Abstract
Children are not small adults. However, the main thesis of this review will be that children's responses to drugs have much in common with the responses in adults and indeed in other mammals. Often, it is assumed that drug effects differ in children but in reality this perception often arises because the drugs have not been adequately studied in paediatric populations of different ages and with different diseases. There may also be difficulties in measuring small but significant effects because the outcome measures are more difficult to assess in children. In some cases, stage of development can alter the action of, and response to, a drug – a truly age-dependent difference in pharmacodynamics. This may be true of both the desired action and adverse events. Examples are given. Programming by drugs is also a phenomenon almost exclusive to early life, i.e. permanent effects result from a stimulus applied at a sensitive point in development (‘critical window’), often in fetal or neonatal life. Again, examples are discussed. Different pathophysiology, different disease variants, different pharmacodynamics, different ‘host’ response and different adverse drug reactions can all explain why some drugs behave differently in children. However, we need to explore ways to avoid re-inventing the wheel by determining how data from adult animal and human models can help inform research and practice for children.
Introduction
Children are certainly not small adults. However, the main thesis of this review will be that, generally, children's responses to drugs have much in common with the responses in adults and indeed in other mammals. It is counter-intuitive to think otherwise. Most basic cellular and physiological processes and receptors are common to all mammals, irrespective of age or stage of development. Children have a loop of Henle and a distal collecting tubule just as adults do, and therefore it is not surprising that diuretics work on a child's kidney. The magnitude of the effect may be different but the basic response has more features in common than there are differences. Often, it is assumed that drug effects differ in children but in reality this perception often arises because the drugs have not been adequately studied in paediatric populations of different ages and with different diseases. There may also be difficulties in measuring small but significant effects because the outcome measures are more difficult to assess in children. For example, a study of an antiasthmatic drug in adults might show a 10% benefit in peak expiratory flow rate. In children under 5 years old who cannot perform such objective measures, a 10% difference may be missed using cruder techniques such as symptom diaries. Finally, part of the reason for the perception that pharmacodynamics are different in children is because the pharmacokinetics may be different at different ages. As a result, the same dose kg−1 does not result in the same circulating concentration because the absorption/metabolism/clearance is different (Box 1).
Developmental pharmacokinetics
Slower GI but faster IM absorption in infancy
More body water vs. lipid in early life
Limited protein binding in infants
Larger liver/body weight ratio in infants
Immature enzymes in neonates
Larger brain/body weight ratio and higher blood–brain barrier permeability in younger children
Immature renal function in infants
Pharmacodynamic and Bayes: designing children's drug studies?
This raises the question of whether we can use the great similarities in basic molecular, cellular and organ mechanisms between children and adults to help short-cut the studies required to take a drug from design to cot side? Does this knowledge alter the prior probability? If an angiotensin converting enzyme inhibitor has already shown efficacy in blood pressure reduction in randomized controlled trials involving thousands of adults, can we use Bayesian statistics to help demonstrate efficacy in children and thus limit the number of children recruited unnecessarily? If we know that there is vitamin D deficiency in secondary hyperparathyroidism due to end-stage renal failure in children, can we use this fact and our great understanding of vitamin D metabolism to help design the smallest reliable study of vitamin D treatment in children?
Pharmacodynamic and surrogate endpoints: designing children's drug studies?
If a gastro-protective drug has already shown efficacy in randomized controlled trials involving thousands of adults with duodenal ulcer disease (phase 3 studies), can we use measurement of gastric pH (phase 2 study) to suggest efficacy in children and not undertake equally large randomized controlled trials? This will only be possible for the ‘same’ disease – i.e. duodenal ulcer disease – and not extrapolation to, for example, gastro-oesophageal reflux. Moreover, it will only be plausible for a disease in which we can show the same pathological process, ideally by tissue diagnosis from biopsy samples, not necessarily for a disease which has the same name in adult and paediatric patients. For example, ‘depression’ and ‘asthma’ are very different entities in adults and children and the efficacy of drug treatment is different in adults and children. In contrast, histologically proven coeliac disease responds to a gluten-free diet in both adults and children and histologically proven Crohn's disease responds to steroids in both adults and children. There are, however, exceptions to this generalization. Acute lymphoblastic leukaemia is an objective pathological diagnosis but the response to chemotherapy is much better in adults than in children.
True pharmacodynamic differences in children
In some cases, stage of development can alter the action of, and response to, a drug – a truly age-dependent difference in pharmacodynamics. This may be true of both the desired action and adverse events (Box 2). Furthermore, and exclusively to fetal life and childhood, a drug can also alter development, temporarily or permanently (‘programming’). Two examples of truly age-dependent differences in pharmacodynamics are given (warfarin and ciclosporin) and corticosteroids are used to illustrate the concept of programming.
Examples of age-dependent adverse drug events
Valproate hepatotoxicity increased in young children (with learning difficulties and receiving multiple AEDs)
Thalidomide only causes phocomelia whilst the limb is forming
Grey baby syndrome – chloramphenicol in young children
Tetracyclines only stain developing enamel
Warfarin – an in vivo study
Plasma concentrations of warfarin, vitamin K1 and vitamin K-dependent proteins and International Normalized Ratio (INR) were measured in 38 prepubertal, 15 pubertal and 81 adult patients given long-term warfarin therapy [1]. The prepubertal, pubertal, and adult patients exhibited comparable mean plasma concentrations of warfarin. However, the prepubertal patients showed significantly lower plasma concentrations of protein C and prothrombin fragments 1 and 2 and higher INR and INR dose−1 than the adults. This augmented response to warfarin in children should be taken into account in estimating warfarin doses for children.
Ciclosporin – an in vitro study
Fifty-six subjects ranging in age from 3 months to 39 years were recruited [2]. Peripheral blood monocytes were separated from whole blood and cultured in the presence of ciclosporin. The peripheral blood monocytes of the infants showed a twofold lower peripheral blood monocyte proliferation and sevenfold lower interleukin-2 expression than peripheral blood monocytes from older subjects. Ciclosporin pharmacodynamics in vitro therefore appear to be related to age. This factor, if neglected, may be a source of iatrogenic risk during paediatric immunosuppressive therapy.
Programming
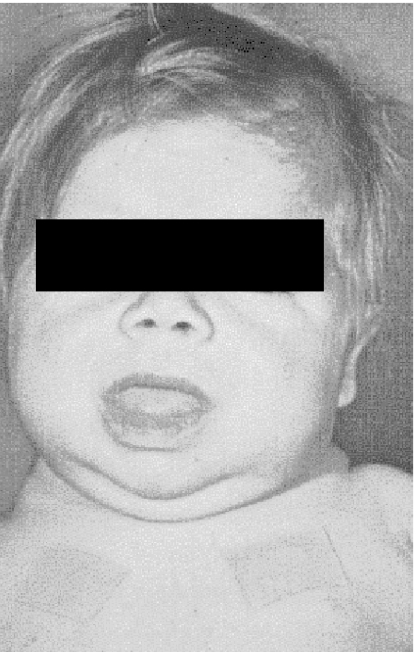
Congenital hypothyroidism, if undetected and untreated, gives rise to lifelong phenotypic changes (see Figure 1) and to permanent learning difficulties. This is an example of programming, i.e. permanent effects result from a stimulus applied at a sensitive point in development (‘critical window’), often in fetal or neonatal life. There is no comparable condition in adulthood because although hypothyroidism occurs in later life, the central nervous system has ceased developing.
Figure 1.
Protruding tongue of infant born with congenital hypothyroidism
Knemometry, a technique which measures changes in lower leg length that can be detected over periods as short as days or even intradaily, shows that inhaled corticosteroids can alter linear bone growth in the short term [3]. However, final height appears unaffected by modest doses [4]. Excessive doses of inhaled steroids can also cause adrenal suppression leading to hyponatraemia and hypoglycaemic seizures, but these effects are reversible on withdrawing the inhaled steroid. Therefore, neither of these is an example of programming due to drugs. However, corticosteroids given to fetal, and perhaps newborn, animals can have profound and permanent effects.
A group of pregnant ewes were given cortisol treatment for 2 days at 27 days of gestation (term approximately 150 days). In the offspring, mean arterial pressure was measured at 1.5 years of age every 10 min for 3 days [5]. Mean arterial blood pressure was significantly higher in the adult female and male offspring at 1.5 years of age when compared with the control offspring. This is evidence that short prenatal exposure to cortisol programmed high blood pressure in the adult female and male offspring of sheep.
Pharmacogenomics
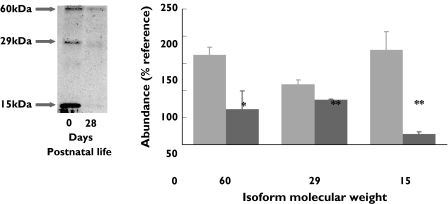
Drugs may have different actions on individuals within an adult population because of genetic polymorphism. Mutations for enzymes or receptors explain fast and slow acetylators and some of the variation in response to β2 agonists, respectively [6]. Much less attention has been given to developmental pharmacogenomics. Some genes are expressed much more in early life than in adults (e.g. the gene for fetal haemoglobin) and such gene switching could give rise to a situation where a drug was effective at one age but not another. In some studies, different patterns of receptor isoforms, arising from post-translational splicing, vary with age (see Figure 2) [7], and again, theoretically, this could give rise to different responses to agonists at different ages.
Figure 2.
The abundance of the different isoforms of prolactin receptor type1 in the perirenal adipose tissue of newborn lambs varies with age. 0 days ( ), 28 days (
), 28 days ( )
)
Summary
The responses of adults and children to many drugs have much in common. We should explore how this can be used to assist parsimonious design of trials in children without losing robustness. Sometimes, there appear to be pharmacodynamic differences when in reality there are pharmacokinetic differences or difficulties in measuring the effect.
There are particular examples which illustrate that the response can differ markedly between adults and children:
Different pathophysiology, e.g. surfactant deficiency.
Different variant of disease, e.g. migraine, epilepsy.
Different pharmacodynamics, e.g. ciclosporin.
Different ‘host’ response, e.g. pneumonia, leukaemia.
Different adverse drug reactions, e.g. thalidomide and tetracyclines.
Acknowledgments
Competing interests: None declared.
References
- 1.Takahashi H, Ishikawa S, Nomoto S, Nishigaki Y, Ando F, Kashima T, Kimura S, Kanamori M, Echizen H. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther. 2000;68:541–55. doi: 10.1067/mcp.2000.110977. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JD, Kearns GL. Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Ther. 1999;66:66–75. doi: 10.1016/S0009-9236(99)70055-X. [DOI] [PubMed] [Google Scholar]
- 3.MacKenzie C. Effects of inhaled corticosteroids on growth. J Allergy Clin Immunol. 1998;101:451–5. doi: 10.1016/s0091-6749(98)70158-7. [DOI] [PubMed] [Google Scholar]
- 4.Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064–9. doi: 10.1056/NEJM200010123431502. [DOI] [PubMed] [Google Scholar]
- 5.Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002;16:1017–26. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- 6.Palmer LJ, Silverman ES, Weiss ST, Drazen JM. Pharmacogenetics of asthma. Am J Respir Crit Care Med. 2002;165:861–6. doi: 10.1164/ajrccm.165.7.2109096. [DOI] [PubMed] [Google Scholar]
- 7.Pearce S, Budge H, Mostyn A, Genever E, Webb R, Ingleton P, Walker AM, Symonds ME, Stephenson T. Prolactin, the prolactin receptor and uncoupling protein abundance and function in adipose tissue during development in young sheep. J Endocrinol. 2005;184:351–9. doi: 10.1677/joe.1.05732. [DOI] [PubMed] [Google Scholar]