Abstract
Introduction
Most of the drugs on the market are originally developed for adults and dosage selection is based on an optimal balance between clinical efficacy and safety. The aphorism ‘children are not small adults’ not only holds true for the selection of suitable drugs and dosages for use in children but also their susceptibility to adverse drug reactions [1]. Since children may not be subject to dose escalation studies similar to those carried out in the adult population, some initial estimation of dose in paediatrics should be obtained via extrapolation approaches. However, following such an exercise, well-conducted PK-PD or PK studies will still be needed to determine the most appropriate doses for neonates, infants, children and adolescents.
Pharmacokinetic-pharmacodynamic modelling to estimate dose in children
Most of the drugs on the market are originally developed for adults and dosage selection is based on an optimal balance between clinical efficacy and safety. The aphorism ‘children are not small adults’ holds true not only for the selection of suitable drugs and dosages for use in children but also for their susceptibility to adverse drug reactions [1]. Since children may not be subject to dose escalation studies similar to those carried out in the adult population, some initial estimation of dose in paediatrics should be obtained via extrapolation approaches. However, following such an exercise, well-conducted pharmacokinetic-pharmacodynamic (PK-PD) or PK studies will still be needed to determine the most appropriate doses for neonates, infants, children and adolescents.
There are two general approaches to conducting PK-PD studies. The classical approach, where a large number of measurements are taken at fixed times from a small number of subjects, and the sparse data sampling or population pharmacokinetic (POPPK) approach, where small numbers of measurements are taken at random times from a large heterogeneous group of subject [2, 3].
POPPK allows the estimation of population and individual parameters as well as intra- and intersubject variability and also the effects of predefined covariates. The decision on which method to use depends on the question to be answered, the availability of patients likely to be recruited for the study, and the practical and ethical problems in obtaining blood samples. The POPPK approach has a number of attractions for studying PK-PD in children: it is less invasive and can thus be considered as more ethical in this age group, and blood sampling times are flexible and can be taken to cause the least inconvenience to the patient and their clinical care. For the purpose of this report, two examples are given to illustrate the application of these approaches to PK-PD determination in the paediatric population. First, a sparse data sampling study of oral midazolam and its 1-hydroxy metabolite for preoperative sedative in children [4], and second, a classical PK-PD study of betaine in the treatment of homocystinuria [5].
Oral midazolam is widely used for preoperative sedation in children. The aim of this study was to investigate the pharmacokinetics and pharmacodyamics (sedation score) of both midazolam (MDZ) and its active metabolite 1-hydroxy-midazolam (1OHMDZ). Only two blood samples were collected at random times from 45 children (age 9 months to 12 years), prior to anaesthetic induction and at end of the surgical procedure. The study was designed around the clinical procedure, as children already had a cannula in situ for collecting blood samples and were asleep or sedated. A simple and practical sedation score (1 = awake, 2 = drowsy/asleep) was recorded at the same time as the first blood sample. The population-PK software P-Pharm was used to analyse the MDZ and 1-OHMDZ data and included the effects of a number of covariates including age, weight, sex and metabolic ratio (1-OHMDZ/MDZ). The PK-PD modelling of the sedation score in relation to plasma MDZ and 1-OHMDZ was carried out using logistic regression analysis.
Despite large variations between individual patients, predicted plasma MDZ and 1-OHMDZ concentrations from the final POPPK model were very close to the actual data. The best PK-PD model included both MDZ and 1-OHMDZ as active moieties and predicted correct sedation scores in 86% of cases. 1-OHMDZ has approximately 50% of the activity of MDZ and can compensate at least in part for the decreased effect of the parent compound due to its increased metabolism in young children. The POPPK results regarding the sedative effects of 1-OHMDZ were consistent with classical PK adult studies [6]. The most important observation was that a median dose level of 0.5 mg kg−1 MDZ resulted in an odds ratio of 4 in favour of score 2 vs. 1, and suggested that a 50% increase in dose would be necessary to achieve sedation in almost all subjects. However, the authors emphasize that the safety of this dose increase would have to be further evaluated.
The second study used a classical design and analysis to investigate the PK and PD of betaine in the treatment of homocystinuria due to cystathionine β-synthase deficiency This is a rare metabolic disease with an incidence of 1 in 300 000 where it would be difficult to recruit sufficient patients to perform a large-scale study. The PK of betaine and its effects on suppressing plasma homocysteine concentration was investigated over a 24-h period in six patients aged 6–17 years. An indirect response model was used to describe the PD effect. Simulations were carried out with the aim of optimizing the betaine dosage regimen. PK-PD simulations indicated minimal benefit from exceeding a twice-daily dosing schedule and a 150 mg kg−1 day−1 dose of betaine (Figure 1).
Figure 1.
Representative plot showing the combined effects of dose frequency and total daily dose on the overall 24-h reduction in plasma homocysteine concentration
The betaine study is an example of a clinical trial simulation; such an approach is being increasingly used in the drug development process in an effort to reduce the number of studies necessary [7]. In the absence of such simulation approaches, recruiting adequate numbers for studying different dosage regimens in rare paediatric diseases would point to the need for national/international collaborations to gain critical mass for PK-PD studies This will require a significant investment in both time and resources.
Regulatory views on PKPD studies
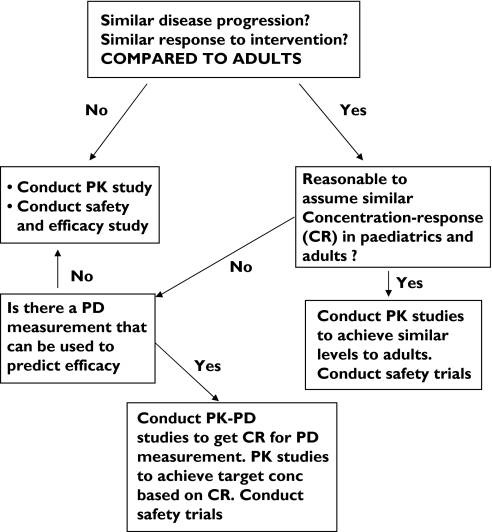
In order to rationalize this whole process, a decision tree has been designed by the Centre for Drug Evaluation and Research (CDER) at the Food and Drug Administration to direct the regulatory decision on the type of study necessary to bridge the knowledge gap between adults’ efficacy and safety data and children [8] (Figure 2).
Figure 2.
Paediatric drug development decision tree for types of PK-PD studies required in children (Center for Drug Evaluation and Research)
The decision tree recommends different types of paediatric studies based on existing knowledge of both the disease and PK-PD of the drug in adults. Where the PK-PD relationship of a drug is similar between adults and children or the PK-PD relationship can be determined then, only PK studies and safety studies are recommended for the bridging and dose determination. This approach is designed to minimize the cost and time required for developing a drug in the paediatric population. The decision process hinges around the definition of ‘similar disease progression’ and ‘similar concentration–response’ between adults and children. For drugs such as antibiotics this is likely to be similar, for certain drugs, e.g. warfarin and cyclosporin, there is published evidence that the PK-PD is different at certain ages, for the majority of drugs the decision is a matter of consensus. A summary of some of the available recent evidence comparing concentration–response between adults and children is shown in Table 1.
Table 1.
Summary of some of the recently reported PK-PD studies undertaken in children
| Drug | Age range | Numberof subjects | Age related change inconcentration response (CR) | Study |
|---|---|---|---|---|
| Bumetanide | Infants, children, young adults | 9 | No detected difference in CR with age | Marshall et al. [9] |
| Cyclosporin | 3 months to 39 years | 56 | Increased CR effect in <1 to 4 age group | Marshall et al. [10] |
| Lansoprazole | 18 days to 14 years | 40 | Increased antisecretory effect in infants <6 months | Tran et al. [11] |
| Midazolam | Pre term 29 weeks | 31 | Decreased CR (sedation response) | de Wildt et al. [12] |
| Mivacurium | 3–6 years, 10–14 years | 10, 10 | No major differences in PK-PD | Stergaard et al. [13] |
| Nizatidine | 5 days to 50 years | 93 | Possible greater CR effect in children? | Abdel-Rahman et al. [14] |
| Ranitidine | 4–11 years | 29 | No major differences in PK-PD | Orenstein et al. [15] |
| Rocuronium | Infants, children, adult | 14, 23, 21 | Greater CR effect in infants compared with children | Saldien et al. [16] |
| Sotolol for SVT | 0.03–41 years | 81 | Increased CR (QTc interval prolongation) in neonates | Laer et al. [17] |
| Warfarin | 1–11 years, 12–18 years, 37–76 years | 38, 15, 81 | Increased CR effect (INR/dose) in 1–11 age group | Takahashi et al.[18] |
The available information gives a number of pointers where caution is needed in extrapolating the concentration–response relationship:
Neonates and infants are particularly difficult groups in which to justify the use of bridging studies because of the rapid developmental changes.
Drugs acting on the developing immune haematopoietic and central nervous systems.
Although much has been achieved to date in terms of our understanding of factors determining PK in children, many gaps still exist in our knowledge of PK-PD across this age group.
New advances in mechanistic and physiologically based pharmacokinetic modelling in paediatrics
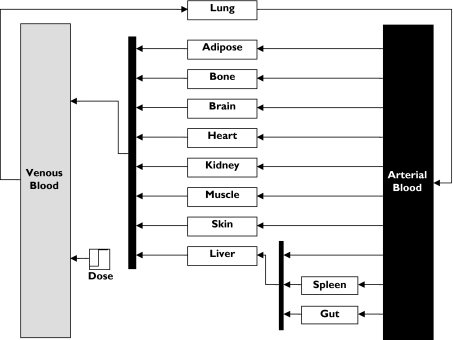
In the developing child there are rapid changes occurring in terms of organ maturation [19], changes in body composition [20] and the ontogeny of drug elimination pathways [21]. All of these changes have a profound effect on the PK of drugs across the paediatric age range. Because of the nonlinear (even nonmonotonic) nature of these changes, simple allometric scaling methods based on body weight or body surface area often fail in the prediction of drug dosage, especially in neonates and infants [22]. Assuming that the PD is similar in paediatrics (see previous section), creating a comparable PK profile requires a more logical approach based on the development of paediatric physiologically based pharmacokinetic (PBPK) models. PBPK models map the complex mechanistic drug movements in the body to a physiologically realistic structure. A PBPK model requires detailed physiological data (Figure 3). However, once the model is built, many input parameters which are drug specific can be obtained from in vitro studies.
Figure 3.
Example of a whole body Physiologically based Pharmacokinetic model
Some models use a combination of classical PK and PBPK models where some components (e.g. CLu) are defined around physiologically based parameters.
PBPK models have been applied to the paediatric population in toxicokinetics to assess both the dose of inhaled organic chemicals in children [23] and the proportional dose of ingested xenobiotics by the mother that the fetus and neonate will receive [24]. Such models, if combined with information on ontogeny of metabolism and excretion mechanisms, can allow predictions to be made on circulating plasma concentrations of xenobiotics as well as dosimetry at the individual organ and tissue level. More recently, PBPK models have been used to predict the PK of caffeine and theophylline in neonates and adults [25]. Although the interindividual variability of model parameters was not incorporated in these models, they could predict the large differences in half-life and clearance between neonates and adults.
Drug metabolism models predicting the hepatic elimination of drugs from in vitro data have been used frequently within the last 10 years [26, 27] but without taking into account any population variability in enzyme abundance and activity. The latter has been a key feature in the design of the Simcyp algorithms.
These algorithms incorporate genetic, physiological, demographic and clinical attributes of patient populations pertinent to in vitro–in vivo extrapolation of xenobiotics into libraries that can be used for automated prediction of drug CLu and drug–drug interactions. The Simcyp model has been successfully applied to the prediction of a number of drug–drug interactions [28–30] and also CLu in adults [31] and children from 2 years of age [32]. The unique feature of the model is that true population variability is incorporated into the model to enable estimates not only of the mean value for a CLu or fold interaction but also of population extremes.
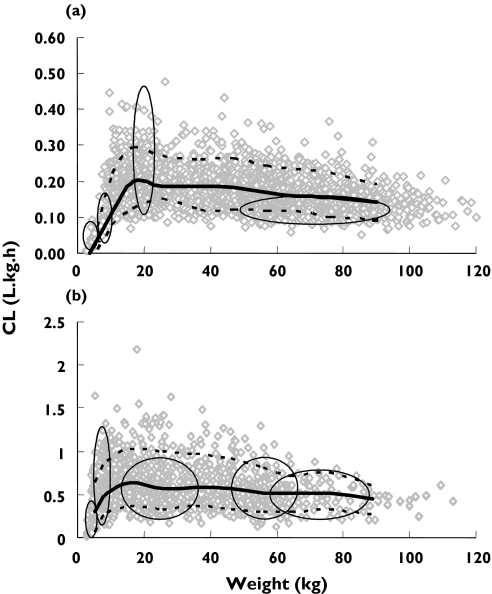
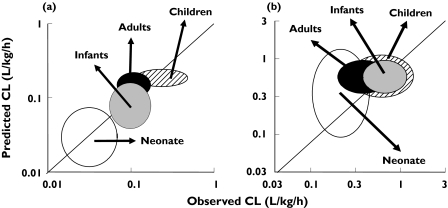
The physiological nature of model parameters means that age-related differences in biological components can be incorporated to simulate paediatric PK from birth onwards. The paediatric PBPK model has been shown to be superior to allometric scaling in the prediction of CLu values for a number of drugs, especially in neonates and infants [21]. Recently the model has been applied to the prediction of CLu for 10 drugs used in children [33]. Simulations were performed in 2000 virtual paediatric patients aged 0–18 years and the predicted CLu for specific age bands compared with actual values from in vivo studies available from the literature. As examples, the results for the age-related changes in oral caffeine and i.v. midazolam CLu are shown in Figure 4a,b. Corresponding observed vs. predicted caffine and midazolam CLu are shown in Figure 5a,b, where the proximity of the centre of the ellipses to the line of unity and circularity of the ellipses are indicative of good predictions of the median values and variability, respectively. Overall, there was close agreement between the observed and predicted CLu values across the age bands so that 70%, 89%, 89% and 94% of predictions for neonates, infants, children and adolescents/adults, respectively, were within twofold of the observed values. Corresponding results for predicted variability were 70%, 67%, 63% and 65% of predicted variability within twofold.
Figure 4.
Changes in caffeine CLu (1a) and midazolam CLu (1b) with body weight (predicted CLu: median = solid line and 95% CI = dashed line; observed CLu= ellipses)
Figure 5.
Concordance between predicted & observed caffeine CLu values (a) and midazolam CLu values (b). Variability (±95% CI) is indicated by the height and width of the ellipses
The next phase in validating models such as Simcyp is to form collaborative work with the pharmaceutical industry in the prediction of CLu for new drugs in children using prospective ‘blind’ studies. While in silico predictions will not replace clinical studies for new drugs, their successful application would provide the best scientific approach for decision making in regard to first time dose in children and as an aid to the design of such studies. Clinical studies under these circumstances become a ‘confirmatory step’ in paediatric drug development, as opposed to the current practice of using these studies as an ‘exploratory step’[34].
The development of paediatric PBPK models such as Simcyp Paediatric reveal areas where more research is required on both the scientific and clinical level. For example, the ‘milligrams of microsomal protein per gram of liver’ value has been determined in adults [35] but not in children. The ontogeny of a number of the hepatic CYP enzymes has been determined in only one or two studies where the number of livers is small; further studies are required both to validate existing data and to fill in some of the age band gaps. For many drugs, clinical pharmacokinetic studies against which to compare data are either very small scale or lacking completely.
Expansion of current models to include a facility for retrograde modelling, whereby with a prior knowledge of the relative contribution of individual CYPs the CLu in paediatrics can be predicted from adult CLu values, would be of practical use. Also, the development of a paediatric disposition model (see Figure 3) will allow prediction of the volume of distribution of drugs in children, with implications for determining first dose rather than just steady-state dose for this age group. The disposition model requires not only information on relative changes in organ and tissue size with age but also their composition. Much of this information has been collated in the ICRP reference main database [36], although in certain instances it is very sparse.
Both the PK-PD and virtual PBPK modelling approaches have real application in the determination of optimal doses in children. In both areas a great deal of research remains to be done. The future challenge is to establish a co-ordinated network of research excellence involving academia, the pharmaceutical industry and government agencies to address both the scientific and clinical gaps in our existing knowledge.
Acknowledgments
Competing interests: None declared.
References
- 1.Johnson TN. The development of drug metabolising enzymes and their influence on the susceptibility to adverse drug reactions in children. Toxicology. 2003;192:37–48. doi: 10.1016/s0300-483x(03)00249-x. [DOI] [PubMed] [Google Scholar]
- 2.Breant V, Charpiat B, Sab JM, Maire P, Jelliffe RW. How many patients and blood levels are necessary for population pharmacokinetic analysis? Eur J Clin Pharmacol. 1996;51:283–8. doi: 10.1007/s002280050199. [DOI] [PubMed] [Google Scholar]
- 3.Lee PID. Design and power of a population pharmacokinetic study. Pharm Res. 2001;18:75–82. doi: 10.1023/a:1011030827847. [DOI] [PubMed] [Google Scholar]
- 4.Johnson TN, Rostami-Hodjegan A, Goddard JM, Tanner MS, Tucker GT. Contribution of midazolam and its 1-hydroxy metabolite to pre-operative sedation in children: a pharmacokinetic-pharmacodynamic analysis. Br J Anaesth. 2002;89:428–37. [PubMed] [Google Scholar]
- 5.Matthews A, Johnson TN, Rostami-Hodjegan A, Chakrapani A, Wraith JE, Moat SJ, Bonham JR, Tucker GT. An indirect response model of homocysteine suppression by betaine: optimising the dosage regimen of betaine in homocystinuria. Br J Clin Pharmacol. 2002;54:140–6. doi: 10.1046/j.1365-2125.2002.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther. 1992;51:715–28. doi: 10.1038/clpt.1992.84. [DOI] [PubMed] [Google Scholar]
- 7.Bonate PL. Clinical trial simulation in drug development. Pharm Res. 2000;17:252–6. doi: 10.1023/a:1007548719885. [DOI] [PubMed] [Google Scholar]
- 8.CDER. Pediatric Decision Tree. [27 April 2004]; http://www.fda.gov/cder/mapp/4000.4.pdf.
- 9.Marshall JD, Wells TG, Letzig L, Kearns GL. Pharmacokinetics and pharmacodynamics of bumetanide in critically ill pediatric patients. J Clin Pharmacol. 1998;38:994–1002. doi: 10.1177/009127009803801102. [DOI] [PubMed] [Google Scholar]
- 10.Marshall JD, Kearns GL. Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Ther. 1999;66:66–75. doi: 10.1016/S0009-9236(99)70055-X. [DOI] [PubMed] [Google Scholar]
- 11.Tran A, Rey E, Pons G, Pariente-Khayat A, d’Athis P, Sallerin V, Dupont C. Pharmacokinetic-pharmacodynamic study of oral lansoprazole in children. Clin Pharmacol Ther. 2002;71:359–67. doi: 10.1067/mcp.2002.122472. [DOI] [PubMed] [Google Scholar]
- 12.de Wildt SN, Kearns GL, Sie SD, Hop WCJ, van den Anker JN. PhD Thesis, Developmental aspects of midazolam metabolism. University of Rotterdam; 2001. Pharmacodynamics of intravenous and oral midazolam in preterm infants. [Google Scholar]
- 13.Stergaard D, Gätke MR, Berg H, Rasmussen SN, Viby-Mogensen J. The pharmacodynamics and pharmacokinetics of mivacurium in children. Acta Anaesthesiol Scand. 2002;46:512–8. doi: 10.1034/j.1399-6576.2002.460507.x. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Rahman SM, Johnson FK, Connor JD, Staiano A, Dupont C, Tolia V, Winter H, Gauthier-Dubois G, Kearns GL. Developmental pharmacokinetics and pharmacodynamics of nizatidine. J Pediatr Gastroenterol Nutr. 2004;38:442–51. doi: 10.1097/00005176-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Orenstein SR, Blumer JL, Faessel HM, McGuire JA, Fung K, Li BUK, Lavine JE, Grunow JE, Treem WR, Ciociola AA. Ranitidine, 75 mg, over-the-counter dose: pharmacokinetic and pharmacodynamic effects in children with symptoms of gastro- oesophageal reflux. Aliment Pharmacol Ther. 2002;16:899–907. doi: 10.1046/j.1365-2036.2002.01243.x. [DOI] [PubMed] [Google Scholar]
- 16.Saldien V, Vermeyen KM, Wuyts FL. Target-controlled infusion of rocuronium in infants, children, and adults: a comparison of the pharmacokinetic and pharmacodynamic relationship. Anesth Analg. 2003;97:44–9. doi: 10.1213/01.ane.0000066262.32103.60. [DOI] [PubMed] [Google Scholar]
- 17.Lear S, Elshoff JP, Meibohm B, Weil J, Mir TS. Development of a Pediatric Dosing Regimen for Sotolol. 9th European Society for Developmental Pharmacology meeting; 2004; Marberg, Germany. [Google Scholar]
- 18.Takahashi H, Ishikawa S, Nomoto S, Nishigaki Y, Ando F, Kashima T, Kimura S, Kanamori M, Echizen H. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther. 2000;68:541–55. doi: 10.1067/mcp.2000.110977. [DOI] [PubMed] [Google Scholar]
- 19.Price PS, Conolly RB, Chaisson CF, Gross EA, Young JS, Mathis ET, Tedder DR. Modeling interindividual variation in physiological factors used in PBPK models of humans. Crit Rev Toxicol. 2003;33:469–503. [PubMed] [Google Scholar]
- 20.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649–80. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 21.Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinetics. 2002;41:959–98. doi: 10.2165/00003088-200241120-00003. [DOI] [PubMed] [Google Scholar]
- 22.Johnson TN, Rostami-Hodjegan A, Tucker GT. A comparison of methods to predict drug clearance in neonates, infants and children. Br J Clin Pharmacol. 2004;57:677–8. [Google Scholar]
- 23.Price K, Haddad S, Krishnan K. Physiological modelling of age specific changes in PK of organic chemicals in children. J Toxicol Environ Health Part A. 2003;66:417–33. doi: 10.1080/15287390306450. [DOI] [PubMed] [Google Scholar]
- 24.Gentry PR, Covington TR, Clewell HJ., III Evaluation of the potential impact of PK differences on tissue dosimetry in offspring during pregnancy and lactation. Reg Toxicol Pharmacol. 2003;38:1–16. doi: 10.1016/s0273-2300(03)00047-3. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, Kozlak M, Smolenski S, Golbe R. PBPK modelling of caffeine and theophylline in neonates and adults: implications for assessing children's risks from environmental agents. J Toxicol Environ Health Part A. 2004;67:297–329. doi: 10.1080/15287390490273550. [DOI] [PubMed] [Google Scholar]
- 26.Houston JB, Carlile DJ. Prediction of hepatic clearance from microsomes, hepatocytes, and liver slices. Drug Metab Rev. 1997;29:891–922. doi: 10.3109/03602539709002237. [DOI] [PubMed] [Google Scholar]
- 27.Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, Rance DJ, Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283:46–58. [PubMed] [Google Scholar]
- 28.Tooley A, Rostami-Hodjegan A, Lennard MS, Tucker GT. Acute inhibition of methadone metabolism by ritonavir: projection of interaindividual variability from in vitro data. Br J Clin Pharmacol. 1999;48:883P–884P. [Google Scholar]
- 29.Yang JS, Rostami-Hodjegan A, Tucker GT. Prediction of fluconazole interaction with midazolam and triazolam: incorporating population variability. Br J Clin Pharmacol. 2001;52:484P. [Google Scholar]
- 30.Yang JS, Rostami-Hodjegan A, Tucker GT. Prediction of ritonavir interaction with sildenafil (Viagra): incorporating population variability. Br J Clin Pharmacol. 2002;53:483P. [Google Scholar]
- 31.Proctor NJ, Smith CY, Rostami-Hodjegan A, Tucker GT. Population clearance prediction of 13 drugs using SIMCYP™. Pharmacologist. 2002;44(Suppl. 1):LB87. [Google Scholar]
- 32.Johnson TN, Sabzghabaee A, Rostami-Hodjegan A, Tucker GT. Prediction of age related changes in midazolam clearance in children using SIMCYP. Br J Clin Pharmacol. 2003;55:432–3P. [Google Scholar]
- 33.Johnson TN, Tucker GT, Rostami-Hodjegan A. Prediction of Drug Clearance from in vitro Data for 12 Drugs in Neonates, Infants and Children. Proceeding of the 7th International ISSX Meeting; 2004; Vancouver, Canada. [Google Scholar]
- 34.Sheiner LB. Learning versus confirming in clinical drug development. Clin Pharmacol Ther. 1997;6:275–91. doi: 10.1016/S0009-9236(97)90160-0. [DOI] [PubMed] [Google Scholar]
- 35.Wilson ZE, Rostami-Hodjegan A, Burn JL, Tooley A, Boyle J, Ellis SW, Tucker GT. Inter-individual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br J Clin Pharmacol. 2003;56:433–40. doi: 10.1046/j.1365-2125.2003.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Commission on Radiological Protection. Report of the Task Force on Reference Man. Oxford: Pergamon Press; 1975. [Google Scholar]