The H1-receptor antagonist fexofenadine is a P-glycoprotein substrate [1]. However, the effects of genetic polymorphisms of the MDR1 (ABCB1) gene, which codes for P-glycoprotein on the disposition of fexofenadine are unclear [2, 3]. In addition, fexofenadine is a substrate of the intestinal uptake transporters organic anion transporting polypeptide 1A2 (OATP1A2, OATP-A) and 2B1 (OATP2B1, OATP-B) [1, 4]. Fruit juices such as grapefruit, orange and apple juice may decrease the oral bioavailability of fexofenadine by inhibiting the activity of OATP1A2 [5]. There seems to be no published data on whether fexofenadine is a substrate of the hepatic uptake transporter OATP1B1 (also known as OATP-C and OATP2), encoded by SLCO1B1. It has been recently demonstrated that certain single-nucleotide polymorphisms (SNP) of SLCO1B1 are associated with a decreased function of OATP1B1 in vitro. For example healthy carriers of the 521T > C allele [6] have an increased systemic exposure to pravastatin [7]. The aim of the present study was to characterize possible relationships between polymorphisms in the SLCO1B1 gene and the pharmacokinetics of fexofenadine in healthy Caucasian subjects. To this end, 20 Caucasian volunteers who had previously participated in a pharmacokinetic study with fexofenadine [3] were genotyped by TaqMan® 5′nuclease assays for the two SLCO1B1 SNPs that were significantly associated with increased pravastatin concentrations in our previous study [7], 521T > C (Val174Ala) and for the promoter SNP −11187G > A.
Of the 20 subjects, two were homozygous and eight were heterozygous for the SLCO1B1521T > C SNP, the remaining 10 subjects having the reference genotype (521TT). Two subjects were heterozygous for the SLCO1B1−11187G > A polymorphism, and the remaining subjects had the reference (−11187GG) genotype. Both heterozygotes for the −11187G > A SNP were also heterozygous for the 521T > C SNP.
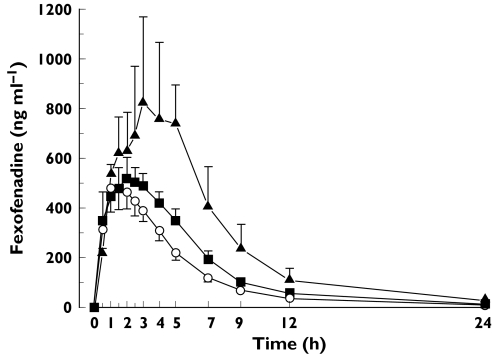
In the two subjects with the 521CC (and −11187GG) genotype, the mean total AUC of fexofenadine was 76.0% higher (P < 0.05; anova followed by the Tukey test) than in the eight subjects with the 521TC genotype and 127% higher (P < 0.01) than in the 10 subjects with the 521TT genotype (Figure 1). The SLCO1B1521T > C polymorphism had no significant influence on the Cmax or t1/2 of fexofenadine. There was a trend (P = 0.13) towards a gene-dose effect regarding the influence of the 521T > C SNP on the apparent nonrenal clearance of fexofenadine. Thus, it was lowest (389 ± 185 ml min−1) in subjects with the 521CC genotype, intermediate (775 ± 401 ml min−1) in those with the 521TC genotype and highest in those with the reference genotype (957 ± 325 ml min−1).
Figure 1.
Mean (± SEM) plasma concentrations of fexofenadine in 20 healthy subjects with different SLCO1B1 521T > C genotypes after a single 180 mg oral dose of fexofenadine HCl. SLCO1B1 521CC subjects (n = 2) (▴); SLCO1B1 521TC subjects (n = 8) (▪); SLCO1B1 521TT subjects (n = 10) (○)
A stepwise multiple regression analysis of data from subjects carrying the SLCO1B1521T > C and −11187G > A SNPs and the MDR13435C > T (exon 26), 2677G > T/A (exon 21), 1236C > T (exon 12) and 61 A > G (exon 2) SNPs indicated that only the SLCO1B1521T > C SNP was an independent predictor of the total AUC of fexofenadine (P < 0.01, r2 = 0.347). The MDR1 SNPs 2677G > T and 3435C > T were assigned into haplotype pairs according to Johne et al. [8] and data from subjects with these haplotypes were put into a stepwise multiple regression analysis with the SLCO1B1 SNP data. It was found that the MDR1 haplotypes had no independent effect on the AUC of fexofenadine (haplotype 2677T/3435C was present in one subject only).
The results of this retrospective study demonstrated that the pharmacokinetics of fexofenadine were influenced by the SLCO1B1 genotype. Fexofenadine has been previously shown to be a substrate of OATP1A2 [1] and OATP2B1 [4].
Tamai et al. reported that OATP1A2 was not expressed in a single sample of human small intestine as analyzed by RT-PCR [9]. However, more recently, expression of OATP1A2 in human intestine was demonstrated [10], a finding supported indirectly by the work of Dresser et al. [5]. These investigators showed that certain fruit juices at 5% of normal strength markedly inhibited OATP1A2 mediated fexofenadine uptake in vitro and that the same juices considerably decreased the oral bioavailability of fexofenadine in vivo. A nonsynonymous SNP associated with a markedly impaired capacity for mediating the cellular uptake of fexofenadine was recently identified within the coding region of the SLCO1A2 gene (encoding for OATP1A2) [10]. Expression of OATP2B1 has been shown immunohistochemically at the apical membrane of human intestinal epithelial cells [11]. Known functionally significant SLCO2B1 SNPs are rare in Caucasians [7], and SLCO2B1 genotypes were not determined in the present study. OATP1B1 is exclusively expressed in the liver [12, 13].
That the SLCO1B1−11187G > A and 521T > C SNPs are associated with a higher pravastatin AUC [7] may be explained by decreased hepatic uptake of the drug, resulting in its slower elimination and lower systemic clearance. The pharmacokinetics of pravastatin and fexofenadine are similar in that these drugs do not undergo significant CYP-mediated biotransformation and are eliminated from the body unchanged mainly by biliary and urinary excretion [14, 15]. The influence of the SLCO1B1521T > C polymorphism on the AUC of fexofenadine suggests that the OATP1B1-mediated uptake of the drug into the liver is a prerequisite for its biliary elimination. This hypothesis is also supported by the trend towards a lower apparent nonrenal clearance of fexofenadine in subjects with the 521CC or 521TC genotype compared with those with the reference genotype.
Studies on the effect of the 3435C > T SNP in the exon 26 of MDR1 on intestinal P-glycoprotein expression and on the disposition of P-glycoprotein substrates, including fexofenadine, have yielded conflicting results [16]. These might be partly explained by the influence of the SLCO1B1 genotype on fexofenadine disposition [2, 3].
In conclusion, we have demonstrated that the pharmacokinetics of fexofenadine are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1). Further studies are required to characterize the interaction between fexofenadine and the hepatic uptake transporter OATP1B1.
Acknowledgments
This study was supported by grants from the Robert Bosch Foundation (Stuttgart, Germany) and the Federal Ministry of Education and Research (Bonn, Germany). M. Niemi was supported by a fellowship from the Alexander von Humboldt Foundation (Bonn).
References
- 1.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–71. [PubMed] [Google Scholar]
- 2.Kim RB, Leake BF, Choo EF, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 3.Drescher S, Schaeffeler E, Hitzl M, et al. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53:526–34. doi: 10.1046/j.1365-2125.2002.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in humans. J Pharmacol Exp Ther. 2004;308:438–45. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 5.Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 6.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276:35669–75. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 7.Niemi M, Schaeffeler E, Lang T, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–40. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 8.Johne A, Kopke K, Gerloff T, et al. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther. 2002;72:584–94. doi: 10.1067/mcp.2002.129196. [DOI] [PubMed] [Google Scholar]
- 9.Tamai I, Nezu J, Uchino H, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–60. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- 10.Lee W, Smith LH, Gervasini G, Leake BF, Kim RB. Identification of nonsynonymous polymorphisms of human organic anion transporting polypeptide-A (OATP-A) associated with altered transport activity [Abstract] Clin Pharmacol Ther. 2004;75:P93. [Google Scholar]
- 11.Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–8. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- 12.Hsiang B, Zhu Y, Wang Z, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–8. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- 13.Marzolini C, Tirona RG, Kim RB. Pharmacogenomics of the OATP and OAT families. Pharmacogenomics. 2004;5:273–82. doi: 10.1517/phgs.5.3.273.29831. [DOI] [PubMed] [Google Scholar]
- 14.Simons FE, Simons KJ. Clinical pharmacology of new histamine H1 receptor antagonists. Clin Pharmacokinet. 1999;36:329–52. doi: 10.2165/00003088-199936050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39:397–412. doi: 10.2165/00003088-200039060-00002. [DOI] [PubMed] [Google Scholar]
- 16.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]