Abstract
Aim
To examine the optimal range of International Normalized Ratio (INR) for Chinese patients receiving warfarin for moderate-intensity anticoagulation.
Methods
This was a retrospective cohort study conducted at the ambulatory setting of a 1400-bed public teaching hospital in Hong Kong. The INR measurements and occurrence of serious or life-threatening haemorrhagic and thromboembolic events among patients newly started on warfarin from 1 January 1999 to 30 June 2001 for indications with target INR 2–3 were analysed. The INR-specific incidence of bleeding and thromboembolism were calculated.
Results
A total of 491 patients were included, contributing to 453 patient-years of observation period. Forty-seven of the 491 patients experienced 25 haemorrhagic events (5.5 per 100 patient-years) and 27 thromboembolic events (6.0 per 100 patient-years). The percentage of patient-time spent within therapeutic INR range (2–3), INR <2 and INR >3 were 50, 44 and 6%, respectively. The incidence of either haemorrhagic or thromboembolic events was lowest (≤4 events per 100 patient-years) at INR values between 1.8 and 2.4.
Conclusions
An INR of 1.8–2.4 appeared to be associated with the lowest incidence rate of major bleeding or thromboembolic events in a cohort of Hong Kong Chinese patients receiving warfarin therapy for moderate-intensity anticoagulation.
Keywords: Warfarin, optimal INR, Chinese patients
Introduction
Warfarin is the most commonly prescribed anticoagulant for prevention and treatment of thromboembolic events [1]. The anticoagulation effect of warfarin is subject to wide inter- and intraindividual variability that possibly leads to haemorrhagic or thromboembolic events despite careful dosage titration. The risks of thromboembolism and haemorrhage depend on the intensity of anticoagulation as measured by the International Normalized Ratio (INR) [2, 3]. Both the British Society for Haematology (BSH) and the American College of Chest Physicians (ACCP) recommend a target INR range of 2–3 (moderate-intensity anticoagulation) for most indications of warfarin, such as prophylaxis and treatment of venous thrombosis, treatment of pulmonary embolism and prevention of systemic embolism secondary to tissue heart valves, valvular heart disease or atrial fibrillation [1, 4]. The exceptions that require high-intensity anticoagulation (INR 2.5–3.5) are patients with mechanical heart-valve prostheses. Despite that both recommendations are mainly derived from clinical trials conducted in western populations, the same recommendations have been adopted for management of anticoagulation therapy in populations of different ethnic backgrounds.
Various studies reporting the association of racial background to warfarin dosage requirement report that Iranian and Asian subjects are more sensitive to warfarin than North American and European subjects [5–7]. A prospective, randomized trial conducted in Japan on patients with nonvalvular atrial fibrillation showed that low-intensity anticoagulation therapy (INR 1.5–2.1) was associated with fewer major haemorrhagic complications than the conventional-intensity treatment (INR 2.2–3.5) for secondary prevention of stroke [8]. It has been reported that Chinese patients require a lower dose of warfarin than Caucasians although the intensity of the anticoagulation is comparable [7, 9–11]. As Chinese patients have a lower warfarin requirement, there are concerns whether the same target INR range for western populations is also the optimal anticoagulation level for Chinese patients. The objective of the present study was therefore to examine the optimal INR range, in which the incidence of both major thromboembolic and bleeding events were lowest, for Chinese patients receiving warfarin for moderate-intensity anticoagulation.
Method
Study design
This was a retrospective cohort study conducted at the ambulatory setting of a 1400-bed public teaching hospital in Hong Kong. The study was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong. The INR measurements and occurrence of warfarin-related major events among the patients receiving warfarin for indications with target INR 2–3 were analysed. Outpatients who were newly receiving warfarin from 1 January 1999 to 30 June 2001 were included in the study. Data were retrieved from medical records until the patients stopped receiving warfarin therapy or 30 June 2001, whichever occurred first. Patients receiving warfarin for indications with target INR 2.5–3.5 (prosthetic heart valves) and patients receiving warfarin for less than 3 months were excluded from the present analysis. Patient demographics, dates and results of all INR assessments, occurrence of warfarin-related major complications including thromboembolic events and bleeding were collected.
The definitions of major events were adopted from Fihn et al.[2]. Major bleeding included gastrointestinal bleeding, gross haematuria, haemoptysis, bleeding leading to cardiopulmonary arrest, to surgical or angiographic intervention, to irreversible sequelae such as myocardial infarction, neurological deficit or massive haemothorax, to systolic hypotension (<90 mmHg), to critical anaemia (haematocrit ≤0.20) or to death. Major thromboembolic events were transient ischaemic attacks, stroke, recurrent deep venous thrombosis, pulmonary embolism and systemic embolism. A major event was included in the present analysis if the INR was obtained at the time of the hospital admission or a measurement of INR was performed in the clinic in less than 7 days before the event.
The percentage of patient-time spent in each INR level was estimated using linear interpolation between measured INR values developed by Rosendaal et al. as described elsewhere [12]. Briefly, the INR value between two measurements over a known period of interval was assumed to vary linearly. Patient-time between two INR measurements was divided into days and allocated equally to all INR values with increment of 0.1 INR over the range of time interval.
The incidence rates of bleeding and thromboembolic events for each specific level of INR was calculated by the following equation [12]:
Results
A total of 491 patients were included in the present study, contributing to 453 patient-years of observation period. The mean age of the patient was 65.8 ± 14.2 years and 237 (48%) patients were male. The main indications for warfarin were atrial fibrillation (72%), deep vein thrombosis (12%), pulmonary embolism (3%), cerebrovascular accident (3%) and rheumatic heart disease (3%).
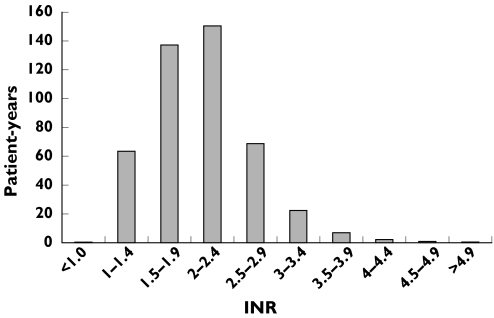
Forty-seven of the 491 patients experienced 25 haemorrhagic events (5.5 per 100 patient-years) and 27 thromboembolic events (6.0 per 100 patient-years). Of these 52 major events, INR was documented at the time of the event in 44 cases and they were used for the analysis of INR-specific incidence rates. Twenty-two of the 44 events were bleedings (5 intracranial; 17 extracranial) and others were thromboembolic events (12 cerebral infarction; 10 peripheral embolism). Five fatal events were all caused by bleeding. There was no significance difference in gender (P = 0.075) and in age (P = 0.342) between patients with major events (67.9 ± 14.6 years) and patients without major events (65.6 ± 14.2 years). The distribution of the patient-years over the INR intervals was shown in Figure 1. The percentage of patient-time spent within therapeutic INR range (2–3), INR <2 and INR >3 were 50, 44 and 6%, respectively.
Figure 1.
Distribution of patient-years among INR intervals
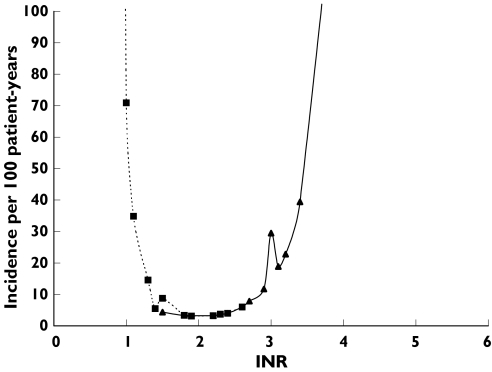
The incidence rates of bleeding and thromboembolic events at specific INR level fell in a narrow U-shaped distribution (Figure 2). The incidence rate of bleeding events increased sharply from four events to 11.7 events per 100 patient-years as INR rose from 2.4 to 2.9, and it further increased to 40 events per 100 patient-years when INR reached 3.4. When the INR fell from 1.8 to 1.5, the thromboembolic event rate increased from 3.3 to 8.7 events per 100 patient years and it reached 71 events per 100 patient-years as INR decreased to 1. In Figure 2, it was estimated that the incidence rate of either bleeding or thromboembolic events was lowest (≤four events per 100 patient-years) at INR values between 1.8 and 2.4. The incidence rate of all events (bleeding and thromboembolism) was about eight events per 100 patients-years at these INR levels. The incidence rates of events in the INR of 1.8–2.4 were significantly lower than the rates in the INR above and below this range (P = 0.037).
Figure 2.
Incidence of major events at specific INR levels. Bleeding event (▴); thromboembolic event (▪).
Discussion
The optimal therapeutic range of anticoagulation therapy varies for different indications and for patients with various characteristics. Bleeding is the major complication of warfarin therapy and it is related to the intensity of anticoagulation [2, 13–15]. In the process of searching for an optimal INR range, studies have therefore focused on establishing the lowest effective therapeutic ranges [16–20]. Randomized trials comparing different target INR ranges are recommended as the most reliable method to establish an optimal anticoagulation intensity, when comparing with other study designs such as indirect comparisons of results from randomized trials, subgroup analyses of anticoagulation group from randomized trials and case-control studies [1]. Based upon clinical evidence predominantly generated in randomized clinical trials, The BSH and the ACCP recommended that a moderate-intensity INR of 2.0–3.0 is effective for most indications [1, 4].
A number of studies have shown lower warfarin requirements in Chinese patients (3 mg day−1) compared with Caucasians (4–6 mg day−1), suggesting that Chinese patients are more sensitive than Caucasians to the anticoagulation effect of warfarin [7, 10, 11, 21–23]. Age and the target intensity of anticoagulation therapy were identified as the two most important factors affecting warfarin dose requirement [11, 23]. Yet little is known about the optimal intensity of anticoagulation, i.e. the INR range in which the incidence of both the major thromboembolic and bleeding events are lowest, in the Chinese population.
In the present study, the INR-specific incidence of major events in a cohort of Chinese patients receiving moderate-intensity anticoagulation therapy was examined. The average age of patients in the present cohort was >65 years and there was no significant difference in age and in gender between the patients with and without major events. Our results showed that the event rate was lowest at an INR of 1.8–2.4 for the present cohort. In this range, the incidence rate of either major bleeding or thromboembolism was ≤ four events per 100 patient-years. The higher mortality rate related to major bleeding events (5 of 22 events were fatal), compared with thromboembolic events (0 fatal events), in this cohort of Chinese patients suggested that the target level of anticoagulation should aim for lower INR.
Cannegieter et al. examined the INR-specific incidence rates of thromboembolism and major bleeding in 1608 Dutch patients with mechanical heart valves and determined the optimal intensity of anticoagulation to be an INR of 2.5–4.9 [24]. The incidence rates of haemorrhagic and thromboembolic events formed a wide U-shaped distribution that the incidence rate at an INR of 2.5–4.9 was only about two events per 100 patient-years. The incidence rate of all events rose sharply when the INR fell below 2.5 or rose to 5.0 or above.
A subgroup analysis of the anticoagulation cohort of the European Atrial Fibrillation Trial, a secondary prevention trial in patients with nonrheumatic atrial fibrillation, was conducted to determine the optimal intensity of oral anticoagulation [25]. The INR-specific incidence rates for occurrence of ischaemic or haemorrhagic complications in 214 patients indicated that the rate was lowest at an INR of 2.0–3.9. The incidence rate of ischaemic events increased to 18 events per 100 patient-years when the INR fell below 2.0. The bleeding incidence rate increased to over 20 events per 100 patient-years when the INR rose to 4.0 or above.
The tolerance of the present Chinese cohort to the anticoagulation effect appeared to be lower than the European patients as the incidence rate of major bleeding increased steeply when the INR rose above 2.4. The incidence rate of all events at an INR of 1.8–2.4 (about eight events per 100 patient-years) in the present cohort were almost 4-fold the rate of all events in the Dutch patients at an INR of 2.5–4.9.
One of the possible explanations for the high bleeding incidence at a moderate level of anticoagulation (INR >2.4) is that the mean age of the present cohort was >65 years and older patients are at higher risk for warfarin-related bleeding [3, 26–32]. Use of herbal medicine is another possible cause for the high sensitivity to anticoagulation effect. The use of herbal medicine was common (26%) among Hong Kong Chinese patients receiving warfarin therapy [33]. A number herbal medications were identified to have antiplatelet and/or antithrombotic effects, and therefore potentially interact with warfarin and increase risk of bleeding [34–43].
Another potential explanation is the polymorphism of cytochrome P450 (CYP) 2C9. Warfarin is a racemic mixture of two enantiomers, S- and R-warfarin, and S-warfarin is 3–5 times more potent than the R-warfarin [44]. The metabolism of S-warfarin is primarily catalysed by CYP2C9 [44, 45]. The functional significance of various mutations on the coding region of the CYP2C9 gene were examined and it has shown that the allelic variants of CYP2C9 affect metabolism clearance of warfarin [46, 47]. CYP2C9 polymorphism was associated with lower warfarin dosage requirement and with increased risk of major bleeding events during the induction phase of warfarin therapy [48–52]. Nevertheless, the frequencies of both known functionally defective CYP2C9*2 and *3 were low in the Chinese population, yet Chinese patients required a 40–50% lower maintenance dose of warfarin than Caucasian patients [23, 53]. This difference is not explainable entirely by variations in age, body weight, sex, dietary vitamin K intake, clinical indications for warfarin use and target level of anticoagulation. It implies that other functionally defective polymorphisms in the coding and noncoding regions of CYP2C9, such as the 5′-flanking region, may also have a significant effect on the phenotype of CYP2C9. Further study is therefore required to determine the association of high warfarin sensitivity in Chinese patients with CYP2C9 polymorphisms.
A limitation of the present study was the retrospective, observational study design that limited the level of evidence presented. Our findings supported further investigation of a low-intensity of anticoagulation compared with the conventional-intensity (INR 2–3) for Chinese patients in randomized clinical trials. The present study was also limited by the nature of retrospective cohort studies that data on some factors affecting the risk of bleeding, such as blood pressure control, vitamin K dietary intake, use of herbal and over-the-counter medications, and concurrent use of antiplatelets or aspirin, could not be gathered. Also, INR values were not documented for eight (15%) of the 52 major events at the time of admission or within 7 days prior to the event, consequently affecting the distribution of incidence of major events over the INR range.
Direct thrombin inhibitors and selective factor Xa inhibitors are recently developed with potential clinical and safety advantages over warfarin for the prevention and management of thromboembolic events [54–56]. Until full-scale pharmacoeconomic evaluations of these new classes of antithrombotic agents are available, warfarin remains the mainstay of drug therapy for prevention and treatment of thrombotic disorders.
In conclusion, a cohort of Hong Kong Chinese patients receiving warfarin therapy for moderate-intensity anticoagulation showed high incidence of major bleeding when INR was >2.4 and an INR of 1.8–2.4 appeared to be associated with the lowest incidence rate (≤ four events per 100 patient-years) of major bleeding or thromboembolic events. The effectiveness and safety of a low-intensity of anticoagulation for Chinese patients should be further examined in randomized clinical trials.
Acknowledgments
Competing interests: None declared.
References
- 1.Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:204S–233S. doi: 10.1378/chest.126.3_suppl.204S. [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation: a multicenter study. Ann Intern Med. 1993;118:511–20. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Van der Meer FJM, Rosendaal FR, Vandenbroucke JP, Briet E. Bleeding complications in oral anticoagulant therapy. Arch Intern Med. 1993;153:1557–62. doi: 10.1001/archinte.153.13.1557. [DOI] [PubMed] [Google Scholar]
- 4.Haemostasis and Thrombosis Task Force of the British Society for Haematology. Guidelines on oral anticoagulation. Br J Haematol. (3rd edn) 1998;101:374–87. [Google Scholar]
- 5.Andalibi P, Farsam H, Amanlou M, Gharouni M. Determination of dosage requirements of warfarin in Iranian patients using HPLC technique. J Clin Pharm Ther. 1998;23:199–202. doi: 10.1046/j.1365-2710.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 6.Blann A, Hewitt J, Siddiqui F, Bareford D. Racial background is a determinant of average warfarin dose required to maintain the INR between 2.0–3 0. Br J Haematol. 1999;107:207–9. doi: 10.1046/j.1365-2141.1999.01672.x. [DOI] [PubMed] [Google Scholar]
- 7.Gan GG, Teh A, Goh KY, Chong HT, Pang KW. Racial background is a determinant factor in the maintenance dosage of warfarin. Int J Hematol. 2003;78:84–6. doi: 10.1007/BF02983247. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi T. Optimal intensity of warfarin therapy for secondary prevention of stroke in patients with non-valvular atrial fibrillation. A multicenter, prospective, randomized trial. Stroke. 2000;31:817–21. doi: 10.1161/01.str.31.4.817. [DOI] [PubMed] [Google Scholar]
- 9.Poller L, Taberner DA. Dosage and control of oral anticoagulants: an international collaborative survey. Br J Haematol. 1982;51:479–85. doi: 10.1111/j.1365-2141.1982.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheung YF, Leung MP. Low dose oral anticoagulation therapy in Chinese children with congential heart disease. J Paediatrics Child Health. 1998;34:563–7. doi: 10.1046/j.1440-1754.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 11.Chenhsu RY, Chiang SC, Chou MH, Lin MF. Long-term treatment with warfarin in Chinese population. Ann Pharmacother. 2000;34:1395–401. doi: 10.1345/aph.19289. [DOI] [PubMed] [Google Scholar]
- 12.Rosendaal FR, Cannegieter SC, van der Meer FJM, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9. [PubMed] [Google Scholar]
- 13.Gohlke-Barwolf C, Acar J, Oakley C, et al. Guidelines for prevention of thromboembolic events in valvular heart disease: Study Group of the Working Group on Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 1995;16:1320–30. doi: 10.1093/oxfordjournals.eurheartj.a060739. [DOI] [PubMed] [Google Scholar]
- 14.Stein PD, Alpert JS, Bussey HI, et al. Antithrombotic therapy in patients with mechanical and biological prosthetic heart valves. Chest. 2001;119(Suppl):220S–227S. doi: 10.1378/chest.119.1_suppl.220s. [DOI] [PubMed] [Google Scholar]
- 15.Landefeld CS, Rosenblatt MW, Goldman L. Bleeding in outpatients treated with warfarin: Relation to the prothrombin time and important remediable lesions. Am J Med. 1989;87:153–9. doi: 10.1016/s0002-9343(89)80690-4. [DOI] [PubMed] [Google Scholar]
- 16.Hull R, Hirsh J, Jay R, et al. Different intensities of oral anticoagulant therapy in the treatment of proximal-vein thrombosis. N Engl J Med. 1982;307:1676–81. doi: 10.1056/NEJM198212303072704. [DOI] [PubMed] [Google Scholar]
- 17.Turpie AGG, Gunstensen J, Hirsh J, et al. Randomized comparison of two intensities of oral anticoagulant therapy after tissue heart valve replacement. Lancet. 1988;1:1242–5. doi: 10.1016/s0140-6736(88)92070-3. [DOI] [PubMed] [Google Scholar]
- 18.Saour JN, Sieck JO, Mamo LAR, et al. Trial of different intensities of anticoagulation in patients with prosthetic heart valves. N Engl J Med. 1990;322:428–32. doi: 10.1056/NEJM199002153220703. [DOI] [PubMed] [Google Scholar]
- 19.Altman R, Rouvier J, Gurfinkel E, et al. Comparison of two levels of anticoagulant therapy in patients with substitute heart valves. J Thorac Cardiovasc Surg. 1991;101:427–31. [PubMed] [Google Scholar]
- 20.Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348:1425–34. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 21.Chan TYK, Tsoi WC, Critchley JAJH. The determinants of warfarin requirements in Chinese patients. Pharmacoepidemiol Drug Saf. 1992;1:281–2. [Google Scholar]
- 22.Fong PC, Lau CP, Tai YT, Chow WH, Cheung KL. Therapeutic quality control of oral anticoagulation in patients with prosthetic heart valves. J Hong Kong Coll Cardiol. 1993;1:13–8. [Google Scholar]
- 23.Yu HCM, Chan TYK, Critchley JAJH, Woo KS. Factors determining the maintenance dose of warfarin in Chinese patients. QJM. 1996;89:127–35. doi: 10.1093/qjmed/89.2.127. [DOI] [PubMed] [Google Scholar]
- 24.Cannegieter SC, Rosendaal FR, Wintzen AR, et al. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11–7. doi: 10.1056/NEJM199507063330103. [DOI] [PubMed] [Google Scholar]
- 25.The European Atrial Fibrillation Trial Study Group. Optimal oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and recent cerebral ischemia. N Eng J Med. 1995;333:5–10. doi: 10.1056/NEJM199507063330102. [DOI] [PubMed] [Google Scholar]
- 26.Beyth RJ, Landefeld CS. Anticoagulants in older patients. A safety perspective. Drugs Aging. 1995;6:45–54. doi: 10.2165/00002512-199506010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Fihn SD, Callahan CM, Martin DC, McDonnell MB, Henikoff JG, White RH. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium Anticoagulation Clinics. Ann Intern Med. 1996;124:970–9. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Anonymous. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: stroke prevention in atrial fibrillation II study. Lancet. 1994;343:687–91. [PubMed] [Google Scholar]
- 29.Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87:144–52. doi: 10.1016/s0002-9343(89)80689-8. [DOI] [PubMed] [Google Scholar]
- 30.Petitti DB, Strom BL, Melmon KL. Prothrombin time ratio and other factors associated with bleeding in patients treated with warfarin. J Clin Epidemiol. 1989;42:759–64. doi: 10.1016/0895-4356(89)90073-5. [DOI] [PubMed] [Google Scholar]
- 31.Launbjerg J, Egeblad H, Heaf J, Nielsen NH, Fugleholm AM, Ladefoged K. Bleeding complications to oral anticoagulant therapy. multivariate analysis of 1010 treatment years in 551 outpatients. J Intern Med. 1991;229:351–5. doi: 10.1111/j.1365-2796.1991.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 32.Coon WW, Willis PW rd. Hemorrhagic complications of anticoagulant therapy. Arch Intern Med. 1974;133:386–92. [PubMed] [Google Scholar]
- 33.Wong RS, Geng G, Chan TY. Use of herbal medicines by patients receiving warfarin. Drug Safety. 2003;26:585–8. doi: 10.2165/00002018-200326080-00004. [DOI] [PubMed] [Google Scholar]
- 34.Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health-System Pharm. 2000;57:1221–30. [PubMed] [Google Scholar]
- 35.Wong AL, Chan TY. Interaction between warfarin and the herb product quilinggao. Ann Pharmacother. 2003;37:836–8. doi: 10.1345/aph.1C503. [DOI] [PubMed] [Google Scholar]
- 36.Chan TY. Interaction between warfarin and danshen (Salvia miltiorrhiza) Ann Pharmacother. 2001;35:501–4. doi: 10.1345/aph.19029. [DOI] [PubMed] [Google Scholar]
- 37.Tam LS, Chan TYK, Leung WK, Critchley JAJH. Warfarin interactions with Chinese traditional medicines; danshen and methyl salicylate medicated oil. Aust NZ J Med. 1995;25:257. doi: 10.1111/j.1445-5994.1995.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 38.Yu CM, Chan JCN, Sanderson JE. Chinese herbs and warfarin potentiation by ‘danshen’. J Intern Med. 1997;241:337–9. doi: 10.1046/j.1365-2796.1997.134137000.x. [DOI] [PubMed] [Google Scholar]
- 39.Izzat MB, Yim APC, El-Zufari MH. A taste of Chinese medicine. Ann Thorac Surg. 1998;66:941–2. doi: 10.1016/s0003-4975(98)00624-9. [DOI] [PubMed] [Google Scholar]
- 40.Chan K, Lo AC, Yeung JH, Woo KS. The effects of danshen (Salvia miltiorrhiza) on warfarin pharmacodynamics and pharmacokinetics of warfarin enantiomers in rats. J Pharm Pharmacol. 1995;47:402–6. doi: 10.1111/j.2042-7158.1995.tb05819.x. [DOI] [PubMed] [Google Scholar]
- 41.Cheng TO. Warfarin danshen interaction. Ann Thorac Surg. 1999;67:892–6. [PubMed] [Google Scholar]
- 42.Shaw D, Leon C, Kolev S, Murray V. Traditional remedies and food supplements: a five year toxicological study (1991–95) Drug Saf. 1997;17:342–56. doi: 10.2165/00002018-199717050-00006. [DOI] [PubMed] [Google Scholar]
- 43.Page RL, Lawrence JD. Potentiation of warfarin by dong quai. Pharmacotherapy. 1999;19:870–6. doi: 10.1592/phco.19.10.870.31558. [DOI] [PubMed] [Google Scholar]
- 44.Chan E, McLachlan A, O'Reilly R, Rowland M. Stereochemical aspects of warfarin drug interactions. Use of a combined pharmacokinetic-pharmacodynamic model. Clin Pharmacol Ther. 1994;56:286–94. doi: 10.1038/clpt.1994.139. [DOI] [PubMed] [Google Scholar]
- 45.Haber LT, Maier A, et al. Genetic polymorphisms in assessing interindividual variability in delivered dose. Reg Toxicol Pharmacol. 2002;35:177–97. doi: 10.1006/rtph.2001.1517. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi H, Kashima T, Nomoto S, et al. Comparisons between in-vitro and in-vivo metabolism of (S) -warfarin: catalytic activities of cDNA-expressed CYP2C9, its Leu359 variant and their mixture versus unbound clearance in patients with the corresponding CYP2C9 genotypes. Pharmacogenetics. 1998;8:365–73. doi: 10.1097/00008571-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Scordo MG, Pengo V, Spina E, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther. 2002;72:702–10. doi: 10.1067/mcp.2002.129321. [DOI] [PubMed] [Google Scholar]
- 48.Loebstein R, Yonath H, Peleg D, et al. Interindividual variability in sensitivity to warfarin – nature or nuture? Clin Pharmacol Ther. 2001;70:159–64. doi: 10.1067/mcp.2001.117444. [DOI] [PubMed] [Google Scholar]
- 49.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–9. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 50.Taube J, Halsall D, Baglin T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood. 2000;96:1816–9. [PubMed] [Google Scholar]
- 51.Higashi MK, Veenstra DL, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287(13):1690–8. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 52.Ogg MS, Brennan B, Meade T, Humphries SE. CYP2C9*3 allelic variant and bleeding complications. Lancet. 1999;354:1124. doi: 10.1016/S0140-6736(05)76918-X. [DOI] [PubMed] [Google Scholar]
- 53.Weibert RT, Palinkas LA. Differences in warfarin dose requirements between Asian and Caucasian patients. Clin Pharmacol. 1991;49:151. [Google Scholar]
- 54.Nutescu EA, Helgason CM. Evolving concepts in the treatment of venous thromboembolism. The role of factor Xa inhibitors. Pharmacotherapy. 2004;24(7 Part 2):82S–87S. doi: 10.1592/phco.24.10.82s.36121. [DOI] [PubMed] [Google Scholar]
- 55.Dager WE, White RH. Pharmacotherapy of heparin-induced thrombocytopenia. Expert Opin Pharmacother. 2003;4:919–40. doi: 10.1517/14656566.4.6.919. [DOI] [PubMed] [Google Scholar]
- 56.Smythe MA, Warkentin TE, Stephens JL, Zakalik D, Mattson JC. Venous limb gangrene during overlapping therapy with warfarin and a direct thrombin inhibitor for immune heparin-induced thrombocytopenia. Am J Hematol. 2002;71:50–2. doi: 10.1002/ajh.10181. [DOI] [PubMed] [Google Scholar]