Abstract
Aims
Approximately 80% of uracil is excreted as β-alanine, ammonia and CO2 via three sequential reactions. The activity of the first enzyme in this scheme, dihydropyrimidine dehydrogenase (DPD), is reported to be the key determinant of the cytotoxicity and side-effects of 5-fluorouracil. The aim of the present study was to re-evaluate the pharmacokinetics of uracil and its metabolites using a sensitive assay and based on a newly developed, physiologically based pharmacokinetic (PBPK) model.
Methods
[2–13C]Uracil was orally administrated to 12 healthy males at escalating doses of 50, 100 and 200 mg, and the concentrations of [2–13C]uracil, [2–13C]5,6-dihydrouracil and β-ureidopropionic acid (ureido-13C) in plasma and urine and 13CO2 in breath were measured by liquid chromatography–tandem mass spectrometry and gas chromatograph–isotope ratio mass spectrometry, respectively.
Results
The pharmacokinetics of [2–13C]uracil were nonlinear. The elimination half-life of [2–13C]5,6-dihydrouracil was 0.9–1.4 h, whereas that of [2–13C]uracil was 0.2–0.3 h. The AUC of [2–13C]5,6-dihydrouracil was 1.9–3.1 times greater than that of [2–13C]uracil, whereas that of ureido-13C was 0.13–0.23 times smaller. The pharamacokinetics of 13CO2 in expired air were linear and the recovery of 13CO2 was approximately 80% of the dose. The renal clearance of [2–13C]uracil was negligible.
Conclusion
A PBPK model to describe 13CO2 exhalation after orally administered [2–13C]uracil was successfully developed. Using [2–13C]uracil as a probe, this model could be useful in identifying DPD-deficient patients at risk of 5-fuorouracil toxicity.
Keywords: 13C-, dihydropyrimidine dehydrogenase (DPD), physiologically based pharmacokinetic (PBPK) model, uracil
Introduction
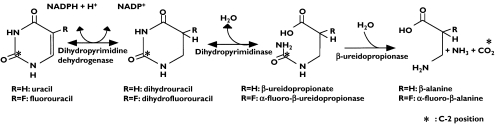
Pyrimidine and purine, which are present endogenously in nucleic acids, nucleotides and their derivatives, display a wide range of physiological functions. Since the catabolism and anabolism of pyrimidines are inextricably linked, their biological fate is complex. Uracil, a pyrimidine base, is metabolized to β-alanine, ammonia and CO2 via three sequential reactions [1, 2] (Figure 1). Uracil is first reduced to dihydrouracil by dihydropyrimidine dehydrogenase (DPD), then hydrolysed to β-ureidopropionic acid by dihydropyrimidinase (DHPase), and finally decarbamoylated to β-alanine by β-ureidopropionase (UP). Of these enzymes, DPD is considered to represent the rate-limiting step [3, 4].
Figure 1.
The metabolism of uracil and 5-fluorouracil in humans
5-Fluorouracil (5-FU) is an anticancer agent in which the hydrogen atom at the C-5 position of uracil is substituted by fluorine (Figure 1). Since the structure of 5-FU is analogous to that of uracil, it is biotransformed to putative, biologically active metabolites, 5-fluoro-2′-deoxyuridine-5′-monophosphate or 5-fluorouridine-5′-triphosphate, by the same anabolic pathway as that of uracil [5, 6]. 5-FU is metabolized by conversion to biologically inactive metabolites by the same enzymes that metabolize uracil [7–10]. When 5-FU is given to patients with genetic DPD deficiency or those taking drugs which inhibit DPD activity, blood concentrations of the drug are markedly elevated, resulting in serious adverse effects [11–14]. The use of diagnostic methods to detect pyrimidine metabolic disorders [15–17] at the start of chemotherapeutic treatment would prevent the development of adverse effects with 5-FU and its prodrugs.
We previously developed a diagnostic product (UBIT®) for Helicobacter pylori infection which measures in vivo urease activity using expired 13CO2 after oral administration of 13C-urea [18–20]. Extending this work, we have developed a new method for diagnosing pyrimidine metabolic disorders using [2–13C]uracil (13C-uracil), prepared by labelling the C-2 position of uracil with 13C, a stable isotope of 12C (Figure 1). We have already applied this method to dogs [21] to show that expired 13CO2 is a good marker of hepatic DPD activity in the enzyme-deficient model. To gain further understanding of pyrimidine catabolism, the pharmacokinetics of 13C-uracil was studied following oral administration under fasting conditions to healthy subjects at escalating doses.
Methods
Subjects
The study protocol was approved by the Ethics Committee of Juntendo University Hospital, and written informed consent was obtained from each participant before enrolment. The subjects were 12 healthy Japanese males (21–57 years old; 56–90 kg) with normal pyrimidine metabolism as determined by measurement [22] of endogenous pyrimidine and dihydropirimidine in urine. Good general health was confirmed by 12-lead electrocardiogram, medical history and physical examination. The study was designed to evaluate the pharmacokinetic profile of 13C-uracil and its metabolites after single oral administration of 13C-uracil using an open label, single-centre, dose escalation design. The subjects were given 13C-uracil on three occasions at escalating doses of 50, 100 and 200 mg under fasted conditions. The subjects were given 13C-uracil as granules with 100 ml of water at approximately 09.00 h. Food was not permitted from 21.00 h on the evening before dosing to 4 h after dosing. Subjects were in a sitting position for 2 h postdose. The order of dosing was 50, 100 and 200 mg and the wash-out period was set at ≥ 5 days. Blood was taken immediately before and at 10, 20, 30, 40, 50, 60 and 90 min and 2, 4, 6, 8 and 12 h after dosing. Urine samples were collected before and at the periods of 0–2, 2–4, 4–8 and 8–12 h after dosing. Breath samples were collected in a bag (volume 300 ml) before and at 10, 20, 30, 40, 50, 60, 80 and 100 min and 2, 3, 4, 6, 8 and 12 h after dosing.
Chemicals
[2-13C]Uracil, [2-13C]5,6-dihydrouracil, β-ureidopropionic acid (ureido-13C), uracil (13C4, 15N2), 5,6-dihydrouracil (13C4, 15N2) and β-ureidopropionic acid (13C4, 15N2) were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). All other solvents and reagents were of the highest grade available.
Determination of 13C-uracil, 13C-DHU and 13C-UPA in plasma and urine by LC-MS/MS
Plasma concentrations of 13C-uracil and its metabolites (13C-DHU and 13C-UPA) were measured by LC-MS/MS (TSQ7000; Thermo Finnigan, San Jose, CA, USA). Isotope-labelled uracil (13C4, 15N2), 5,6-dihydrouracil (13C4, 15N2) and β-ureidopropionic acid (13C4, 15N2) were used as internal standards. 13C-Uracil and 13C-DHU were extracted from 0.5 ml of plasma using 4 ml of acetonitrile after the addition of 0.5 ml of a saturated aqueous ammonium sulphate solution. Samples were then centrifuged at 2200 g for 10 min. The organic layer was evaporated to dryness, the residue reconstituted in 200 µl of purified water, and a 50-µl aliquot was injected into LC-MS/MS. A Develosil RPAQUEAUS column (5 µm, 2.0 mm i.d. × 150 mm; Nomura Chemical Co., Ltd, Seto, Japan) and a mobile phase of water were used to separate the analytes. 13C-UPA was extracted from 0.2 ml of plasma by solid-phase extraction on silica after deproteinization. After evaporation of the eluate to dryness, the residue was reconstituted in 200 µl of purified water. This solution (30 µl) was injected onto the LC-MS/MS fitted with two columns in series, a Develosil RPAQUEAUS (5 µm, 2.0 mm i.d. × 150 mm) and a Capcell pak SCX UG80 (5 µm, 2.0 mm i.d. × 50 mm; Shiseido Co., Ltd, Tokyo, Japan). The mobile phase was an aqueous solution of 10 m m ammonium formate (pH 3.5). Protonated molecular ions [M+1]+ of the analytes including the internal standard, formed by atmospheric pressure chemical ionization, were fragmented, and the selected product ions were monitored (selected reaction monitoring). The calibration curves were linear over the ranges 5–250 ng ml−1 for 13C-uracil and 13C-DHU and 50–2000 ng ml−1 for 13C-UPA. The percent recoveries of isotope-labelled uracil (13C4, 15N2), 5,6-dihydrouracil (13C4, 15N2) and β-ureidopropionic acid (13C4, 15N2) from human plasma were 83–105%, 56–74% and 23–24%, respectively. The limit of quantification (LOQ), defined as the lowest concentration with a coefficient of variation (CV) of < 20% and accuracy within ± 20%, was 5 ng ml−1 for 13C-uracil and 13C-DHU and 50 ng ml−1 for 13C-UPA. Precision, estimated as CV, was < 15% and accuracy was within ± 15% for the analytes at all concentrations except the LOQ.
Urinary concentrations of each compound were measured using LC-MS/MS. 13C-Uracil and 13C-DHU were extracted from 0.2 ml of urine using 5 ml of ethyl acetate after the addition of 0.1 ml of a saturated aqueous ammonium sulphate solution. The samples were then centrifuged at 1800 g for 10 min. After the organic layer was evaporated to dryness, the residue was reconstituted in 200 µl of purified water and 30 µl of the solution was injected onto the LC-MS/MS. 13C-UPA was extracted from 0.1 ml of the urine by solid-phase extraction on silica after deproteinization. After evaporation of the solid-phase extraction eluate to dryness, the residue was reconstituted in 200 µl of purified water and 30 µl was injected onto the LC-MS/MS. The chromatographic conditions were similar to those for the plasma samples. The calibration curves for these analytes were linear over the range of 0.1–5 µg ml−1, and the LOQ was 0.1 µg ml−1. The percent recoveries of isotope-labelled uracil (13C4, 15N2), 5,6-dihydrouracil (13C4, 15N2) and β-ureidopropionic acid (13C4, 15N2) from human urine were 57–64%, 63–74% and 20–30%, respectively. Precision was < 15% and accuracy was within ± 15% at all concentrations except the LOQ.
Analysis of 13CO2 in expired air by gas chromatography isotope ratio mass spectrometry (IRMS)
13CO2 concentrations in expired air were determined using a gas chromatograph-IRMS (model ABCA-G; PDZ-Europa Ltd, Cheshire, UK). 13CO2/12CO2 ratios were expressed as δ13C value (permil, ‰) relative to the Pee Dee Belemnite Limestone standard, and changes in the δ13C value as Δ13C (‰) were compared with the baseline using the following equations:
where Δ13Ct (‰) is the difference between respiratory δ13Ct measured at time t and baseline δ13C0 following the administration of 13C-uracil.
Pharmacokinetic analysis
Pharmacokinetic parameters for 13C-uracil and its metabolites were calculated using noncompartmental pharmacokinetic analysis (WinNonlin Standard version 3.1; Pharsight Co., Mountain View, CA, USA). Maximum plasma concentration (Cmax), time to Cmax (tmax) and the area under the plasma concentration vs. time curve up to 12 h after administration (AUC12 h) were determined. The apparent terminal-phase slope (λz) was estimated by linear regression of the semilogarithmic curve of plasma concentration vs. time. The terminal elimination half-life (t½) was calculated as 0.693/λz. AUC∞ was calculated by dividing the last measured concentration (Clast) by λz. The apparent total clearance (CL/F) is dose/AUC∞, and the apparent volume of distribution (Vd/F) is CL/F/λz. The cumulative amount excreted into the urine for 12 h (Ae) was used to estimate the renal clearance (CLR) from the expression Ae/AUC12 h. The total amount of 13CO2 recovered in the breath (m) was calculated from 13CO2 excretion curves based on the method of Ghoos et al.[23], in which CO2 production was assumed to be 300 mmol m−2 h−1.
Statistical analysis
The relationships between the dose and the pharmacokinetic parameters (Cmax, AUCt, and AUC∞) were analysed by using a predictive power model based on the equation
Statistical analysis was performed with SAS software, version 8.2 (SAS Institute Japan, Tokyo, Japan).
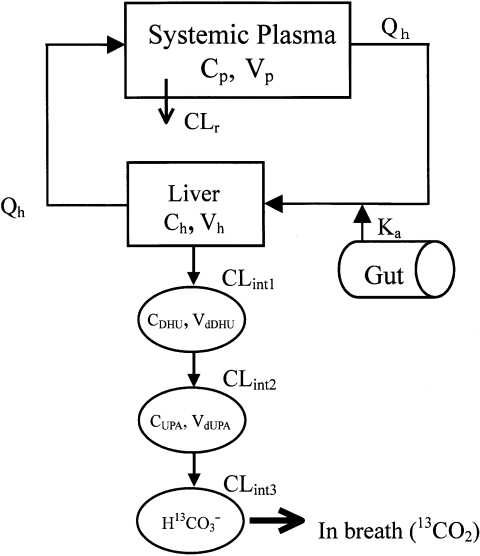
Model development
A physiologically based pharmacokinetic (PBPK) model model (Figure 2) was constructed to describe the time course of plasma concentrations of 13C-uracil and its metabolites, and 13CO2 in expired air. This model incorporates Michaelis–Menten catabolic and first-order degradation processes. The differential equations for the PBPK model were as follows:
Figure 2.
A physiologically based pharmacokinetic model describing the concentration–time profiles for 13C-uracil, its metabolites and 13CO2 in expired air
For 13C-uracil
| (1) |
| (2) |
| (3) |
For 13C-DHU
| (4) |
For 13C-UPA
| (5) |
For 13CO2
| (6) |
| (7) |
where Vh is the volume of the liver; Vp is the volume of distribution in rapidly equilibrating tissues, including the systemic plasma compartment of 13C-uracil; VdDHU and VdUPA are the pseudo-distribution volumes of 13C-DHU and 13C-UPA, respectively; Ch is the concentration of 13C-uracil in liver; Cp,CDHU and CUPA are the plasma concentrations of 13C-uracil, 13C-DHU and 13C-UPA, respectively; QH is the hepatic blood flow rate; Kp is the liver-to-blood concentration ratio of 13C-uracil; CLR is the renal clearance of 13C-uracil; CLint is intrinsic metabolic clearance; Vmax and Km are the maximum rate of 13C-uracil metabolism and the Michaelis–Menten constant, respectively; fp is the unbound fraction of 13C-uracil in plasma; keDHU and keUPA are the degradation rate constants of 13C-DHU and 13C-UPA, respectively; XH13CO3- is the amount of H13CO3−; ke is the excretion rate constant of 13CO2; and P1 and P2 are constants.
These equations are based on the following assumptions:
The gastrointestinal absorption of 13C-uracil follows a first-order process.
13C-Uracil is eliminated by the liver and kidney, and is converted sequentially in the liver to 13C-DHU, 13C-UPA and H13CO3−.
13C-uracil is eliminated via a single irreversible and saturable Michaelis–Menten process.
The elimination of the metabolites of 13C-uracil follows first-order irreversible kinetics.
The elimination of 13C-DHU, 13C-UPA and H13CO3−is described by a single-compartment model.
This assumption can be justified from the following findings: (i) a single-compartment model describes the pharmacokinetics of 13CO2-H13CO3− after co-administration of sodium bicarbonate [24]; (ii) the kinetics of 13CO2 after administration of 13C-compounds are best described by a one-compartment model [25–27].
Equation holds true because Δ13C (‰) is proportional to the amount of H13CO3− in the body [28]. The physiological data used, namely Vh = 1070 ml, QH = 1190 ml min−1 and haematocrit value = 0.55, were obtained from the literature. Kp, fp and Fa were set at 1.0 according to our preliminary experiments (data not shown), and CLR at 120 ml min−1 on the basis of our urinary excretion analysis.
The pharmacokinetic software SAAM II (SAAM Institute Inc., Seattle, WA, USA) was used for nonlinear least squares analysis to fit the parameters Vp, ke, Km and Vmax to the set of plasma concentrations of 13C-uracil for dose-escalation experiments using equations. Using these fixed parameters, the parameters VdDHU, VdUPA, keDHU,keUPA, ke, P1 and P2 were subsequently estimated by SAAM II using equations 4–7.
Results
Pharmacokinetics
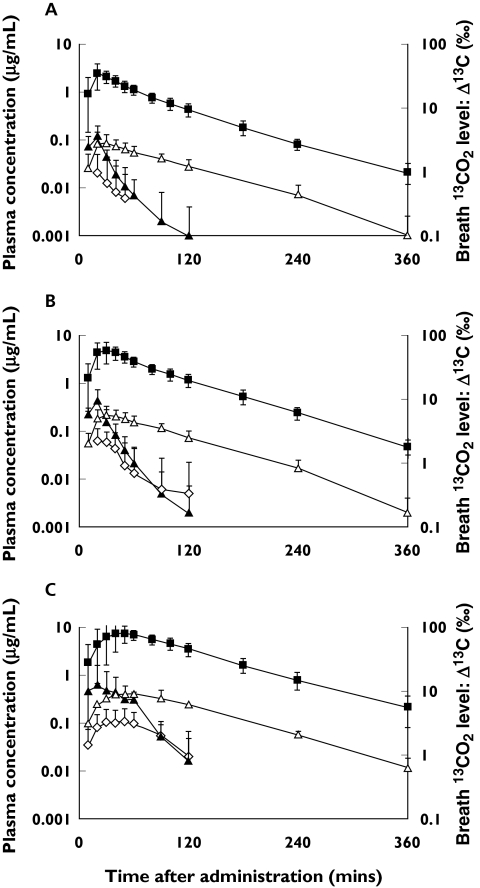
Tables 1, 2 and 3 show the pharmacokinetic parameters for 13C-uracil, 13C-DHU and 13C-UPA in the plasma, respectively, and Tables 4 and 5 the urinary excretion and expiratory 13CO2 excretion data. Figure 3 shows the concentration vs. time curves for 13C-uracil and its metabolites, and the Δ13C in the expired air vs. time curve.
Table 1. Pharmacokinetic parameters for 13C-uracil in plasma.
| Dose | Cmax (µg ml−1) | AUC12 h (µg h ml−1) | AUC∝ (µg h ml−1) | tmax (h) | λz (1 h−1) | t1/2 (h) | CL/F (l h−1) | Vd/F (l) |
|---|---|---|---|---|---|---|---|---|
| 50 mg | 0.127 | 0.047 | 0.053 | 0.36 | 3.66 | 0.26 | 1082 | 466 |
| ±0.083 | ±0.022 | ±0.021 | ±0.10 | ±1.71 | ±0.22 | ±410 | ±500 | |
| 100 mg | 0.534 | 0.165 | 0.170 | 0.39 | 5.25 | 0.21 | 772 | 296 |
| ±0.430 | ±0.109 | ±0.107 | ±0.18 | ±2.92 | ±0.19 | ±368 | ±423 | |
| 200 mg | 1.205 | 0.545 | 0.567 | 0.54 | 2.91 | 0.32 | 464 | 296 |
| ±0.899 | ±0.325 | ±0.312 | ±0.25 | ±1.19 | ±0.26 | ±265 | ±480 |
Values: mean ± SD (n = 12).
Table 2. Pharmacokinetic parameters for 13C-DHU in plasma.
| Dose | Cmax (µg ml−1) | AUC12 h (µg h ml−1) | tmax (h) | λz (1 h−1) | t1/2 (h) |
|---|---|---|---|---|---|
| 50 mg | 0.102 | 0.144 | 0.49 | 0.68 | 1.10 |
| ±0.050 | ±0.048 | ±0.15 | ±0.18 | ±0.34 | |
| 100 mg | 0.251 | 0.378 | 0.56 | 0.78 | 0.91 |
| ±0.094 | ±0.135 | ±0.18 | ±0.11 | ±0.14 | |
| 200 mg | 0.551 | 1.054 | 0.93 | 0.60 | 1.37 |
| ±0.255 | ±0.449 | ±0.50 | ±0.19 | ±0.74 |
Values: mean ± SD (n = 12).
Table 3. Pharmacokinetic parameters for 13C-DHU in plasma.
| Dose | Cmax (µg ml−1) | AUC12 h (µg h ml−1) | tmax (h) | λz (1 h−1) | t1/2 (h) |
|---|---|---|---|---|---|
| 50 mg | 0.023 | 0.006 | 0.46 | – | – |
| ±0.035 | ±0.014 | ±0.16 | – | – | |
| 100 mg | 0.076 | 0.034 | 0.50 | 1.17 | 0.87 |
| ±0.040 | ±0.042 | ±0.19 | ±0.88 | ±0.61 | |
| 200 mg | 0.149 | 0.126 | 0.82 | 1.12 | 1.05 |
| ±0.081 | ±0.105 | ±0.45 | ±0.67 | ±1.05 |
–, Not calculated. Values: mean ± SD (n = 12).
Table 4. Urinary excretion and kinetic parameters for 13C-uracil and its metabolites.
| Substance | Parameter | 50 mg | 100 mg | 200 mg |
|---|---|---|---|---|
| 13C-uracil | Ae (mg) | 0.35 ± 0.19 | 1.21 ± 0.80 | 4.52 ± 2.80 |
| CLR (l h−1) | 6.8 ± 1.6 | 7.2 ± 2.1 | 7.7 ± 1.4 | |
| Excretion (%/dose) | 0.7 ± 0.4 | 1.2 ± 0.8 | 2.3 ± 1.4 | |
| 13C-DHU | Ae (mg) | 0.10 ± 0.05 | 0.28 ± 0.14 | 0.83 ± 0.44 |
| CLR (l h−1) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.3 | |
| Excretion (%/dose) | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 | |
| 13C-UPA | Ae (mg) | 0.21 ± 0.10 | 0.53 ± 0.31 | 1.33 ± 0.91 |
| CLR (l h−1) | 17.1 ± 8.7 | 13.4 ± 6.3 | 9.1 ± 4.1 | |
| Excretion (%/dose) | 0.4 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.4 |
13C-DHU: 5,6-dihydrouracil (2-13C)]13C-UPA,β-ureidopropionic acid (ureido-13C). Values: mean ± SD (n = 12).
Table 5. Expiratory excretion parameters for 13CO2 (Δ13C).
| Dose | Cmax (‰) | AUC12 h (‰ h) | AUC∝ (‰ h) | tmax (h) | λz (1 h−1) | t1/2 (h) | m (% dose−1) |
|---|---|---|---|---|---|---|---|
| 50 mg | 37.8 | 53.3 | 54.2 | 0.43 | 0.59 | 1.27 | 75.5 |
| ±11.7 | ±5.3 | ±5.2 | ±0.15 | ±0.18 | ±0.36 | ±7.4 | |
| 100 mg | 67.9 | 106.4 | 107.8 | 0.54 | 0.56 | 1.41 | 75.9 |
| ±17.3 | ±9.7 | ±9.9 | ±0.19 | ±0.21 | ±0.59 | ±3.3 | |
| 200 mg | 104.8 | 213.8 | 216.2 | 0.89 | 0.55 | 1.34 | 76.4 |
| ±20.8 | ±25.0 | ±24.9 | ±0.44 | ±0.15 | ±0.37 | ±6.1 |
m, Amount of 13CO2 recovered in the breath. Values: mean ± SD (n = 12).
Figure 3.
Plasma concentration–time curves for 13C-uracil and its metabolites, and Δ13C–time curves in expired air after oral administration of 13C-uracil at doses of (A) 50 mg, (B) 100 mg, and (C) 200 mg to 12 healthy males. (▴; 13C-uracil, Δ; 13C-DHU, ◊; 13C-UPA, ▪; 13CO2, mean ± SD, n = 12)
13C-Uracil was absorbed rapidly after oral dosing to attain Cmax within 0.54 h, and then declined rapidly in plasma, with a short half-life of less than 0.32 h. The major metabolite of 13C-uracil in plasma at all doses was 13C-DHU, with the relative ratios of the AUC12 h of 13C-DHU to 13C-uracil at 50, 100 and 200 mg being 3.1, 2.3 and 1.9, respectively. 13C-UPA was a minor metabolite, and the relative ratios of the AUC12 h of 13C-UPA to 13C-uracil at 50, 100 and 200 mg were 0.13, 0.21 and 0.23, respectively. Plasma concentrations of 13C-DHU were higher than those of 13C-uracil from 50 min after administration. At all doses, the elimination half-life of 13C-DHU was much longer than that of 13C-uracil. A predictive power model was used to evaluate the dose-proportionality of Cmax, AUC12 h and AUC∞ values. None of these parameters was dose-proportional over the range of 50–200 mg [the 95% confidence interval (CI) for the slope of the regression line (β) did not include unity]. Nonlinearity in pharmacokinetics was clearly present in 10 out of the 12 subjects, although there was considerable interindividual variability between subjects.
The contribution of renal clearance to the total body clearance was negligible (Table 4).
The Δ13C of 13CO2 in expired air vs. time curve was similar to that of 13C-DHU in plasma (Figure 3). The recovery of 13C in expired air was approximately 80% at each dose, which is in agreement with previous reports [8–10] on the deposition of 5-FU. Cmax was found not to be dose-proportional over the range of 50–200 mg, but AUC∞ and AUC12 h were proportional to dose [the 95% CIs of the slopes (β) for these parameters were 0.93–1.06 and 0.94–1.06].
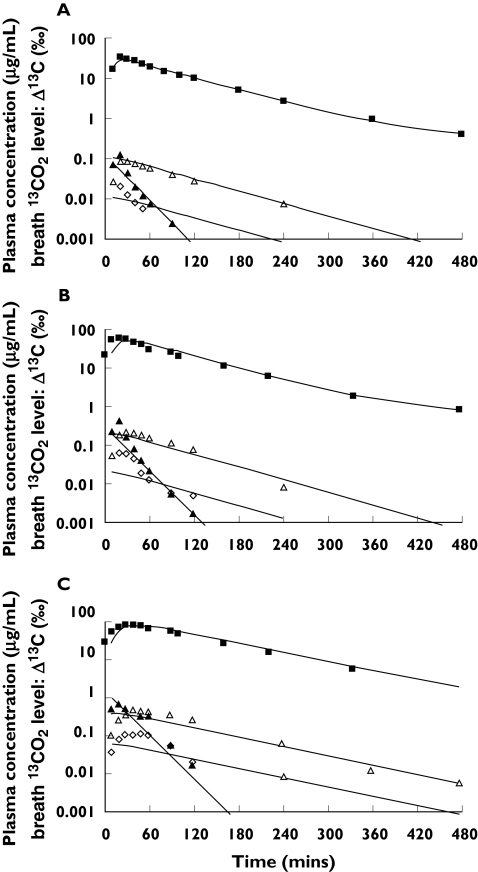
The predicted concentration–time courses of 13C-uracil, 13C-DHU, 13C-UPA and Δ13C (‰) are shown in Figure 4. Satisfactory agreement between the predicted curve and experimental data was obtained. The exception was the relationship for 13C-UPA, which had a higher limit of quantification (50 ng ml−1) than with 13C-uracil and 13C-DHU (5 ng ml−1), thus introducing some uncertainty into the data. The pharmacokinetic parameters estimated by nonlinear least squares regression are listed in Table 6. The clearance down each metabolic step was calculated as follows: intrinsic clearance (CLint1) for the first step catalysed by DPD was assumed to be Vmax/Km, clearance (CLint2) for the second step catalysed by DHPase to be VdDHU·keDHU, and clearance (CLint3) for the third step catalysed by UP to be VdUPA·keUPA. We have assumed that, since 13C-DHU and 13C-UPA are generated sequentially only in the liver, their clearance represents intrinsic hepatic clearance. The rank order of metabolic clearances was CLint3 > CLint1 > CLint2 (Table 6).
Figure 4.
Model fits for the mean plasma concentration–time data for 13C-uracil and its metabolites, and Δ13C in the expired air after oral administration of 13C-uracil at doases of (A) 50 mg, (B) 100 mg, and (C) 200 mg to 12 healthy males. (▴; 13C-uracil, Δ; 13C-DHU, ◊; 13C-UPA, ▪; 13CO2). Solid lines represent the predicted values calculated by the PBPK model shown in Figure 2
Table 6. Parameters estimated from simultaneous fitting of mean data for 13C-uracil and its metabolites and 13CO2 in expired air.
| Dose | |||
|---|---|---|---|
| Parameter | 50 mg | 100 mg | 200 mg |
| Km (µg ml−1) | – | 1.60* | – |
| Vmax (µg min−1) | – | 33900* | – |
| Vp (l) | – | 17.2* | – |
| ka (min−1) | – | 0.259* | – |
| VdDHU (l) | – | 408† | – |
| VdUPA (l) | – | 10.0† | – |
| keDHU (min−1) | 0.0119 | 0.0126 | 0.00948 |
| keUPA (min−1) | 4.58 | 5.00 | 2.54 |
| ke (min−1) | 0.151 | 0.0781 | 0.0578 |
| P1 | 0.264 | 0.627 | 0.365 |
| P2 | 0.00904 | 0.00458 | 0.00368 |
| CLint1 (l min−1)‡ | – | 21.2 | – |
| CLint2 (l min−1)§ | 4.86 | 5.14 | 3.87 |
| CLint3 (l min−1)¶ | 45.8 | 50.0 | 25.4 |
–, Not calculated.
Calculated using plasma concentrations of 13C-uracil at the doses of 50, 100 and 200 mg by equations.
Calculated using plasma concentrations of 13C-uracil and its metabolites and 13CO2 in expired air at the dose of 100 mg by equations.
Calculated as Vmax/Km.
Calculated as VdDHU·keDHU.
Calculated as VdUPA·keUPA.
Discussion
DPD is reported to be the rate-limiting enzyme in the metabolism of pyrimidine and its analogues under in vivo conditions [29–32]. However, this view has been disputed by others [2, 10, 33]. Wasternack [33] stated that ‘In most papers hitherto published, the first step in degradation has been considered as rate-limiting. However, recent results argue against this concept’ and ‘Under in vivo conditions little information is available because intermediates of degradation are not detectable.’ Daher et al.[10] reported that ‘all steps in the sequential 3-step reactions in pyrimidine metabolism have a potential to be rate-limiting and also it is still unclear which enzyme is rate-limiting’. In the present study, assuming that these discrepancies might be attributable problems in the analysis of pyrimidine and its metabolites in plasma or urine, we re-evaluated the pharmacokinetics of 13C-uracil and its metabolites using the high-resolution methods of LC-MS/MS and IRMS.
The results showed that 13C-uracil was reduced to 13C-DHU by DPD in the first catabolic step, which caused its rapid elimination from plasma. DHPase then mediated the conversion of 13C-DHU to 13C-UPA, but the relatively slow rate of this reaction meant that 13C-DHU remained in plasma for much longer than 13C-uracil. Subsequently, since 13C-UPA was rapidly biotransformed to H13CO3− by UP, the Δ13C in the expired air vs. time curves were similar to the plasma concentration vs. time curves of 13C-DHU.
We developed a PBPK model to describe the pharmacokinetics of uracil. PBPK models have been shown to be useful in quantitative evaluation of the metabolism and transport processes of drugs and endogenous substrates under physiological conditions [34–37]. The derived intrinsic clearances for each metabolic step are consistent with the rapid conversion of uracil to DHU, the slow biotransformation of DHU to UPA, and the rapid conversion of UPA to HCO3−.
In contrast, Wasternack [33] suggested that the efflux of dihydropyrimidines from mitochondria into cytosol is rate-limiting owing to the cytosolic location of DPD. Thus, uncertainty remains regarding the rate-limiting step in the disposition of pyrimidines in vivo, which may involve factors such as the transportation of 13C-uracil and its metabolites into hepatocytes, the location of the three enzymes responsible for its metabolism, and coenzymes such as NADH, NADPH [38].
Sumi et al.[39] reported two cases of dihydropyrimidinuria among 21 200 infants in whom urinary pyrimidine and dihydropyrimidine concentrations were measured, and concluded that a defect in DHPase was a probable risk factor for an adverse response to 5-FU therapy. Hamajimaet al.[40] subsequently analysed the DHPase gene and demonstrated six types of gene mutation. In addition to these reports, 5-fluoro-5,6-dihydrouracil, the substrate of DHPase and the metabolite of 5-FU, is reported to have antitumour cytotoxic activity [41], suggesting its involvement in the toxicity of 5-FU. Given that the metabolic characteristics of 5-FU [42, 43] and uracil are similar, the present results for uracil should be applicable to 5-FU. Therefore, we came to the tentative hypothesis that the in vivo rate-limiting enzyme for 5-FU metabolism is not DPD but DHPase. This hypothesis is supported by a study [9] of [6-3H]5-FU that produced pharmacokinetic profiles of the drug and its metabolites comparable to our results for 13C-uracil. However, in DPD-deficient subjects, DPD is likely to be the rate-limiting enzyme for overall pyrimidine catabolism.
In conclusion, we investigated the metabolic fate of 13C-uracil, its metabolites and the end product 13CO2 in expired air. Our PBPK model described the nonlinear pharmacokinetics of uracil and its metabolites well, and showed that of the three enzymes involved in pyrimidine degradation, DHPase is the least active in vivo in humans. Further study is required to show whether the analysis of 13CO2 in expired air after administration of 13C-uracil will allow the identification of patients at risk of a severe adverse response to treatment with 5-FU.
Acknowledgments
Competing interests: None declared.
References
- 1.Sonoda T, Tatibana M. Metabolic fate of pyrimidines and purines in dietary nucleic acids ingested by mice. Biochim Biophys Acta. 1978;521:55–66. doi: 10.1016/0005-2787(78)90248-4. [DOI] [PubMed] [Google Scholar]
- 2.Traut TW, Loechel S. Pyrimidine catabolism: individual characterization of the three sequential enzymes with a new assay. Biochemistry. 1984;23:2533–9. doi: 10.1021/bi00306a033. [DOI] [PubMed] [Google Scholar]
- 3.Canellakis ES. Pyrimidine metabolism. Enzymatic pathway of uracil and thymine degradation. J Biol Chem. 1956;221:315–31. [PubMed] [Google Scholar]
- 4.Fritzson P. Catabolism of C14-labelled uracil, dihydrouracil and β-ureidopropionic acid in rat liver slices. J Biol Chem. 1957;226:223–8. [PubMed] [Google Scholar]
- 5.Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16:215–37. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 6.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–64. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 7.Ho DH, Townsend L, Luna MA, Bodey GP. Distribution and inhibition of dihydrouracil dehydrogenase activities in human tissues using 5-fluorouracil as a substrate. Anticancer Res. 1986;6:781–4. [PubMed] [Google Scholar]
- 8.Woodcock TM, Martin DS, Damin LA, Kemeny NE, Young CW. Combination clinical trials with thymidine and fluorouracil: a phase I and clinical pharmacologic evaluation. Cancer. 1980;45:1135–43. doi: 10.1002/1097-0142(19800315)45:5+<1135::aid-cncr2820451318>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47:2203–6. [PubMed] [Google Scholar]
- 10.Daher GC, Harris BE, Diasio RB. Metabolism of pyrimidine analogues and their nucleosides. Pharmacol Ther. 1990;48:189–222. doi: 10.1016/0163-7258(90)90080-l. [DOI] [PubMed] [Google Scholar]
- 11.Lyss AP, Lilenbaum RC, Harris BE, Diasio RB. Severe 5-fluorouracil toxicity in a patient with decreased dihydropyrimidine dehydrogenase activity. Cancer Invest. 1993;11:239–40. doi: 10.3109/07357909309024846. [DOI] [PubMed] [Google Scholar]
- 12.Morrison GB, Bastian A, Dela Rosa T, Diasio RB, Takimoto CH. Dihydropyrimidine dehydrogenase deficiency: a pharmacogenetic defect causing severe adverse reactions to 5-fluorouracil-based chemotherapy. Oncol Nurs Forum. 1997;24:83–8. [PubMed] [Google Scholar]
- 13.Okuda H, Nishiyama T, Ogura K, Nagayama S, Ikeda K, Yamaguchi S, Nakamura Y, Kawaguchi Y, Watabe T. Lethal drug interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. Drug Metab Dispos. 1997;25:270–3. [PubMed] [Google Scholar]
- 14.Okuda H, Ogura K, Kato A, Takubo H, Watabe T. A possible mechanism of eighteen patient deaths caused by interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. J Pharmacol Exp Ther. 1998;287:791–9. [PubMed] [Google Scholar]
- 15.Bakkeren JA, De Abre RA, Sengers RC, Gabreels FJ, Maas JM, Renier WO. Elevated urine, blood and cerebrospinal fluid levels of uracil and thymine in a child with dihydrothymine dehydrogenase deficiency. Clin Chim Acta. 1984;140:247–56. doi: 10.1016/0009-8981(84)90206-7. [DOI] [PubMed] [Google Scholar]
- 16.Berger R, Stoker-de Vries SA, Wadman SK, Duran M, Beemer FA, de Bree PK, Weits-Binnerts JJ, Penders TJ, van der Woude JK. Dihydropyrimidine dehydrogenase deficiency leading to thymine-uraciluria. An inborn error of pyrimidine metabolism. Clin Chim Acta. 1984;141:227–34. doi: 10.1016/0009-8981(84)90014-7. [DOI] [PubMed] [Google Scholar]
- 17.Wilcken B, Hammond J, Berger R, Wise G, James C. Dihydropyrimidine dehydrogenase deficiency: a further case. J Inherit Metab Dis. 1985;8:115–6. doi: 10.1007/BF01811485. [DOI] [PubMed] [Google Scholar]
- 18.Graham DY, Klein PD, Evans DJ, Jr, Evans DG, Alpert LC, Opekun AR, Boutton TW. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet. 1987;1:1174–7. doi: 10.1016/s0140-6736(87)92145-3. [DOI] [PubMed] [Google Scholar]
- 19.Cutler AF, Havstad S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136–41. doi: 10.1016/0016-5085(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 20.Goddard AF, Logan RP. Urea breath tests for detecting Helicobacter pylori. Aliment Pharmacol Ther. 1997;11:641–9. doi: 10.1046/j.1365-2036.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 21.Inada M, Hirao Y, Koga T, Itose M, Kunizaki J, Shimizu T, Sato H. Relationships among plasma [2–13C]uracil concentrations, breath 13CO2-expiration, and DPD activity in the liver in normal and DPD-deficient dogs. Drug Metab Dispos. 2005;33:381–7. doi: 10.1124/dmd.104.001032. [DOI] [PubMed] [Google Scholar]
- 22.Sumi S, Kidouchi K, Kondou M, Hayashi K, Dobashi K, Kouwaki M, Togari H, Wada Y. Possible prediction of adverse reactions to fluorouracil by the measurement of urinary dihydrothymine and thymine. Int J Mol Med. 1998;2:477–82. doi: 10.3892/ijmm.2.4.477. [DOI] [PubMed] [Google Scholar]
- 23.Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–7. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 24.Meineke I, De Mey C, Eggers R, Bauer FE. Evaluation of the 13CO2 kinetics in humans after oral application of sodium bicarbonate as a model for breath testing. Eur J Clin Invest. 1993;23:91–6. doi: 10.1111/j.1365-2362.1993.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 25.Maes BD, Ghoos YF, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Combined carbon-13-glycine/carbon-14-octanoic acid breath test to monitor gastric emptying rates of liquids and solids. J Nucl Med. 1994;35:824–31. [PubMed] [Google Scholar]
- 26.Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology. 1997;112:1155–62. doi: 10.1016/s0016-5085(97)70126-4. [DOI] [PubMed] [Google Scholar]
- 27.Duan LP, Braden B, Caspary WF, Lembcke B. Influence of cisapride on gastric emptying of solids and liquids monitored by 13C breath tests. Dig Dis Sci. 1995;40:2200–6. doi: 10.1007/BF02209007. [DOI] [PubMed] [Google Scholar]
- 28.Cornetta AM, Simpson KW, Strauss-Ayali D, McDonough PL, Gleed RD. Use of a [13C]urea breath test for detection of gastric infection with Helicobacter spp in dogs. Am J Vet Res. 1998;59:1364–9. [PubMed] [Google Scholar]
- 29.Gonzalez FJ, Fernandez-Salguero P. Diagnostic analysis, clinical importance and molecular basis of dihydropyrimidine dehydrogenase deficiency. Trends Pharmacol Sci. 1995;16:325–7. doi: 10.1016/s0165-6147(00)89065-3. [DOI] [PubMed] [Google Scholar]
- 30.Ignoffo RJ. Novel oral fluoropyrimidines in the treatment of metastatic colorectal cancer. Am J Health Syst Pharm. 1999;56:2417–28. doi: 10.1093/ajhp/56.23.2417. [DOI] [PubMed] [Google Scholar]
- 31.Diasio RB. Clinical implications of dihydropyrimidine dehydrogenase on 5-FU pharmacology. Oncology (Huntingdon) 2001;15:21–6. [PubMed] [Google Scholar]
- 32.Diasio RB, Johnson MR. The role of pharmacogenetics and pharmacogenomics in cancer chemotherapy with 5-fluorouracil. Pharmacology. 2000;61:199–203. doi: 10.1159/000028401. [DOI] [PubMed] [Google Scholar]
- 33.Wasternack C. Degradation of pyrimidines and pyrimidine analogs—pathways and mutual influences. Pharmacol Ther. 1980;8:629–51. doi: 10.1016/0163-7258(80)90079-0. [DOI] [PubMed] [Google Scholar]
- 34.Charnick SB, Kawai R, Nedelman JR, Lemaire M, Niederberger W, Sato H. Perspectives in pharmacokinetics. Physiologically based pharmacokinetic modeling as a tool for drug development. J Pharmacokinet Biopharm. 1995;23:217–29. doi: 10.1007/BF02354273. [DOI] [PubMed] [Google Scholar]
- 35.Sato H, Sugiyama Y, Sawada Y, Iga T, Hanano M. Physiologically based pharmacokinetics of radioiodinated human beta-endorphin in rats. An application of the capillary membrane-limited model. Drug Metab Dispos. 1987;15:540–50. [PubMed] [Google Scholar]
- 36.Nagata O, Murata M, Kato H, Terasaki T, Sato H, Tsuji A. Physiological pharmacokinetics of a new muscle-relaxant, inaperisone, combined with its pharmacological effect on blood flow rate. Drug Metab Dispos. 1990;18:902–10. [PubMed] [Google Scholar]
- 37.Sato H, Terasaki T, Mizuguchi H, Okumura K, Tsuji A. Receptor-recycling model of clearance and distribution of insulin in the perfused mouse liver. Diabetologia. 1991;34:613–21. doi: 10.1007/BF00400989. [DOI] [PubMed] [Google Scholar]
- 38.Smith AE, Yamada EW. Dihydrouracil dehydrogenase of rat liver. Separation of hydrogenase and dehydrogenase activities. J Biol Chem. 1971;246:3610–7. [PubMed] [Google Scholar]
- 39.Sumi S, Imaeda M, Kidouchi K, Ohba S, Hamajima N, Kodama K, Togari H, Wada Y. Population and family studies of dihydropyrimidinuria: prevalence, inheritance mode, and risk of fluorouracil toxicity. Am J Med Genet. 1998;78:336–40. doi: 10.1002/(sici)1096-8628(19980724)78:4<336::aid-ajmg6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 40.Hamajima N, Kouwaki M, Vreken P, Matsuda K, Sumi S, Imaeda M, Ohba S, Kidouchi K, Nonaka M, Sasaki M, Tamaki N, Endo Y, De Abreu R, Rotteveel J, van Kuilenburg A, van Gennip A, Togari H, Wada Y. Dihydropyriminase deficiency: structural organization, chromosomal localization, and mutation analysis of the human dihydropyrimidinase gene. Am J Hum Genet. 1998;63:717–26. doi: 10.1086/302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diasio RB, Schuetz JD, Wallace HJ, Sommadossi JP. Dihydrofluorouracil, a fluorouracil catabolite with antitumor activity in murine and human cells. Cancer Res. 1985;45:4900–3. [PubMed] [Google Scholar]
- 42.Collins JM, Dedrick RL, King FG, Speyer JL, Myers CE. Nonlinear pharmacokinetic models for 5-fluorouracil in man: intravenous and intraperitoneal routes. Clin Pharmacol Ther. 1980;28:235–46. doi: 10.1038/clpt.1980.156. [DOI] [PubMed] [Google Scholar]
- 43.Wagner JG, Gyves JW, Stetson PL, Walker-Andrews SC, Wollner IS, Cochran MK, Ensminger WD. Steady-state nonlinear pharmacokinetics of 5-fluorouracil during hepatic arterial and intravenous infusions in cancer patients. Cancer Res. 1986;46:1499–506. [PubMed] [Google Scholar]