Abstract
Aims
Previous isobolographic analysis revealed that coadministration of morphine and oxycodone produces synergistic antinociception in laboratory rodents. As both opioids can produce ventilatory depression, this study was designed to determine whether their ventilatory effects were synergistic when coadministered to healthy human subjects.
Methods
A placebo-controlled, randomized, crossover study was performed in 12 male volunteers. Ventilatory responses to hypoxaemia and hypercapnia were determined from 1-h intravenous infusions of saline (‘placebo’), 15 mg morphine sulphate (M), 15 mg oxycodone hydrochloride (O), and their combination in the dose ratios of 1 : 2, 1 : 1, 2 : 1. Drug and metabolite concentrations in serial peripheral venous blood samples were measured by high-performance liquid chromatography–MS/MS.
Results
‘Placebo’ treatment was without significant ventilatory effects. There were no systematic differences between active drug treatments on either the slopes or intercepts of the hypoxaemic and hypercapnia ventilation responses. During drug treatment, the mean minute ventilation at PetCO2 = 55 mmHg (VE55) decreased to 74% of the subjects’ before treatment values (95% confidence interval 62, 87), 68% (57, 80), 69% (59, 79), 68% (63, 73), and 61% (52, 69) for M15, M10/O5, M7.5/O7.5, M5/O10 and O15, respectively. Recovery was more prolonged with increasing oxycodone doses, corresponding to its greater potency and lower clearance compared with morphine.
Conclusions
Although adverse ventilatory effects of these drugs were found as expected, no unexpected or disproportionate effects of any of the morphine and oxycodone treatments were found that might impede their use in combination for pain management.
Keywords: opioid effect, stimulated hypercapnic response, stimulated hypoxaemic response, ventilatory depression
Morphine and oxycodone are commonly used in the treatment of moderate-to-severe pain. It is traditionally taught that these opioids are agonists at central µ-opioid receptors and this association determines both their analgetic and respiratory effects [1]. Used clinically, the analgetic potency of oxycodone is approximately 1.5 times that of morphine [2–4]. Previous research in laboratory rodents using isobolographic analysis of a wide range of doses revealed that the combination of morphine and oxycodone can act synergistically to produce a greater antinociceptive effect than that expected from the additive actions of the individual opioids [5]. A double-blind, randomized, crossover study undertaken in patients with cancer-related pain found that there was a 38% lower requirement for immediate-release morphine as breakthrough medication, together with less nausea and vomiting, when patients received controlled-release (CR) oxycodone rather than morphine for pain control [6]. These findings also are suggestive of a synergistic interaction between morphine and oxycodone for producing analgesia, but without greater side-effects. By contrast, a recent study in healthy volunteers found that combined oral administration of small doses of morphine and oxycodone did not produce synergistic antinociception when assessed using the cold pressor test [7]. However, as a dose–response relationship for the administered opioids was not demonstrated, these findings are difficult to interpret [7].
Whereas morphine is the prototypical µ-opioid agonist, behavioural antinociceptive and cross-tolerance studies indicate that pain-relieving effects of oxycodone are mediated by putative κ-opioid receptors [8, 9], and these studies are supported by radioligand binding data showing that oxycodone has high-affinity binding for κ2-opioid binding sites in rat brain homogenate depleted of µ- and δ-opioid receptors [10]. If the synergistic antinociceptive activity of the morphine–oxycodone combination in rats [5] is caused by reinforcing actions on distinctly different opioid receptor populations, it is important to determine whether depression of ventilation could also be synergistic, or be associated with unexpected pharmacokinetics, in order to assess the safety of these two opioids administered in combination to humans. Depression of ventilation is of particular concern, as it is the most serious acute opioid adverse effect that sometimes limits the clinical use of opioids, and thus the pain relief achievable.
Morphine-like drugs reduce the central nervous system (CNS) responsiveness to hypercapnia and hypoxia. From a functional perspective, opioids can produce clinically significant ventilatory depression if they increase the hypercapnic set point and/or decrease the hypoxic set point (intercepts) and/or decrease the gain (slope) of the ventilatory drive in response to hypercapnia and/or hypoxaemia [11–14]. A number of studies have described effects of morphine and oxycodone on ventilation in humans under experimental and clinical circumstances when administered individually [1, 15, 16, 17, 18, 19], but apparently not in combination, and recent studies have described the effects of morphine in comparison with its metabolite morphine-6-glucuronide (M6G) in laboratory animals and humans [20]. A particular aspect emerging from such studies is the role of experimental design in revealing different information about opioid effects on resting ventilation during the breathing of room air compared with ventilation stimulated by the imposition of exogenous carbon dioxide [21]. Whereas the former mimics the clinical situation of an opioid-induced adverse effect, it does not provide a suitable platform for pharmacodynamic measurements due to confounding influences of concomitant local events such as sedation; the latter, on the other hand, facilitates quantitative pharmacodynamic measurements, but under contrived conditions.
The aim of the present study was to determine whether the effects of combinations of morphine and oxycodone on hypercapnia- and hypoxaemia-stimulated breathing responses were synergistic in healthy human subjects, not undergoing any concurrent medical procedures.
Materials and methods
Drugs
Morphine sulphate for injection (hereafter referred to as morphine) was used from the hospital pharmacy at the Royal North Shore Hospital (Sydney, Australia). Intravenous oxycodone hydrochloride (hereafter referred to as oxycodone), currently not locally approved for clinical use, was prepared by the manufacturing facility of Royal Brisbane Hospital Pharmacy and approved for this specified research use by the Australian Therapeutic Goods Administration under the Clinical Trial Notification (CTN) scheme. A ‘placebo’ solution of normal saline (0.9% NaCl) was also used for comparison.
Subjects
The study protocol was approved by the Human Research Ethics Committee of the Royal North Shore Hospital and written informed consent was obtained from all subjects. The volunteer subjects were 12 male nonsmokers, aged 18–45 years, within 15% of optimal body weight, who had no significant medical illnesses, no known allergy to opioids, and were not taking regular medication containing opioids, benzodiazepines, antidepressants, major tranquilizers, or drugs known to alter hepatic metabolism significantly. Although 12 subjects were treated in the study, two of these did not complete all active drug treatments and were subsequently excluded from the data analysis. Males were chosen to preclude known gender-related differences in ventilatory response and opioid effect [22].
Study design
The main objective was to determine whether there were significant differences in ventilatory responses between the various active drug treatments. Subjects were instructed to avoid taking any sedatives and medications, refrain from consuming alcohol for 24 h before and after each study, to fast on the morning of the study, and were provided with a meal when the study finished. Placebo effects were considered possible [23], and opioid effects were expected at the active drug doses used [1, 15, 16, 17, 18, 19, 20]. Moreover, inter- and intrasubject variability is well known in ventilatory response measurements [13, 24]. Hence the order of administration of ‘placebo’ and active drug treatment, and of active drug treatments, was randomized for each subject with all measures being made and analysed for each subject's study session. At each study session, 1 week apart, each subject received an intravenous (i.v.) 1-h infusion of normal saline (‘placebo’), and an i.v. 1-h infusion of active drug which consisted of morphine 15 mg (M15), oxycodone 15 mg (O15), morphine 10 mg +oxycodone 5 mg (M10/O5), oxycodone 10 mg +morphine 5 mg (M5/O10), or morphine 7.5 mg +oxycodone 7.5 mg (M7.5/O7.5). The order and identity of the various treatments remained blinded to subjects and relevant investigators until the data had been entered into a computer storage system.
Ventilatory responses
The effects of the various treatments were evaluated on the stimulated hypoxaemic and hypercapnic ventilatory responses using a previously described non-invasive method [25, 26] adapted to locally available and contemporary apparatus. Pneumotachography was performed with a research pneumotachograph head (Biopac Inc., Santa Monica, CA, USA) through which the subject inspired; the pressure data produced were decoded in the computer and saved to disk in real time, to produce a flow-time chart of the subject's ventilation, which was analysed later. Inspired and end tidal carbon dioxide concentrations (PetCO2) and arterial blood oxygen saturation ascertained by pulse oximetry (SpO2) were measured concurrently (POET II® Monitor; Johnson & Johnson Criticare, Waukesha, WI, USA); these were stored electronically with the ventilation data.
The gas mixtures used for making hypercapnic and hypoxaemic states were breathed by the subject from a standard anaesthetic machine that had been locally modified to allow the high flow rates required for calibrating the pneumotachography apparatus. These were stored in premeasured concentrations in large weather balloons (that acted like Douglas bags) for fresh gas reservoirs as a demand valve supply system. Hypoxic gas mixtures, produced by mixing nitrogen and air in the inspired gases, were modified in real time during the subject's breathing of this mixture to enable rapid changes to be made to gas concentrations in response to observed SpO2. Unused hypoxic gas mixture was kept in a smaller similar bag for spontaneous breathing upstream of the demand valve, so that an adequate supply of gas was available in the event of the subject's taking sudden deep breaths.
Study procedures
For each study session, an i.v. Teflon cannula was placed in each arm of the -one for the ‘placebo’/drug treatments, the other for collection of blood samples and for administration of normal saline solution for hydration. Subjects were then given the assigned active drug or ‘placebo’ by infusion at a constant rate of 50 ml over 1 h using a syringe driver. Heart rate, blood pressure and SpO2 values were monitored non-invasively at 5-min intervals throughout the study. Every 20 min during and following the ‘placebo’/active drug infusions, subjects were instructed to breathe as naturally as possible through a well-fitting and sealed mask attached to a breathing system that measured and recorded their breathing. At these times, blood samples were collected for drug analyses. Spontaneously volunteered comments and investigators’ observations about subjective effects were noted.
Each set of measurements to determine the hypoxaemic and hypercapnic ventilation responses took 11 min, and this limited the frequency of data collection to 20-min intervals, bearing in mind the comfort of the subjects. The mask was applied for 1 min, and normal ventilation was recorded for 3 min. It took ∼3 min to stabilize a subject's SpO2 at 80% followed by a 1-min reading, then ∼2 min to stabilize PetCO2 followed by a 1-min reading. Hypoxaemia developed over 2–3 min, typically requiring an inspired oxygen concentration of 10–15%; this gradually decreased the subject's SpO2 to 80%, which was maintained for 1 min to allow the hypoxic ventilatory response to be measured during ventilation under hypoxaemic conditions. However, if a subject showed an SpO2 of <70%, the run was aborted and the subject was given 50% oxygen to breathe until an SpO2 of 97–99% was restored. Hypercapnia was produced by the subject breathing 40 mmHg CO2 in oxygen-enriched air (about 5% CO2, 35% O2, balance nitrogen) for 3 min via a demand-valve type supply system such that PetCO2 increased to ∼50 mmHg. It was found that SpO2 and PetCO2 normally self-corrected within 1–2 min of breathing room air; SpO2 always self-corrected within a minute of the subject being placed on the hypercarbic gas mixture (which had enriched oxygen) or on 50% oxygen in the event of a large decrease in saturation. PetCO2 normally self-corrected within 1–2 min of breathing room air.
Quantification of morphine, oxycodone and their metabolites in plasma
Morphine, morphine 3- and 6-glucuronides, oxycodone, normorphine, normorphine-3-glucuronide and noroxycodone were separated from plasma proteins and quantified using high-performance liquid chromatography (HPLC) with mass spectrometric detection. Briefly, aliquots of plasma (100 µl) were added to polypropylene tubes (10 ml), followed by internal standard (hydromorphone HCl, 2 ng µl−1, 100 µl). Samples were deproteinated by addition of acetonitrile (3.0 ml, containing 1% formic acid), followed by vortex mixing (10 s). After centrifugation, the supernatant was decanted into clean Eppendorf™ tubes and the liquid evaporated using a Savant centrifugal vacuum dryer. The dried residues were reconstituted using deionized water (18.2 MΩ, 50-µl aliquots), followed by vortex mixing (30 s). Sample aliquots (20 µl) were then injected onto the HPLC-MS/MS (Shimadzu VP HPLC system, and a Perkin-Elmer API 300 mass spectrometer) and the relevant analytes were separated using a stainless steel column (Zorbax SB-C18, 5 µm, 2.1 × 50 mm) and an isocratic mobile phase (methanol : 0.1% formic acid = 9 : 91, at a flow rate of 0.15 ml min−1). The total run time for each sample chromatogram was 4.5 min.
The assay was highly sensitive and specific as each analyte was identified and quantified using selected ion monitoring according to the m/z (mass : charge) ratio of the parent ion to the daughter ion (Table 1). Morphine-3- and 6-glucuronides were resolved chromatographically (retention times of 2.1 and 2.4, respectively) as these two analytes had identical m/z ratios. Recoveries and lower limits of quantification for morphine, morphine-3-glucuronide, morphine-6-glucuronide, normorphine, normorphine-3-glucuronide, oxycodone and noroxycodone are shown in Table 1. Standard curves comprising six to seven concentrations of each analyte were processed in random order with each batch of human plasma samples. For each subject at each testing session, a ‘blank’ plasma sample was collected before dosing in addition to the samples collected after dosing. Quality control samples containing known concentrations of each analyte of interest at the lower limit of quantification (LLOQ), middle and upper concentrations of the standard curves were also included with each chromatographic run. Regression analysis was used to produce standard curves, which were accepted if the correlation coefficients were ≥0.995. Plasma concentrations of drugs were inversely determined from the standard curve that was processed with each batch of plasma samples. The between-day percent coefficients of variation (%CV) for precision at the LLOQ determined from the quality control samples included with each chromatographic run were <15% for morphine and metabolites and <17.5% for oxycodone and noroxycodone. Between-day accuracy (% deviation from the known true concentrations) at the LLOQ was <10.5% for morphine and metabolites and <3% for oxycodone and noroxycodone.
Table 1.
Summary of analytical methodology performance for morphine, morphine 3- and 6-glucuronides (M3G, M6G, respectively), oxycodone and their N-demethylated metabolites, normorphine and noroxycodone, in plasma
| Drug | Mor | Nmor | M3G | M6G | NM3G | IS-Hmor | Oxy | Noxy |
|---|---|---|---|---|---|---|---|---|
| Mrm signal | 286/201 | 272/209 | 462/286 | 462/286 | 447/272 | 286/185 | 316/299 | 302/277 |
| Recovery (%) | 74 ± 7 | 68 ± 4 | 58 ± 4 | 40 ± 10 | 30 ± 4 | 37 ± 4 | 53 ± 3 | 51 ± 1 |
| Calibration range plasma concentration (mg l−1) | 0.078–5 | 0.039–2.5 | 0.156–10 | 0.039–2.5 | 0.039–2.5 | – | 0.078–5 | 0.078–2.5 |
| LOQ (pg on column) | 156 | 78 | 313 | 78 | 78 | – | 156 | 156 |
Mor, Morphine; Nmor, normorphine; M3G, morphine 3-glucuronide; M6G, morphine 6-glucuronide; IS-Hmor, internal standard-hydromorphone; Oxy, oxycodone; Noxy, noroxycodone; Mrm, m/z (mass : charge) ratio of the parent ion to the daughter ion; LOQ, limit of quantification.
Data processing and statistical analysis
Ventilatory effect data for comparing effects from the ‘placebo’/active drug treatments comprised the slope and intercept of the hypoxaemic ventilation response [minute ventilation ( VE) vs.%SpO2) curve (limited to normal, where SpO2=97–99%, and hypoxaemia where SpO2=79–81%), the slope and abscissa intercept of the hypercapnic ventilation response (VEvs. PetCO2) curve (where the abscissa intercept is the apnoeic threshold), and the minute ventilation at a PetCO2 of 55 mmHg (VE55). The data for each subject's study session were analysed by treatment (‘placebo’/active drug) and period (before, during and after active drug treatment), which does not take into account individual pharmacokinetics, and by treatment and plasma drug concentrations, which does.
For analysis by period, individual data were collected into the averages of the three serial measurements made 20 min apart in each successive 1-h period. Because subjects were randomized to receive either a ‘placebo’ or active drug treatment for the first 1-h infusion period, the resultant data were subjected to an interim analysis of variance (anova) that determined there was not a significant effect of ‘placebo’. This being not found, the data were then assigned into ‘before’, ‘during’ and ‘after’ drug treatment periods for further statistical processing. For analysis by drug concentrations, individual and group ventilation response data were plotted against time, and against plasma drug concentration, examining for correlations. Subsequent data analysis indicated that VE55 was the most sensitive parameter on which to analyse between-treatment differences; unweighted linear regression equations were constructed for the relationship between VE55 and plasma drug concentration expressed as morphine equivalents (= morphine concentration +1.5 × oxycodone concentration) [2–4]. Analysis of the plasma drug concentration area-under-the-curve from 0 to 120 min (AUC120) was determined by application of the trapezoidal rule.
The null hypotheses tested were that there was no difference in relevant respective ventilatory effects between drug treatments, between treatment and ‘placebo’, between treatment sequences (‘placebo’ preceding, or following, active drug), with order of active drug treatments, and between time period (before, during and after infusion). P < 0.05 was taken as weak evidence for rejection of the null hypothesis, and P < 0.01 was taken as strong evidence. Repeated measures anova was performed with treatment and period as within-subject effects and subject as the repeated measure. When there was a significance main effect, differences in mean values between treatments were determined by the method of least significant differences and linear contrasts. Statistix for Windows (v7 and v8; Analytical Solutions, Tallahassee, FL, USA) was used.
Results
Missing values occurred occasionally with all treatments, mostly with the hypoxaemic response (nine instances vs. two instances with hypercapnia), because it was difficult to induce a SpO2 of 80% in these healthy subjects.
Subjective responses
The subjective side-effects to the drug treatments were typical of opioid analgesics but were mild, e.g. drowsiness, tingling, feeling of warmth. Most side-effects were apparently random, although some subjects tended to experience recurring side-effects, e.g. itching, or nausea. Overall, subjective side-effects increased with increasing oxycodone dose.
Comparison of ventilatory responses vs. drug treatments
As noted above, there was no significant effect of ‘placebo’ infusion, and there was no sequence effect of active treatments, i.e. whether administered before or after ‘placebo’.
Hypoxaemic response
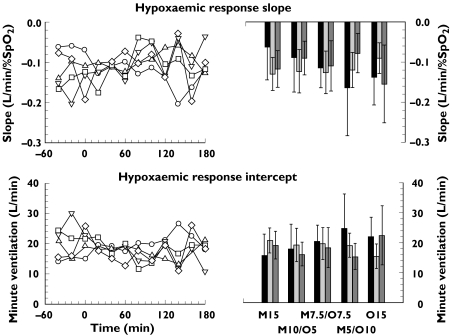
A consistent treatment effect was not demonstrated for either slope or intercept of the hypoxaemic response (see Figure 1). There were no significant differences between active drug treatments (slope P = 0.59; intercept P = 0.85). Although there were significant treatment–period interactions (hypoxaemia slope P = 0.005; intercept P = 0.004), these were considered not meaningful as they resulted only from a reduction in the variability of values observed during drug treatment compared with the other periods.
Figure 1.
Hypoxaemic response for the five opioid drug treatments. Left panels: time course of the median slope (upper panel) and intercept (lower panel). Right panels: mean and 95% confidence intervals of the slope and intercept for the periods before, during and after drug treatment. Repeated measures analysis of variance on these ventilatory responses with treatment and period as within subject factors with subject as the repeated measure found no significant differences between treatments or periods.
Abbreviations: M15 = morphine 15 mg, M10/O5 = morphine 10 mg + oxycodone 5 mg; M7.5/O7.5 = morphine 7.5 mg + oxycodone 7.5 mg; M5/O10 = morphine 5 mg + oxycodone 10 mg; O15 = oxycodone 15 mg. M15 (○); M10/O5 (▵); M7.5/O7.5 (▿); M5/O10 (□); O15 (⋄); before (▪); during ( ); after (
); after ( )
)
Hypercapnic response
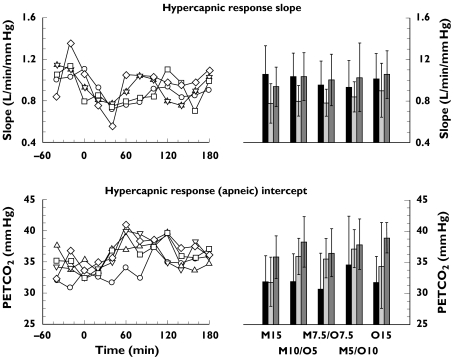
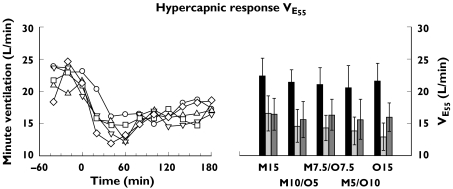
There was a consistent decrease in slope of the hypercapnic response during all active drug treatments, with general recovery after treatment. This was accompanied by an increase of the abscissa intercept (apnoeic threshold) during all treatments that persisted after drug treatment (slope P = 0.048: before =after >during; intercept P = 0.0006: after >during >before; see Figure 2). There were no significant differences between active drug treatments (P = 0.85 and 0.24, respectively). Similarly, there was a consistent decrease of VE55 during all treatments, with partial recovery after drug treatment (P < 0.0001: before >after >during; see Figure 3), but not between active drug treatments (P = 0.24). During drug treatment, VE55 decreased significantly to a mean of 74% of the respective values before drug treatment (95% confidence interval 62, 87), 68% (57, 80), 69% (59, 79), 68% (63, 73), and 61% (52, 69) for M15, M10/O5, M7.5/O7.5, M5/O10 and O15, respectively. After drug treatment mean values of VE55 were 75% (61, 88), 73% (61, 84), 78% (69, 87), 76% (67, 86) and 75% (65, 84) of the respective values before drug treatment.
Figure 2.
Hypercapnic response for the five opioid drug treatments. Left panels: time course of the median slope (upper panel) and intercept (lower panel). Right panels: mean and 95% confidence intervals of the slope and abscissa intercept (apnoeic threshold) for the periods before, during and after drug treatment. Repeated measures analysis of variance on these ventilatory responses with treatment and period as within subject factors with subject as the repeated measure found significant differences between periods, but not between active drug treatments.
Abbreviations: M15 = morphine 15 mg, M10/O5 = morphine mg + oxycodone 5 mg; M7.5/O7.5 = morphine 7.5 mg + oxycodone 7.5 mg; M5/O10 = morphine 5 mg + oxycodone 10 mg; O15 = oxycodone 15 mg. M15 (○); M10/O5 (▵); M7.5/O7.5 (▿); M5/O10 (□); O15 (⋄); before (▪); during ( ); after (
); after ( )
)
Figure 3.
Hypercapnic response for the five opioid drug treatments determined by the extrapolated minute ventilation at a PetCO2 of 55 mmHg (VE55). Left panel: time course of the median value. Right panel: mean and 95% confidence intervals of the values for the periods before, during and after drug treatment. Repeated measures analysis of variance on tis ventilatory response with treatment and period as within subject factors with subject as the repeated measure found significant differences between periods, but not between active drug treatments.
Abbreviations: M15 = morphine 15 mg, M10/O5 = morphine mg + oxycodone 5 mg; M7.5/O7.5 = morphine 7.5 mg + oxycodone 7.5 mg; M5/O10 = morphine 5 mg + oxycodone 10 mg; O15 = oxycodone 15 mg. M15 (○); M10/O5 (▵); M7.5/O7.5 (▿); M5/O10 (□); O15 (⋄); before (▪); during ( ); after (
); after ( )
)
Comparison of ventilatory responses vs. plasma drug concentrations
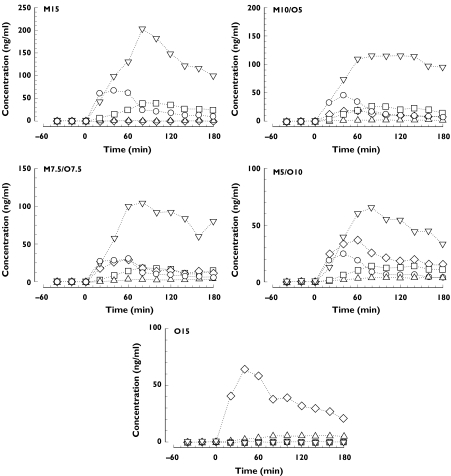
Measured plasma concentrations of the drugs and metabolites are shown in Figure 4. Both drug and metabolite AUC120 was linearly proportional to dose (Table 2) and did not significantly differ between drugs (morphine: slope 326 ± 13 SE, r2 = 0.82, P < 0.0001; oxycodone: slope 279 ± 20 SE, r2 = 0.57, P < 0.0001). Although there were significant differences in mean plasma drug concentrations between subjects (P = 0.028), there were no significant differences between treatments during infusion (P = 0.36); significant differences were found between treatments (P = 0.0004) after infusion, with concentrations being directly correlated with the oxycodone dose.
Figure 4.
Median plasma concentrations of morphine (○) and its 3- (▿) and 6-glucuronide (□) metabolites (respectively, Mor, M3G and M6G), oxycodone (◊) and noroxycodone (▵) (respectively, Oxy and Noxy).
Abbreviations: M15 = morphine 15 mg, M10/O5 = morphine mg + oxycodone 5 mg; M7.5/O7.5 = morphine 7.5 mg + oxycodone 7.5 mg; M5/O10 = morphine 5 mg + oxycodone 10 mg; O15 = oxycodone 15 mg
Table 2.
Analysis of morphine and oxycodone area under the plasma concentration–time curve to 120 min (median AUC120) in relation to dose composition
| Treatment | Mor (ng min ml−1) | M3G (ng min ml−1) | M6G (ng min ml−1) | Oxy (ng min ml−1) | Noxy (ng min ml−1) |
|---|---|---|---|---|---|
| M15 | 5653 | 21977 | 4558 | 0 | 0 |
| M10/O5 | 3500 | 16349 | 3063 | 2031 | 228 |
| M7.5/O7.5 | 2659 | 13080 | 2143 | 3014 | 408 |
| M5/O10 | 1885 | 7819 | 1690 | 3988 | 476 |
| O15 | 0 | 0 | 0 | 6838 | 714 |
| Dose-normalized median AUC120 values | |||||
|---|---|---|---|---|---|
| ng min ml−1 per mg morphine) | ng min ml−1 per mg morphine) | ng min ml−1 per mg morphine) | ng min ml−1 per mg oxycodone) | ng min ml−1 per mg oxycodone) | |
| M15 | 377 | 1465 | 304 | 0 | 0 |
| M10/O5 | 350 | 1635 | 306 | 456 | 48 |
| M7.5/O7.5 | 355 | 1744 | 286 | 399 | 48 |
| M5/O10 | 377 | 1564 | 338 | 402 | 54 |
| O15 | 0 | 0 | 0 | 406 | 46 |
M15, Morphine 15 mg; M10/O5, morphine mg +oxycodone 5 mg; M7.5/O7.5, morphine 7.5 mg +oxycodone 7.5 mg; M5/O10, morphine 5 mg +oxycodone 10 mg; O15, oxycodone 15 mg; Mor, morphine; M3G, morphine 3-glucuronide; M6G, morphine 6-glucuronide; Oxy, oxycodone; Noxy, noroxycodone.
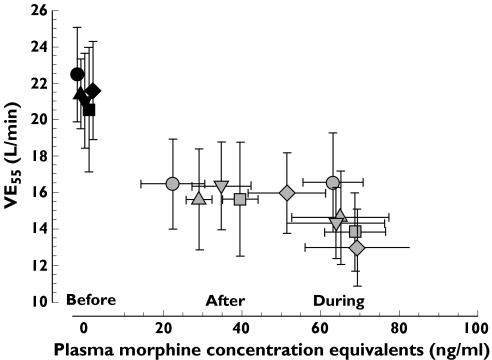
VE55 was found to be the most sensitive ventilatory response variable for comparing the individuals and treatments in relation to drug plasma concentrations (Figure 5). Unweighted linear regression analysis of VE55 on plasma morphine equivalents showed that the regression lines were not distinguishable on the basis of treatment.
Figure 5.
Overplots of mean and 95% confidence intervals of extrapolated minute ventilation at PetCO2 55 mmHg (VE55) vs. plasma morphine equivalents (morphine + 1.5x oxycodone concentrations) for each treatment. The values for “before” drug treatment (time = 0) are slightly staggered to show the values of VE55 for drug concentration = 0.
Abbreviations: M15 = morphine 15 mg, M10/O5 = morphine mg + oxycodone 5 mg; M7.5/O7.5 = morphine 7.5 mg + oxycodone 7.5 mg; M5/O10 = morphine 5 mg + oxycodone 10 mg; O15 = oxycodone 15 mg. M15 (•); M10/O5 (▴); M7.5/O7.5 (▾); M5/O10 (▪); O15 (♦)
Discussion
The original evidence of antinociceptive synergy from the combination of morphine and oxycodone came from a study in which these drugs were administered by intracerebroventricular, subcutaneous and intraperitoneal routes to rats [5]. Combination subcutaneous dose ratios of 1 : 3, 1 : 1 and 3 : 1 were each found to be more potent than either morphine or oxycodone alone, with isobolographic analysis revealing marked antinociceptive synergy between these two opioids [5]. This is supported by a more recent clinical finding of 38% less consumption of immediate-release morphine for breakthrough pain in patients administered CR oxycodone rather than CR morphine for control of cancer pain [6]. The present study was designed to determine whether there was also synergy in the (adverse) ventilatory responses to combinations of these opioids. Although evidence of expected opioid-related ventilatory depression was found when tested in healthy human subjects, systematic evidence of unexpected or synergistic ventilatory effects from the opioid combinations was not found. Thus, an improvement in the analgetic effect, without concomitant increase in side-effects, suggests that there may potentially be a clinical advantage in the use of combinations of these opioids for pain pharmacotherapy.
Study design considerations
The study was complex and a number of considerations needed to be incorporated into its design. It was decided that the study design should be minimally invasive, and that only healthy subjects should be used to enable the use of large enough doses of the opioids to provoke an adverse response, but with safety through intensive supervision. Moreover, a triple blinded (subject, investigator, data recorder) protocol was used to test for placebo response. A previous, crossover design study investigated changes in unstimulated breathing pattern and derived respiratory variables resulting from i.v. infusion of morphine or oxycodone in healthy human volunteers: this found qualitatively similar, but more potent, effects of oxycodone [1], consistent with its greater analgetic potency [2–4]. Because ventilatory responses to opioids are known to be both gender-related [22] and variable both within and between subjects [13, 24], we used only male subjects and also used a crossover study design, with saline ‘placebo’ infused to assess responses in each study session. In one reported study, an acute hypoxaemic response to a sudden perturbation of decreased inspired oxygen content (as in the present study) produced measured hypoxaemic response slopes ranging from 0.24 to 1.87 l min−1 %−1 SpO2 in 10 healthy young subjects, with within-subject coefficients of variation ranging from 12 to 246%, and with an overall mean between-subjects coefficient of variation of 48%[24]. Such high within-subject contribution to opioid drug effect is usually ascribed to measurement error [7], to natural variability within subjects [24], and to variation in drug disposition [27].
A 1-h duration of i.v. infusion was decided upon to mimic the time course of clinically used intramuscular injections, to allow immediate cessation of drug treatment in the event of an emergency, and to minimize unpleasant CNS effects associated with rapid i.v. injections. Because morphine equilibrates relatively slowly between plasma and the CNS [28], a slow i.v. administration would promote temporal correlation between the peripheral venous blood drug concentrations and the various CNS responses by allowing time for re-establishment of carbon dioxide dynamics [14, 21]. The measured metabolites were not included in the ensuing analyses because of their uncertain contributions to effect. However, neither the analgetic nor ventilatory effects of morphine in small bolus doses have been found to be manifestly altered by morphine-3-glucuronide in healthy volunteers [29]. Although systemically administered M6G does cause depression of ventilation, it is considerably less potent than morphine [20, 29]. Moreover, in the present study, the plasma M6G concentrations were similar to those of morphine, and its rate of blood–CNS equilibration is slower than that of morphine [30]. After systemic administration of oxycodone to humans, noroxycodone is the principal metabolite in the systemic circulation with negligible concentrations of oxymorphone, the O-demethylated metabolite, being present [31]. As the intrinsic antinociceptive potency of noroxycodone is much less than that of either morphine or oxycodone [2–4, 32], and its secondary amine is fully ionized at physiological pH, it is unlikely to reach the CNS in sufficient concentration to contribute significantly to the respiratory effects of oxycodone. Taken together, it is reasonable to infer that the opioid metabolites contributed negligibly to the responses measured in this study.
Subjective responses
It is relevant to note that subjective effects, all known effects of opioid analgesics, were remarkably minor considering that full ‘clinical’ doses of the drugs were used and that the subjects were encouraged to report any effects. When this did occur, the effects tended to recur in the same individuals in a similar manner to that found in patients having opioid-based pain management after surgery [33, 34].
Ventilatory responses
Opioids exert a number of effects on behavioural control of ventilation, e.g. ventilatory rate and PaO2 may be decreased, and PetCO2 may be increased [35]. These are easier to measure than the effects on chemical control of ventilation, but they are not unambiguous direct measures of opioid effect [1, 11–14] because they may be influenced by concomitant behavioural factors, e.g. sleepiness in subjects undergoing prolonged study. The chemical control outcome variables, decreases in the set point and/or gain of the acute hypoxaemic and hypercapnic ventilation responses, represent clinically serious opioid-derived effects. The expected responses to hypoxaemia were that the slope of the hypoxaemic ventilation response ( VEvs.%SpO2) curve would become less negative and/or the intercept would be decreased; those to hypercapnia were that the slope of the hypercapnic ventilation response (VEvs. PetCO2) curve would be decreased and/or the apnoeic threshold would be increased. The response to hypercapnia has been studied more frequently than that to hypoxaemia. The latter is considered a secondary or ‘back-up’ chemosensitive reflex that protects the subject from hypoventilation. It has been suggested that morphine has a greater effect on the set point compared with its effect on the gain [11, 12]. The effects of morphine on VE are mediated by changes in the tidal volume and frequency of breathing. However, analgetic doses of the opioids (as used in this study) often produce no consistent effects, unlike excessive doses that predominantly affect the frequency [1, 11, 12]. Nevertheless, because of ease of measurement, bradypnoea is the most common ventilatory effect of opioids measured clinically.
We found that it was difficult to generate a consistent hypoxaemic response in these healthy subjects and the results were not dependable; however, the hypercapnic response reflected the expected opioid effects [35]. In particular, the changes in VE55 provided a suitable basis for a simple linear pharmacokinetic–pharmacodynamic analysis from which the treatment potencies could be compared. This found no active drug treatment differences but it underscores the importance of considering pharmacokinetics when considering such relationships. These results are also consistent with a previous report of a greater potency of oxycodone on ventilation with the need to prematurely terminate oxycodone infusions in four of six healthy volunteers compared with none of six volunteers who received a similar infusion rate of morphine [1]. However, the plasma concentrations of oxycodone were also approximately double those of morphine [1], both being considerably greater than found in the present study. Differences in the pharmacokinetics of morphine and oxycodone are substantial. The mean total body clearance of morphine is around twice that of oxycodone [36, 37], thus it is not surprising that the ventilatory effects dissipate more slowly after cessation of drug administration with the greater dose of oxycodone.
In summary, although adverse ventilatory effects of these drugs were found as expected, no unexpected or disproportionate effects of any of the tested combinations of morphine and oxycodone were found that might impede use of these two opioids in combination for pain management.
Acknowledgments
The authors are pleased to acknowledge the contributions of their colleagues A/Prof. I. Power MD, Ms A. Zammit RN, B. McDonald RN, Ms A. Pollard RN, Ms A. Gregg RN, Ms A. O’Connor RN, Ms M. Millar RN and Ms B. Fryirs BSc, Ms M. Gibson BPharm, all of the Royal North Shore Hospital, to this project. This project was partly supported by AusIndustry R&D START grant with Sigma Pharmaceuticals Pty Ltd, Melbourne, Australia, and by the Centre for Anaesthesia and Pain Management Research Ltd, Sydney, Australia.
References
- 1.Leino K, Mildh L, Lertola K, Seppala T, Kirvela O. Time course of changes in breathing pattern in morphine- and oxycodone-induced respiratory depression. Anaesthesia. 1999;54:835–840. doi: 10.1046/j.1365-2044.1999.00946.x. [DOI] [PubMed] [Google Scholar]
- 2.Kalso E, Poyhia R, Onnela P, Linko K, Tigerstedt I, Tammisto T. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand. 1991;35:642–646. doi: 10.1111/j.1399-6576.1991.tb03364.x. [DOI] [PubMed] [Google Scholar]
- 3.Heiskanen T, Kalso E. Controlled-release oxycodone and morphine in cancer-related pain. Pain. 1997;73:37–45. doi: 10.1016/s0304-3959(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 4.Bruera E, Belzile M, Pituskin E, et al. Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. J Clin Oncol. 1998;16:3222–3229. doi: 10.1200/JCO.1998.16.10.3222. [DOI] [PubMed] [Google Scholar]
- 5.Ross FB, Wallis SC, Smith MT. Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain. 2000;84:421–428. doi: 10.1016/s0304-3959(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 6.Lauretti GR, Oliviera GM, Pereira NL. Comparison of sustained release morphine with sustained-release oxycodone in advanced cancer patients. Br J Cancer. 2003;89:2027–2030. doi: 10.1038/sj.bjc.6601365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grach M, Massalha W, Pud D, Adler R, Eisenberg E. Can coadministration of oxycodone and morphine produce analgesic synergy in humans? An experimental cold pain study. Br J Clin Pharm. 2004;58:235–242. doi: 10.1111/j.1365-2125.2004.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross FB, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be kappa opioid receptor mediated. Pain. 1997;73:151–157. doi: 10.1016/S0304-3959(97)00093-6. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen CK, Ross FB, Smith MT. Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the Dark Agouti rat. J Pharmacol Exp Ther. 2000;295:91–99. [PubMed] [Google Scholar]
- 10.Nielsen CK. Studies on the Preclinical Pharmacology of Oxycodone. 2003 PhD Thesis. The University of Queensland. [Google Scholar]
- 11.Borison HL. Central nervous respiratory depressants—control-systems approach to respiratory depression. Pharmac Ther B. 1977;3:211–226. doi: 10.1016/0306-039x(77)90033-2. [DOI] [PubMed] [Google Scholar]
- 12.Borison HL. Central nervous respiratory depressants—narcotic analgesics. Pharmac Ther B. 1977;3:227–237. doi: 10.1016/0306-039x(77)90034-4. [DOI] [PubMed] [Google Scholar]
- 13.Jordan C. Assessment of the effects of drugs on respiration. Br J Anaesth. 1982;54:763–782. doi: 10.1093/bja/54.7.763. [DOI] [PubMed] [Google Scholar]
- 14.Dahan A, Teppema LJ. Influence of anaesthesia and analgesia on the control of breathing. Br J Anaesth. 2003;91:40–49. doi: 10.1093/bja/aeg150. [DOI] [PubMed] [Google Scholar]
- 15.Berkenbosch A, Teppema LJ, Olievier CN, Dahan A. Influences of morphine on the ventilatory response to isocapnic hypoxia. Anesthesiology. 1997;86:1342–1349. doi: 10.1097/00000542-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Houghton IT, Aun CS, Wong YC, Chan K, Lau JT, Oh TE. The respiratory depressant effect of morphine. A comparative study in three ethnic groups. Anaesthesia. 1994;49:197–201. [PubMed] [Google Scholar]
- 17.Tallgren M, Olkkola KT, Seppala T, Hockerstedt K, Lindgren L. Pharmacokinetics and ventilatory effects of oxycodone before and after liver transplantation. Clin Pharmacol Ther. 1997;61:655–661. doi: 10.1016/S0009-9236(97)90100-4. [DOI] [PubMed] [Google Scholar]
- 18.Tarkkila P, Tuominen M, Lindgren L. Comparison of respiratory effects of tramadol and oxycodone. J Clin Anaesth. 1997;9:582–585. doi: 10.1016/s0952-8180(97)00147-5. [DOI] [PubMed] [Google Scholar]
- 19.Skarke C, Jarrar M, Erb K, Schmidt H, Geisslinger G, Lötsch J. Respiratory and miotic effects of morphine in healthy volunteers when P-glycoprotein is blocked by quinidine. Clin Pharmacol Ther. 2003;74:303–311. doi: 10.1016/S0009-9236(03)00220-0. [DOI] [PubMed] [Google Scholar]
- 20.Romberg R, Olofsen E, Sarton E, Teppema L, Dahan A. Pharmacodynamic effect of morphine-6-glucuronide versus morphine on hypoxic and hypercapnic breathing in healthy volunteers. Anesthesiology. 2003;99:788–798. doi: 10.1097/00000542-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Gross JB. When you breathe IN you inspire, when you DON’T breathe, you…expire. Anesthesiology. 2003;99:767–770. doi: 10.1097/00000542-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sarton E, Teppema L, Dahan A. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology. 1999;90:1329–1338. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90:205–215. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Robbins PA. Methodological and physiological variability within the ventilatory response to hypoxia in humans. J Appl Physiol. 2000;88:1924–1932. doi: 10.1152/jappl.2000.88.5.1924. [DOI] [PubMed] [Google Scholar]
- 25.Johnson A, Löfström JB. A new method for studying the ventilatory response in patients. Acta Anaesth Scand. 1990;34:440–446. doi: 10.1111/j.1399-6576.1990.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson A. ‘Breathe Without Pain’: Clinical Ventilatory Regulation. Linköping University Medical Dissertation. 1992. no. 349,
- 27.Bouillon T, Schmidt C, Garstka G, et al. Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of alfentanil. Anesthesiology. 1999;91:144–155. doi: 10.1097/00000542-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Ederoth P, Tunblad K, Bouw R, et al. Blood–brain barrier transport of morphine in patients with severe brain trauma. Br J Clin Pharmacol. 2004;57:427–435. doi: 10.1046/j.1365-2125.2003.02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penson RT, Joel SP, Bakhshi K, Clark SJ, Langford RM, Slevin ML. Randomized placebo-controlled trial of the activity the morphine glucuronides. Clin Pharmacol Ther. 2000;68:667–676. doi: 10.1067/mcp.2000.111934. [DOI] [PubMed] [Google Scholar]
- 30.Meineke I, Freudenthaler S, Hofmann U, et al. Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br J Clin Pharmacol. 2002;54:592–603. doi: 10.1046/j.1365-2125.2002.t01-1-01689.x. [DOI] [PubMed] [Google Scholar]
- 31.Heiskanen T, Olkkola KT, Kalso E. Effects of blocking CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacol Ther. 1998;64:603–611. doi: 10.1016/S0009-9236(98)90051-0. [DOI] [PubMed] [Google Scholar]
- 32.Leow KP, Smith MT. The antinociceptive potencies of oxycodone, noroxycodone and morphine after intracerebroventricular administration to rats. Life Sci. 1994;54:1229–1236. doi: 10.1016/0024-3205(94)00849-3. [DOI] [PubMed] [Google Scholar]
- 33.Woodhouse A, Hobbes AFT, Mather LE, Gibson M. A comparison of morphine, pethidine and fentanyl in the postsurgical patient-controlled analgesia environment. Pain. 1996;64:115–121. doi: 10.1016/0304-3959(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 34.Woodhouse A, Ward ME, Mather LE. Intra-subject variability in patient-controlled analgesia (PCA): is the patient equally satisfied with morphine, pethidine and fentanyl? Pain. 1999;80:545–553. doi: 10.1016/S0304-3959(98)00247-4. [DOI] [PubMed] [Google Scholar]
- 35.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–223. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- 36.Milne RW, Nation RL, Somogyi AA. The disposition of morphine and its 3- and 6-glucuronide metabolites in humans and animals, and the importance of the metabolites to the pharmacological effects of morphine. Drug Metab Rev. 1996;28:345–472. doi: 10.3109/03602539608994011. [DOI] [PubMed] [Google Scholar]
- 37.Leow KP, Cramond T, Smith MT. Pharmacokinetics and pharmacodynamics of oxycodone when given intravenously and rectally to adult patients with cancer pain. Anaesth Analg. 1995;80:296–302. doi: 10.1097/00000539-199502000-00016. [DOI] [PubMed] [Google Scholar]