (Table 3). Of the 11 other tests measuring search related functions, only the alphabetic cross-out test (% errors) showed a impairment in 50% of the results (Table 3).
Figure 4.
Visual, visuomotor & auditory effects of SSRI and amitriptyline. Low dose ( ), med dose (
), med dose ( ), high dose (•), med dose (
), high dose (•), med dose ( ), high dose (▪)
), high dose (▪)
Figure 5.
SSRI effects on Choice reaction time. Low dose ( ), med dose (
), med dose ( ), high dose (•)
), high dose (•)
Motor
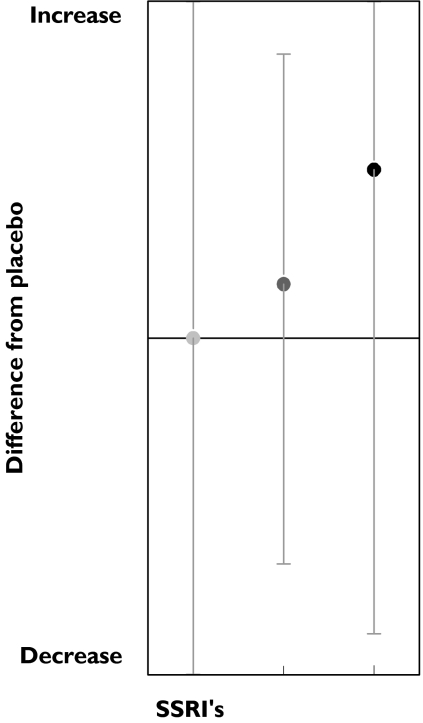
Motor function was assessed by a total of six tests, of which only three were performed more than four times. Contrary to the trends for inverted dose–response relationships observed with the cognitive tests, tests of motor speed often showed dose-related improvements. This was observed for both the symbol copying test and tapping rate (Figure 6; Table 3). In contrast, amitriptyline impaired both tests. However, the ‘Feinmotorik test’ showed a decrease in 22% of the results after SSRI administration. An inverted dose-related effect was suggested: medium doses improved 25% of the results and impaired 25%, while the high dose showed impairments in 33% of the results, but no improvements.
Figure 6.
SSRI effects on Tapping rate. Low dose ( ), med dose (
), med dose ( ), high dose (•)
), high dose (•)
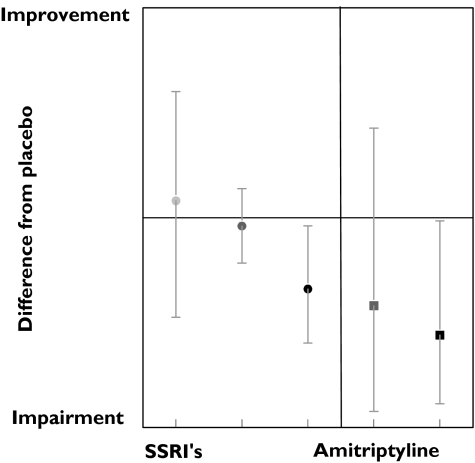
Subjective
Subjective responses to SSRI administration were reported 192 times, although only 35 showed significant changes from placebo. There were hardly any responses at low and medium dose ranges, but impairments were rather consistent with high doses (Figure 7). Although the number of studies is smaller, amitriptyline seemed to cause subjective impairments in lower dose ranges (Figure 7). Only a few mood scales responded to SSRI administration, namely VAS affectivity, VAS contentedness and ‘Von Zerssen Befindlichkeits Scala’, although only in the medium and/or high dose range. The results for the test responses are presented in Table 3.
Figure 7.
Subjective effects of SSRI and amitriptyline. Low dose ( ), med dose (
), med dose ( ), high dose (•), med dose (
), high dose (•), med dose ( ), high dose (▪)
), high dose (▪)
Neurophysiological
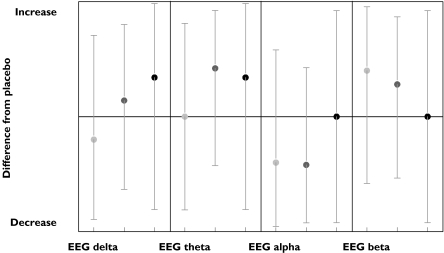
EEG total power was decreased in 80% of the results with an inverted dose–response relation (Table 3). The results for the separate EEG bands are depicted in Figure 8. Sleep effects were evaluated with sleep latency and REM measurements, but only the latter was evaluated more than four times. Delta REM (SSRI REM duration corrected for placebo) decreased markedly and suggested a dose–response relationship as depicted in Figure 9.
Figure 8.
SSRI effects on EEG. Low dose ( ), med dose (
), med dose ( ), high dose (•)
), high dose (•)
Figure 9.
SSRI effects on REM sleep. Med dose ( ), high dose (•)
), high dose (•)
Neuroendocrine
Neuroendocrine responses to SSRI administration were observed in the medium and in the high dose range (Table 3). Five different hormones were evaluated, but only 14 results were reported in total. Melatonin was the only hormone that was investigated often enough for separate evaluation and increased after SSRI administration. Medium and high SSRI doses caused an increase in 33% of the studies, low doses showed no change.
Physiological
The following physiological tests responded in more then 20% of the studies:
pupil diameter increased in all dose ranges, however, without an apparent dose–response relation;
skin conductance increased in 50% of the results. An inverted dose–response relation was suggested (both studies with low doses showed an increase; two of three medium dose studies showed increases; three high dose studies showed no change compared with placebo);
salivation decreased in 36% of the studies (medium dose; 33%, high dose; 50%).
Multiple dose
The literature search yielded 22 different multiple dose studies using seven different SSRIs, published since 1986. In the multiple dose studies reviewed, dosing was performed for more then 13 consecutive days in order to reach steady state. Ninety different tests were used, on average 4.1 tests per study. On average 14 subjects participated in each study (ranging from eight to 29 subjects). Since all doses were within the mid range no dose–response relationship could be observed. All 90 tests were performed fewer then five times and were therefore not individually analysed. Six test-clusters included at least five test results, of which four did not respond to SSRI treatment.
Memory
A total of nine tests measuring memory showed a consistent but weak response; 22% of the results were impaired at mid-therapeutic SSRI doses.
Subjective
Tests measuring subjective effects yielded 56 results. Tests measuring subjective sleep effects were grouped as ‘scale sleep’, which showed a reduction in 25% out of the eight results.
Neurophysiological
Six neurophysiological tests were grouped as ‘eye movements saccadic’ and showed an improvement in 50% of the results. Sleep effects of SSRI treatment were measured four times contrary to many sleep studies with ‘classic’ antidepressants [4]. Although performed only four times, delta REM sleep (REM duration corrected for placebo) showed an impairment in 50% of these studies.
Neuroendocrine
Eleven tests measured neuroendocrine effects. No significant responses were observed.
Discussion
Limited effects were observed after administration of SSRIs to healthy subjects. Although 171 different tests were reported in 56 studies, only 35 tests were used more than five times, allowing some general conclusions. None of these tests seemed to comply with all the stated requirements for useful biomarkers. Only five tests showed a response to SSRI administration in 50% or more of the studies. This improved somewhat, when dosages were taken into account: 11 tests showed a response in more than 50% of the studies at low (subtherapeutic) SSRI-doses. Most CNS-functions showed an inverted dose–response relationship, with slight stimulation at low single doses, and slight impairment or no effects at high doses.
Most psychological domains were slightly stimulated, especially after low single doses. These stimulating effects generally seemed to diminish with increased doses (an inverted dose–response relation). This is in contrast with the profound impairment observed after the most frequently used positive control, amitriptyline. Recent reviews also showed more pronounced effects of other psychoactive agents on the CNS [1, 2].
To evaluate the tests that were performed infrequently, we grouped tests in clusters. Any manipulation of data might obscure information: tests that in fact represent the ‘ideal’ biomarker could be masked by other nonresponsive tests in the same group. However, rejection of these tests on the basis of limited experience would have ignored possibly valuable information. Only EEG alpha and theta frequencies, REM sleep duration and the critical flicker fusion test, and subjective effects at high doses, showed reasonably consistent responses across different studies and drugs.
Several factors contributed to the lack of profound effects in healthy subjects after SSRI administration. First, the effects of single doses of SSRIs in clinically relevant doses are probably limited in healthy subjects. The clinical effects of SSRIs are usually only noticeable after several weeks of treatment. This suggests that the administration of SSRI triggers adaptive mechanisms that are responsible for the therapeutic effect, as suggested previously by Cowen et al. [8]. Similar phenomena could account for the absence of neuroendocrine responses in multiple dose studies, and the diminution of the slight stimulatory effects, observed in most single dose studies. Multiple dose studies in healthy subjects, although much more relevant to clinical practice, face considerable ethical and practical limitations. These limitations probably account for the small number of multiple dose studies observed in this review – which in turn impair firm conclusions about potentially useful biomarkers for multiple dose effects.
Second, SSRIs seem to cause slight stimulating effects, while most tests are more sensitive to CNS-impairment – at least in healthy subjects. The observed stimulating effects of low doses of SSRIs are compatible with acute serotonergic reuptake inhibition. Higher SSRI doses also activate other pharmacological activities, notably noradrenergic effects that are generally associated with sedation [9]. This might explain the disappearance of cognitive stimulation that seemed to occur with increasing SSRI doses. Therefore, if a new SSRI causes slight stimulant effects across a range of CNS-domains in healthy subjects, this may indicate that a low therapeutic dose level has been reached. CNS-depression may be an indication for side-effects.
Although the numbers of studies and individual tests are small, most cognitive and subjective tests and clusters showed inverted dose–response relationships. Motor speed, however, showed a positive dose–response relation: increasing doses resulted in improved speed. This applied to tests exclusively measuring motor speed, since the Feinmotorik test results showed an inverted dose–response relation. There is no clear explanation for this apparent discrepancy, which could be spurious in view of the large variability and small changes caused by SSRIs.
EEG profiles after SSRI administration differed markedly from profiles induced by amitriptyline administration. Low doses of SSRIs had virtually no effect on low frequency bands of the EEG (delta and theta), and caused small decreases in alpha- and increases in beta-frequencies (both in 60% of studies). These findings are compatible with CNS-stimulation. At high doses, the power of the low frequencies increased, and the alpha and beta changes disappeared. Similar findings have been reported previously by Saletu et al. [10].
Amitriptyline was not used often enough in the studies included in this review, to allow conclusions about EEG-effect. Amitriptyline typically causes increases in delta-, theta- and beta 2-frequencies, and decreases in alpha and beta 1-frequencies; a profile compatible with drug-induced sedation also reported by Saletu et al. [14].
The stimulating effects of SSRIs are also shown by the results of the CFF test. The results improved with low, medium and high SSRI doses (in 75%, 40% and 43% of the studies, respectively). These results indicate that the CFF test is one of the more useful methods to be employed in single-dose SSRI-studies in healthy subjects. The CFF test is primarily a test of attention, although it can be affected by other modalities like pupil size and retinal function. In part therefore, the CFF-results may be due to pupillary dilation, which was observed in the majority of studies with all SSRI-doses. Other CNS-domains that respond fairly consistently to single SSRI-doses are memory, visual, visuomotor, auditory, motor and subjective.
REM sleep duration was markedly decreased in single dose, as well as in multiple dose studies. This suggests that the shortening of REM sleep correlates with SSRI action and the therapeutic effects. However, REM sleep measurements have only been reported four times in the multiple dose review, which limits the conclusions. The subjective experience of sleep was impaired, which could be related to the slight stimulant effects at low and medium SSRI-doses, and which agrees with the reported REM sleep phase reduction reported for antidepressants [6, 7, 11–14].
The value of CNS-testing in early Phase I studies with SSRIs could be questioned, considering the limited effects of SSRIs in healthy subjects. For any new CNS-active agent, tests of the central nervous system can show inadvertent dose-related effects, which is essential information for the further development of the drug. In the current review, none of the methods were specifically related to the therapeutic range of the SSRIs. However, many tests seemed to show small stimulant effects at low therapeutic doses. This often did not reach statistical significance, which could be due to the limited sensitivity of most methods for CNS-stimulation. A battery of tests that are sensitive to CNS-stimulation in several domains, particularly attention (critical flicker fusion), visuomotor coordination and memory, seems to have the highest chance of picking up the effect of low doses of a novel SSRI. The EEG may show a reduction in the alpha-band and a slight increase in the beta-band, but this is also seen with other CNS-active agents like benzodiazepines [2].
In summary, the effects of SSRIs in healthy subjects are limited, compared with a tricyclic antidepressant like amitriptyline and other CNS-active agents [1, 2]. No test seems to be specifically related to the mechanism of action of SSRIs. There are indications for a slight stimulant effect at low therapeutic doses. These are best detected with a test battery that is sensitive to general CNS-stimulation, but such tests only comprise a very small portion of the almost 200 different methods that were found in current review.
Acknowledgments
This review was produced on behalf of the Biomarker Working Group of the German Association for Applied Human Pharmacology (Arbeitsgemeinschaft für angewandte Humanpharmakologie; AGAH).
References
- 1.de Visser SJ, van der Post J, Pieters MSM, Cohen AF, van Gerven JMA. Biomarkers for the effects of antipsychotic drugs in healthy volunteers. Br J Clin Pharmacol. 2001;51:119–32. doi: 10.1111/j.1365-2125.2001.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Visser SJ, van der Post JP, de Waal PP, Cornet F, Cohen AF, van Gerven JMA. Biomarkers for the effects of benzodiazepines in healthy volunteers. Br J Clin Pharmacol. 2003;55:39–50. doi: 10.1046/j.1365-2125.2002.t01-10-01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spreen O, Strauss E. A compendium of neuropsychological tests; Administration, norms, and commentary. Second Edition. New York: Oxford University Press, Inc.; 1998. [Google Scholar]
- 4.Bond AJ. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–18. [Google Scholar]
- 5.Herrmann WM. International Pharmaco EEG Group (IPEG). Recommendations for EE G and evoked potential mapping. Neuropsychobiol. 1989;22:170–6. doi: 10.1159/000118612. [DOI] [PubMed] [Google Scholar]
- 6.Rijnbeek B, de Visser SJ, Franson KL, Cohen AF, van Gerven JMA. REM sleep reduction as a biomarker for the effects of antidepressants in healthy volunteers. J Psychopharmacol. 2003;17(2):196–203. doi: 10.1177/0269881103017002008. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa Y, Kotorii M, Kotorii T, Ohshima M, Hasuzawa H. Individual variations in response of human REM sleep to amitriptyline and haloperidol. Electroencephalogr Clin Neurophysiol. 1977;42:769–75. doi: 10.1016/0013-4694(77)90230-9. [DOI] [PubMed] [Google Scholar]
- 8.Cowen PJ, Sargent PA. Changes in plasma prolactin during SSRI treatment: evidence for a delayed increase in 5-HT neurotransmission. J Psychopharmacol. 1997;11:345–8. doi: 10.1177/026988119701100410. [DOI] [PubMed] [Google Scholar]
- 9.Ogura C, Kishimoto A, Mizukawa R, Matsubayashi M, Omura F, Kunimoto N. Comparative study of the effects of 9 antidepressants on several physiological parameters in healthy volunteers. Neuropsychobiology. 1987;17:139–44. doi: 10.1159/000118354. [DOI] [PubMed] [Google Scholar]
- 10.Saletu B, Frey R, Krupka M, Anderer P, Grunberger J, See WR. Sleep laboratory studies on the single-dose effects of serotonin reuptake inhibitors paroxetine and fluoxetine on human sleep and awakening qualities. Sleep. 1991;14:439–47. doi: 10.1093/sleep/14.5.439. [DOI] [PubMed] [Google Scholar]
- 11.Sharpley AL, Williamson DJ, Attenburrow ME, Pearson G, Sargent P, Cowen PJ. The effects of paroxetine and nefazodone on sleep: a placebo controlled trial. Psychopharmacology (Berl) 1996;126:50–4. doi: 10.1007/BF02246410. [DOI] [PubMed] [Google Scholar]
- 12.Riemann D, Velthaus S, Laubenthal S, Muller WE, Berger M. REM-suppressing effects of amitriptyline and amitriptyline-N-oxide after acute medication in healthy volunteers: results of two uncontrolled pilot trials. Pharmacopsychiatry. 1990;23:253–8. doi: 10.1055/s-2007-1014515. [DOI] [PubMed] [Google Scholar]
- 13.Schlosser R, Roschke J, Rossbach W, Benkert O. Conventional and spectral power analysis of all-night sleep EEG after subchronic treatment with paroxetine in healthy male volunteers. Eur Neuropsychopharmacol. 1998;8:273–8. doi: 10.1016/s0924-977x(97)00080-1. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson AN, Pascoe PA. 5-Hydroxytryptamine and noradrenaline uptake inhibition: studies on sleep in man. Neuropharmacology. 1986;25:1079–83. doi: 10.1016/0028-3908(86)90153-x. [DOI] [PubMed] [Google Scholar]
- 15.Green JF, King DJ, Trimble KM. Antisaccade and smooth pursuit eye movements in healthy subjects receiving sertraline and lorazepam. J Psychopharmacol. 2000;14:30–6. doi: 10.1177/026988110001400103. [DOI] [PubMed] [Google Scholar]
- 16.Hindmarch I, Bhatti JZ. Psychopharmacological effects of sertraline in normal, healthy volunteers. Eur J Clin Pharmacol. 1988;35:221–23. doi: 10.1007/BF00609258. [DOI] [PubMed] [Google Scholar]
- 17.Saletu B, Grunberger J, Linzmayer L. On central effects of serotonin re-uptake inhibitors: quantitative EEG and psychometric studies with sertraline and zimelidine. J Neural Transm. 1986;67:241–66. doi: 10.1007/BF01243351. [DOI] [PubMed] [Google Scholar]
- 18.Ilic TV, Korchounov A, Ziemann U. Complex modulation of human motor cortex excitability by the specific serotonin re-uptake inhibitor sertraline. Neurosci Lett. 2002;319:116–20. doi: 10.1016/s0304-3940(01)02563-0. [DOI] [PubMed] [Google Scholar]
- 19.Seifritz E, Baumann P, Muller MJ, et al. Neuroendocrine effects of a 20-mg citalopram infusion in healthy males. A placebo-controlled evaluation of citalopram as 5-HT function probe. Neuropsychopharmacology. 1996;14:253–63. doi: 10.1016/0893-133X(95)00117-V. [DOI] [PubMed] [Google Scholar]
- 20.Itil TM, Menon GN, Bozak MM, Itil KZ. CNS effects of citalopram, a new serotonin inhibitor antidepressant (a quantitative pharmaco-electroencephalography study) Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:397–409. [PubMed] [Google Scholar]
- 21.Nathan PJ, Sitaram G, Stough C, Silberstein RB, Sali A. Serotonin, noradrenaline and cognitive function: a preliminary investigation of the acute pharmacodynamic effects of a serotonin versus a serotonin and noradrenaline reuptake inhibitor. Behav Pharmacol. 2000;11:639–42. doi: 10.1097/00008877-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Clemmesen L, Jensen E, Min SK, Bolwig TG, Rafaelsen OJ. Salivation after single-doses of the new antidepressants femoxetine, mianserin and citalopram. A cross-over study. Pharmacopsychiatry. 1984;17:126–32. doi: 10.1055/s-2007-1017422. [DOI] [PubMed] [Google Scholar]
- 23.Penttila J, Syvalahti E, Hinkka S, Kuusela T, Scheinin H. The effects of amitriptyline, citalopram and reboxetine on autonomic nervous system. A randomised placebo-controlled study on healthy volunteers. Psychopharmacology (Berl) 2001;154:343–9. doi: 10.1007/s002130000664. [DOI] [PubMed] [Google Scholar]
- 24.Lader M, Melhuish A, Frcka G, Fredricson OK, Christensen V. The effects of citalopram in single and repeated doses and with alcohol on physiological and psychological measures in healthy subjects. Eur J Clin Pharmacol. 1986;31:183–90. doi: 10.1007/BF00606656. [DOI] [PubMed] [Google Scholar]
- 25.Liechti ME, Geyer MA, Hell D, Vollenweider FX. Effects of MDMA (ecstasy) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology. 2001;24:240–52. doi: 10.1016/S0893-133X(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 26.von Bahr C, Ursing C, Yasui N, Tybring G, Bertilsson L, Rojdmark S. Fluvoxamine but not citalopram increases serum melatonin in healthy subjects – an indication that cytochrome P450 CYP1A2 and CYP2C19 hydroxylate melatonin. Eur J Clin Pharmacol. 2000;56:123–27. doi: 10.1007/s002280050729. [DOI] [PubMed] [Google Scholar]
- 27.Tse WS, Bond AJ. Serotonergic involvement in the psychosocial dimension of personality. J Psychopharmacol. 2001;15:195–8. doi: 10.1177/026988110101500313. [DOI] [PubMed] [Google Scholar]
- 28.Rammsayer T. Dopaminergic and serotoninergic influence on duration discrimination and vigilance. Pharmacopsychiatry. 1989;22(Suppl 1):39–43. doi: 10.1055/s-2007-1014623. [DOI] [PubMed] [Google Scholar]
- 29.d'Ardhuy XL, Boeijinga PH, Renault B, et al. Effects of serotonin-selective and classical antidepressants on the auditory P300 cognitive potential. Neuropsychobiology. 1999;40:207–13. doi: 10.1159/000026621. [DOI] [PubMed] [Google Scholar]
- 30.Ramaekers JG, Muntjewerff ND, O'Hanlon JF. A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. Br J Clin Pharmacol. 1995;39:397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteleone P, Orazzo C, Natale M, Maj M. Lack of effect of short-term fluoxetine administration on nighttime plasma melatonin levels in healthy subjects. Biol Psychiatry. 1994;35:139–42. doi: 10.1016/0006-3223(94)91205-x. [DOI] [PubMed] [Google Scholar]
- 32.McGuirk J, Silverstone T. The effect of the 5-HT re-uptake inhibitor fluoxetine on food intake and body weight in healthy male subjects. Int J Obes. 1990;14:361–72. [PubMed] [Google Scholar]
- 33.Parise G, Bosman MJ, Boecker DR, Barry MJ, Tarnopolsky MA. Selective serotonin reuptake inhibitors: Their effect on high-intensity exercise performance. Arch Phys Med Rehabil. 2001;82:867–71. doi: 10.1053/apmr.2001.23275. [DOI] [PubMed] [Google Scholar]
- 34.Wilson SJ. effects of 5 weeks of administration of fluoxetine and dotheipin in normal volumteers on sleep, daytime sedation, psychomotor performance and mood. J Psychopharmacol. 2002;16:321. doi: 10.1177/026988110201600406. j. psychopharmacology. [DOI] [PubMed] [Google Scholar]
- 35.Gelfin Y, Gorfine M, Lerer B. Effect of clinical doses of fluoxetine on psychological variables in healthy volunteers. Am J Psychiatry. 1998;155:290–2. doi: 10.1176/ajp.155.2.290. [DOI] [PubMed] [Google Scholar]
- 36.McGuirk J, Silverstone T. The effect of the 5-HT re-uptake inhibitor fluoxetine on food intake and body weight in healthy male subjects. Int J Obes. 1990;14:361–72. [PubMed] [Google Scholar]
- 37.Ramaekers JG, Muntjewerff ND, O'Hanlon JF. A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. Br J Clin Pharmacol. 1995;39:397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonne O, Krausz Y, Aharon Y, Gelfin Y, Chisin R, Lerer B. Clinical doses of fluoxetine and cerebral blood flow in healthy volunteers. Psychopharmacology (Berl) 1999;143:24–8. doi: 10.1007/s002130050915. [DOI] [PubMed] [Google Scholar]
- 39.Demisch K, Demisch L, Nickelsen T, Rieth R. The influence of acute and subchronic administration of various antidepressants on early morning melatonin plasma levels in healthy subjects: increases following fluvoxamine. J Neural Transm. 1987;68:257–70. doi: 10.1007/BF02098502. [DOI] [PubMed] [Google Scholar]
- 40.Hasbroucq T, Rihet P, Blin O, Possamai CA. Serotonin and human information processing: fluvoxamine can improve reaction time performance. Neurosci Lett. 1997;229:204–8. doi: 10.1016/s0304-3940(97)00451-5. [DOI] [PubMed] [Google Scholar]
- 41.Phillips MA, Bitsios P, Szabadi E, Bradshaw CM. Comparison of the antidepressants reboxetine, fluvoxamine and amitriptyline upon spontaneous pupillary fluctuations in healthy human volunteers. Psychopharmacology (Berl) 2000;149:72–6. doi: 10.1007/s002139900334. [DOI] [PubMed] [Google Scholar]
- 42.Linnoila M, Stapleton JM, George DT, Lane E, Eckardt MJ. Effects of fluvoxamine, alone and in combination with ethanol, on psychomotor and cognitive performance and on autonomic nervous system reactivity in healthy volunteers. J Clin Psychopharmacol. 1993;13:175–80. [PubMed] [Google Scholar]
- 43.Flett SR, Szabadi E, Bradshaw CM. A comparison of the effects of fluvoxamine and amitriptyline on autonomic functions in healthy volunteers. Eur J Clin Pharmacol. 1992;42:529–33. doi: 10.1007/BF00314863. [DOI] [PubMed] [Google Scholar]
- 44.Wilson WH, Higano H, Papadatos Y, Kelwala S, Ban TA. A double-blind placebo-controlled study to compare the autonomic effects of fluvoxamine with those of amitriptyline and doxepin in healthy volunteers. Br J Clin Pharmacol. 1983;15(Suppl 3):385S–392S. doi: 10.1111/j.1365-2125.1983.tb02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curran HV, Shine P, Lader M. Effects of repeated doses of fluvoxamine, mianserin and placebo on memory and measures of sedation. Psychopharmacology (Berl) 1986;89:360–3. doi: 10.1007/BF00174375. [DOI] [PubMed] [Google Scholar]
- 46.Rihet P, Hasbroucq T, Blin O, Possamai CA. Serotonin and human information processing: an electromyographic study of the effects of fluvoxamine on choice reaction time. Neurosci Lett. 1999;265:143–6. doi: 10.1016/s0304-3940(99)00231-1. [DOI] [PubMed] [Google Scholar]
- 47.Saletu B, Grunberger J, Rajna P. Pharmaco-EEG profiles of antidepressants. Pharmacodynamic studies with fluvoxamine. Br J Clin Pharmacol. 1983;15(Suppl 3):369S–383S. doi: 10.1111/j.1365-2125.1983.tb02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saletu B, Grunberger J, Anderer P, Linzmayer L, Zyhlarz G. Comparative pharmacodynamic studies with the novel serotonin uptake-enhancing tianeptine and -inhibiting fluvoxamine utilizing EEG mapping and psychometry. J Neural Transm. 1996;103:191–216. doi: 10.1007/BF01292627. [DOI] [PubMed] [Google Scholar]
- 49.Demisch K, Demisch L, Nickelsen T, Rieth R. The influence of acute and subchronic administration of various antidepressants on early morning melatonin plasma levels in healthy subjects: increases following fluvoxamine. J Neural Transm. 1987;68:257–70. doi: 10.1007/BF02098502. [DOI] [PubMed] [Google Scholar]
- 50.Ochs HR, Greenblatt DJ, Verburg-Ochs B, Labedski L. Chronic treatment with fluvoxamine, clovoxamine, and placebo: interaction with digoxin and effects on sleep and alertness. J Clin Pharmacol. 1989;29:91–5. doi: 10.1002/j.1552-4604.1989.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 51.Spigset O, Mjorndal T. Effect of fluvoxamine on platelet 5-HT2A receptors as studied by [3H]lysergic acid diethylamide ([3H]LSD) binding in healthy volunteers. Psychopharmacology (Berl) 1997;133:39–42. doi: 10.1007/s002130050368. [DOI] [PubMed] [Google Scholar]
- 52.Spigset O, Mjorndal T. The effect of fluvoxamine on serum prolactin and serum sodium concentrations: relation to platelet 5-HT2A receptor status. J Clin Psychopharmacol. 1997;17:292–7. doi: 10.1097/00004714-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 53.van Laar MW, van Willigenburg AP, Volkerts ER. Acute and subchronic effects of nefazodone and imipramine on highway driving, cognitive functions, and daytime sleepiness in healthy adult and elderly subjects. J Clin Psychopharmacol. 1995;15:30–40. doi: 10.1097/00004714-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Frewer LJ, Lader M. The effects of nefazodone, imipramine and placebo, alone and combined with alcohol, in normal subjects. Int Clin Psychopharmacol. 1993;8:13–20. doi: 10.1097/00004850-199300810-00002. [DOI] [PubMed] [Google Scholar]
- 55.Wilson WM, Maughan RJ. Evidence for a possible role of 5-hydroxytryptamine in the genesis of fatigue in man: administration of paroxetine, a 5-HT re-uptake inhibitor, reduces the capacity to perform prolonged exercise. Exp Physiol. 1992;77:921–4. doi: 10.1113/expphysiol.1992.sp003660. [DOI] [PubMed] [Google Scholar]
- 56.Parise G, Bosman MJ, Boecker DR, Barry MJ, Tarnopolsky MA. Selective serotonin reuptake inhibitors: Their effect on high-intensity exercise performance. Arch Phys Med Rehabil. 2001;82:867–71. doi: 10.1053/apmr.2001.23275. [DOI] [PubMed] [Google Scholar]
- 57.Nathan PJ, Norman TR, Burrows GD. Nocturnal plasma melatonin concentrations in healthy volunteers: effect of single doses of d-fenfluramine, paroxetine, and ipsapirone. J Pineal Res. 1996;21:55–8. doi: 10.1111/j.1600-079x.1996.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 58.Kerr JS, Fairweather DB, Mahendran R, Hindmarch I. The effects of paroxetine, alone and in combination with alcohol on psychomotor performance and cognitive function in the elderly. Int Clin Psychopharmacol. 1992;7:101–8. [PubMed] [Google Scholar]
- 59.Bitsios P, Szabadi E, Bradshaw CM. Comparison of the effects of venlafaxine, paroxetine and desipramine on the pupillary light reflex in man. Psychopharmacology (Berl) 1999;143:286–92. doi: 10.1007/s002130050949. [DOI] [PubMed] [Google Scholar]
- 60.Deijen JB, Orlebeke JF, Loriaux SM, de Vries J. The effects of paroxetine and maprotiline on mood, perceptual-motor skills, and eye movements in healthy volunteers. Acta Psychiatr Scand Suppl. 1989;350:41. [PubMed] [Google Scholar]
- 61.Robbe HW, O'Hanlon JF. Acute and subchronic effects of paroxetine 20 and 40 mg on actual driving, psychomotor performance and subjective assessments in healthy volunteers. Eur Neuropsychopharmacol. 1995;5:35–42. doi: 10.1016/0924-977x(94)00130-4. [DOI] [PubMed] [Google Scholar]
- 62.Brauer LH, Rukstalis MR, de Wit H. Acute subjective responses to paroxetine in normal volunteers. Drug Alcohol Depend. 1995;39:223–30. doi: 10.1016/0376-8716(95)01173-2. [DOI] [PubMed] [Google Scholar]
- 63.Schlosser R, Wetzel H, Dorr H, Rossbach W, Hiemke C, Benkert O. Effects of subchronic paroxetine administration on night-time endocrinological profiles in healthy male volunteers. Psychoneuroendocrinology. 2000;25:377–88. doi: 10.1016/s0306-4530(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 64.Roschke J, Kogel P, Schlosser R, et al. Analysis of sleep EEG microstructure in subchronic paroxetine treatment of healthy subjects. Psychopharmacology (Berl) 1997;132:44–9. doi: 10.1007/s002130050318. [DOI] [PubMed] [Google Scholar]
- 65.Hergovich N, Aigner M, Eichler HG, Entlicher J, Drucker C, Jilma B. Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin Pharmacol Ther. 2000;68:435–42. doi: 10.1067/mcp.2000.110456. [DOI] [PubMed] [Google Scholar]
- 66.Schmitt JA, Kruizinga MJ, Riedel WJ. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. J Psychopharmacol. 2001;15:173–9. doi: 10.1177/026988110101500304. [DOI] [PubMed] [Google Scholar]
- 67.Gordon C, Whale R, Cowen PJ. Sertraline treatment does not increase plasma prolactin levels in healthy subjects. Psychopharmacology (Berl) 1998;137:201–2. doi: 10.1007/s002130050610. [DOI] [PubMed] [Google Scholar]
- 68.Tranter R, Healy H, Cattell D, Healy D. Functional effects of agents differentially selective to noradrenergic or serotonergic systems. Psychol Med. 2002;32:517–24. doi: 10.1017/s0033291701005086. [DOI] [PubMed] [Google Scholar]
- 69.Longmore J, Banjar W, Bradshaw CM, Szabadi E. Effects of a controlled-release formulation of trazodone on psychomotor and autonomic functions in healthy volunteers: comparison with trazodone (conventional formulation), amitriptyline and placebo. Eur J Clin Pharmacol. 1988;34:97–9. doi: 10.1007/BF01061427. [DOI] [PubMed] [Google Scholar]
- 70.Semlitsch HV, Anderer P, Saletu B, Binder GA, Decker KA. Acute effects of the novel antidepressant venlafaxine on cognitive event-related potentials (P300), eye blink rate and mood in young healthy subjects. Int Clin Psychopharmacol. 1993;8:155–66. doi: 10.1097/00004850-199300830-00004. [DOI] [PubMed] [Google Scholar]
- 71.O'Hanlon JF, Robbe HW, Vermeeren A, van Leeuwen C, Danjou PE. Venlafaxine's effects on healthy volunteers' driving, psychomotor, and vigilance performance during 15-day fixed and incremental dosing regimens. J Clin Psychopharmacol. 1998;18:212–21. doi: 10.1097/00004714-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Saletu B, Grunberger J, Anderer P, Linzmayer L, Semlitsch HV, Magni G. Pharmacodynamics of venlafaxine evaluated by EEG brain mapping, psychometry and psychophysiology. Br J Clin Pharmacol. 1992;33:589–601. doi: 10.1111/j.1365-2125.1992.tb04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patat A, Troy S, Burke J, et al. Absolute bioavailability and electroencephalographic effects of conventional and extended-release formulations of venlafaxine in healthy subjects. J Clin Pharmacol. 1998;38:256–67. doi: 10.1002/j.1552-4604.1998.tb04423.x. [DOI] [PubMed] [Google Scholar]
- 74.O'Hanlon JF, Robbe HW, Vermeeren A, van Leeuwen C, Danjou PE. Venlafaxine's effects on healthy volunteers' driving, psychomotor, and vigilance performance during 15-day fixed and incremental dosing regimens. J Clin Psychopharmacol. 1998;18:212–21. doi: 10.1097/00004714-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Rudorfer MV, Scheinin M, Karoum F, Ross RJ, Potter WZ, Linnoila M. Reduction of norepinephrine turnover by serotonergic drug in man. Biol Psychiatry. 1984;19:179–193. [PubMed] [Google Scholar]
- 76.von Knorring L, Mornstad H. Saliva secretion rate and saliva composition as a model to determine the effect of antidepressant drugs on cholinergic and noradrenergic transmission. Neuropsychobiology. 1986;15:146–54. doi: 10.1159/000118258. [DOI] [PubMed] [Google Scholar]
- 77.Knorring L, Mornstad H, Forsgren L, Holmgren S. Acute effects of different antidepressant drugs on saliva secretion and accommodation range. Pharmacopsychiatry. 1986;19:106–10. doi: 10.1055/s-2007-1017165. [DOI] [PubMed] [Google Scholar]
- 78.Linnoila M, Johnson J, Dubyoski K, Buchsbaum MS, Schneinin M, Kilts C. Effects of antidepressants on skilled performance. Br J Clin Pharmacol. 1984;18(Suppl 1):109S–120S. doi: 10.1111/j.1365-2125.1984.tb02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]