Abstract
Aims
To estimate the diffusion of new safety information concerning postmenopausal hormonal replacement therapy (HRT) into prescribing practice in the Netherlands and to assess the impact of revised guidelines on the long-term treatment of HRT.
Design
Cross-sectional study.
Setting
Community pharmacy dispensing data from a population of approximately 450 000 patients in the northern and eastern part of the Netherlands.
Population
Women aged 45–69 years to whom at least one HRT prescription was dispensed between 1 January 2000 and 1 January 2004.
Main outcome measures
Annual and quarter prevalences of HRT and the proportion of new HRT users, switchers and continuous HRT users per quarter.
Results
The prevalence of HRT prescribing decreased significantly from 107/1000 [95% confidence interval (CI) 104, 110] in 2000 to 87/1000 (95% CI 84, 89) in 2003. The decreasing prevalence was especially evident among the younger age groups and was most pronounced among users of oestrogen/progestagen combinations. The publication of the Women Health Initiative Study (WHI) was followed by a modest decrease in prescribing of HRT, whereas prescribing of HRT declined dramatically after publication of the Million Women Study (MWS) in August 2003. Among the continuous HRT users in the 4th quarter of 2002, 55% used HRT longer than 3 years. This percentage was 53 in the 4th quarter of 2003.
Conclusions
In contrast to the release of the WHI study results, publication of the MWS was followed by a dramatic fall in prescribing of HRT in the Netherlands. Despite the new recommendation that long-term HRT use should be discouraged, the proportion of long-term users did not change after the publication of the MWS.
Keywords: hormone replacement therapy, risk, drug utilization, prescribing
Introduction
The Women Health Initiative [1–3] and the Million Women Study [4] investigators recently reported increased rates of breast cancer, coronary heart diseases, stroke, dementia and venous thromboembolism, and decreased rates of hip fracture and colorectal diseases in postmenopausal women using long-term hormone replacement therapy (HRT), especially when using combined oestrogen/progestagen preparations. Summarizing all these endpoints, risks of HRT outweighed benefits. These findings contrasted with prior observational studies that showed cardiovascular benefits next to the symptomatic relief of perimenopausal symptoms [5–7]. These observational studies had resulted in widespread clinical belief in and increased use of HRT in the last two decades.
Both the Women's Health Initiative Study (WHI) and the Million Women Study (MWS) attracted much attention in the medical as well as the lay press. In the Netherlands, in contrast with the UK and USA, the WHI study did not receive as much media coverage as was the case with the MWS. The latter study instigated the revision of clinical guidelines on HRT in the Netherlands. In August 2003, almost immediately after the publication of the MWS, the Dutch associations of gynaecologists and general practitioners clearly stated that HRT should be used only for short duration in women with severe complaints [8, 9].
Several studies have shown that new safety evidence generally rather slowly diffuses into clinical practice [10, 11]. However, Hersh and colleagues showed that clinical practice in the USA responded rapidly after the first publication of the WHI study in July 2002; a sharp and continuing decline in HRT prescribing was visible after July 2002 [12]. Other studies from the USA [13] and New Zealand [14], both based on surveys among menopausal women, have shown a substantial decline in the proportion of women using HRT after the release of the WHI study. Yet questions remain. Is the decrease in HRT use solely attributable to fewer women who initiate treatment, are women discontinuing treatment at a high rate? Which women, long- or short-term users, followed the advice to discontinue treatment? And is the decline of use after the WHI study also evident in European countries?
The objective of the present study was to estimate the extent and speed of diffusion of the new safety information concerning HRT resulting from the WHI and MWS into prescribing practice in the Netherlands and, moreover, to assess the impact of revised clinical guidelines on long-term HRT treatment.
Methods
Setting
This study was performed with the InterAction database (IADB), which contains prescription drug dispensing data from community pharmacies in the northern and eastern part of the Netherlands. The IADB covers all prescriptions from an estimated population of 220 000 from 1994 to 1999, and was expanded to approximately 450 000 after 1999 [15, 16]. Each prescription record contains information about the drug, date of dispensing, amount dispensed, dose regimen and the prescribing physician. All drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification. Each patient has a unique, though anonymous identifier. Date of birth and gender of patients are available. Due to a high patient-pharmacy commitment in the Netherlands and sophisticated pharmacy software, the medication records for each patient are virtually complete [17]. This database comprises all prescriptions, regardless of insurance or reimbursement status, apart from drugs dispensed during hospitalizations. Note that almost all HRT preparations are fully reimbursed. For transdermal oestrogen/progestagen preparations a patient's co-payment is required.
Study population and design
All women aged 45–69 years, to whom at least one HRT prescription was dispensed during the study period 1 January 2000 to 1 January 2004, were selected from the IADB. Prescriptions for HRT were classified into eight categories (Table 1). Prevalence of HRT prescribing was estimated per year (2000, 2001, 2002 and 2003) and was defined as the number of women (aged 45–69) to whom any HRT prescription was dispensed per 1000 women in the population covered by the IADB. Annual prevalence was stratified per HRT category. In addition, we calculated prevalence per 3 months (quarter) from the 1st quarter in 2000 through the 4th quarter in 2003 stratified by HRT category and 5-year age categories.
Table 1. HRT categories with type of progestagens and oestrogens.
| Hormone category | Type of oestrogen | Type of progestagen |
|---|---|---|
| 1.Oral oestrogens | Estradiol (hemihydrate) | – |
| Estradiol (valerate) | ||
| Estriol | ||
| Ethinylestradiol | ||
| Conjugated oestrogens | ||
| 2.Oral oestrogen/progestagen combinations | Estradiol (hemihydrate) | Cyproteron (acetate) |
| Estradiol (valerate) | Dydrogesteron | |
| Conjugated oestrogens | Norethisteron (acetate) | |
| Medroxyprogesteron (acetate) | ||
| Medrogeston | ||
| Norgestrel | ||
| Dienogest | ||
| 3.Transdermal oestrogens | Estradiol (hemihydrate) | – |
| 4.Transdermal oestrogen/progestagen combinations | Estradiol | Levonorgestrel |
| Norethisteron (acetate) | ||
| 5.Tibolon | ||
| 6.Formulation with progestagens only (oral/injection) | – | Dydrogesteron |
| Lynestrenol | ||
| Norethisteron | ||
| Medroxyprogesteron | ||
| Progesteron | ||
| Medrogeston | ||
| 7.Vaginal preparations | Estradiol | – |
| Estriol | ||
| Conjugated equine oestrogens | ||
| 8.Other oestrogen formulations (nasal/implant/depot) | Estradiol (hemihydrate) | – |
| Estradiol (valerate) |
Per quarter all women (aged 45–69) to whom a HRT prescription was dispensed (‘index prescription’) were either categorized as new user, switcher or continuous user based on the use of HRT in the previous 12 months. New users were defined as women, to whom a first HRT prescription was dispensed, i.e. having no prescription for HRT in the previous 12 months, despite being registered for at least 1 year in the IADB. Switchers were women who had a prescription for a HRT preparation from another category in the previous 12 months. Continuous users were women whose last HRT prescription in the previous 12 months was from the same HRT category. Data from 1999 were used to categorize HRT users for the year 2000. Per quarter the number of new users, switchers and continuous users among all HRT users during that quarter was calculated.
Results
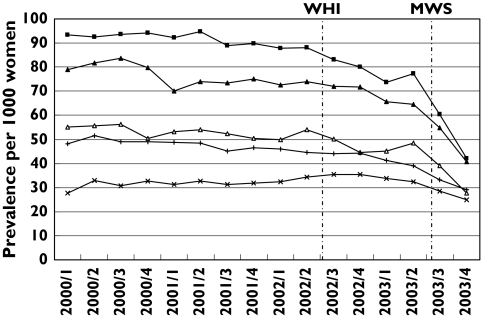
The annual prevalence of HRT from 2000 through 2003 among women aged 45–69 is presented in Table 2. The prevalence of prescribing of any HRT decreased significantly from 107/1000 [95% confidence interval (CI) 104, 110 per 1000] in 2000 to 87/1000 (95% CI 84, 89 per 1000) in 2003. Regarding the HRT categories, apart from tibolon, vaginal preparations and other oestrogen formulations, the annual prevalence in 2003 of all HRT categories is lower compared with prior years. Figure 1 shows the course of HRT prevalence per quarter stratified per 5-year age categories. We found the highest prevalences of HRT prescribing in women aged 50–60 years, being almost twice as high compared with women in the 45–49 and 60–64 age category. Lowest prevalences were found in women aged 65–69 years; in this age group more than 50% of the prescribed HRT preparations concerned vaginal preparations with oestrogens. In all age groups the prevalence drops sharply in the 3rd and 4th quarters of 2003, i.e. immediately after the publication of the MWS. This decline is most evident in women younger than 60. When we compared HRT prevalence in the 4th with that in the 2nd quarter of 2003 we noted in women aged 45–49, 50–54 and 55–59 a decrease of, respectively, 42%, 46% and 37%, while this decline is, respectively, 26% and 22% in the group 60–64 and 65–69 age groups. Influence on prescribing after publication of the WHI study in July 2002 is less clear, although the HRT prevalence in the youngest age categories (45–49 and 50–54) also shows a decrease of, respectively, 15% and 9% in the 4th quarter of 2002 (i.e. after publication of the WHI study) compared with the 2nd quarter of 2002 (before the WHI publication).
Table 2. Prevalence of HRT prescribing per category by women aged 45–69 from 2000 to 2003 (expressed per 1000 women of 45–69 years old in study population).
| Hormone category | 2000 | 2001 | 2002 | 2003 |
|---|---|---|---|---|
| 1.Oral oestrogens | 24.0 | 20.8 | 18.8 | 15.1 |
| 2.Oral oestrogen/progestagen combinations | 28.7 | 29.5 | 30.4 | 24.3 |
| 3.Transdermal oestrogens | 15.6 | 13.1 | 10.7 | 8.6 |
| 4.Transdermal oestrogen/progestagen combinations | 1.5 | 1.3 | 1.3 | 0.9 |
| 5.Tibolon | 8.9 | 9.2 | 9.3 | 8.7 |
| 6.Formulation with progestagens only (oral/injection) | 23.4 | 21.2 | 19.6 | 16.4 |
| 7.Vaginal preparations | 19.8 | 20.3 | 20.4 | 19.7 |
| 8.Other oestrogen formulations (nasal/implant/depot) | 0.8 | 0.8 | 1.1 | 0.9 |
| Any HRT | 107.0 | 103.4 | 100.2 | 86.5 |
| Total study population | 56304 | 58870 | 57560 | 60542 |
Figure 1.
Quarter prevalences of HRT prescribing per age category from 2000 through 2003 (expressed per 1000 women per age category). 45–49 yrs (▵); 50–54 yrs (▪); 55–59 yrs (▴); 60–64 yrs (+); 65–69 yrs (×)
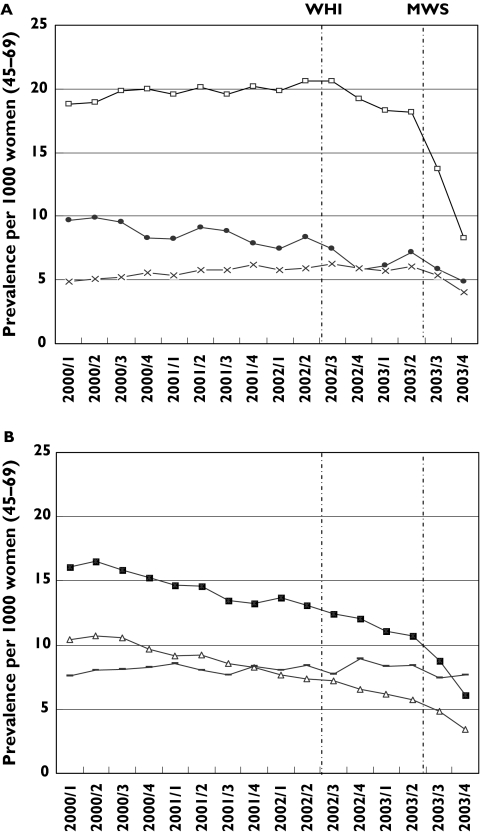
Quarter prevalences per HRT category between January 2000 and January 2004 are presented in Figure 2a, b. From January 2000 to July 2003 (just before publication of the MWS) the prevalence of oral combined oestrogen/progestagen and tibolon and vaginal preparations remained relatively stable. However, in case of oral oestrogen/progestagen combinations there appears to be a sudden reduction in prescribing even before the publication of the WMS. During the same period between January 2000 and July 2003 the prevalence of oral oestrogens, transdermal oestrogens and progestagens show a declining trend. For oral oestrogens the prevalence decreased from about 16/1000 in 2000 to approximately 11/1000 in the 2nd quarter of 2003.
Figure 2.
(A) (top) and (B) (bottom). Prevalence of HRT prescribing per category from January 2000 through December 2004. [Prevalence of transdermal oestrogen/progestagen combinations (category 4) and other oestrogen formulations (category 8) are not presented, due to very low numbers.] A: Oral oestrogen/progestagen combinations (□); formulations with progestagen only (•);tibolon (×). B: Oral oestrogens (▪); transdermal oestrogens (▵); vaginal preparations ( )
)
Following the publication of MWS in August 2003, prevalences of all HRT categories, except for vaginal preparations, declined through the last quarter of 2003. For oral oestrogens as well as for transdermal oestrogens, the declining trend is stronger compared with the period before August 2003. The decline in prevalence is most evident for the combined oestrogen/progestagen prescriptions. The quarter prevalence fell from around 18 per 1000 before the publication of the MWS to around 8/1000 at the end of 2003.
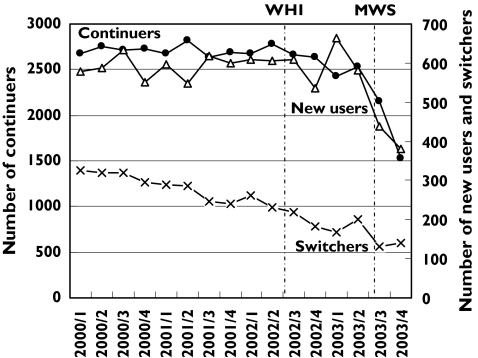
In Figure 3 the total number of women per quarter to whom HRT was prescribed is divided into new users, switchers and continuers. After the publication of the WHI study a small decrease is seen in the total number of women receiving any HRT prescription from above 3500 before the WHI study to around 3300 the year after. A sharp fall is visible after the publication of the MWS in August 2003. We also estimated the decrease in the number of women in the three defined groups (new users, switchers or continuers) by comparing the number of new users, switchers and continuers in the 4th quarter of 2003 with that of 4th quarter of 2002. We estimated a fall of 25% among switchers, 42% among continuers and 29% among new users. Among the continuers in the 4th quarter of 2002, 55% had prescriptions for HRT of the same category dispensed for longer than 3 years. This percentage was 53 in the 4th quarter of 2003, illustrating that the proportion of long-term users did not decrease after publication of the MWS and the new Dutch guideline recommendations advising only a short-term use of HRT. Among the small number of switchers a diversity of switches between the different HRT categories occurred. No specific changes in patterns of switch regimens during the study period could be observed.
Figure 3.
Number of HRT users (aged 45–69) per quarter from 2000 through 2003, classified as number of new users, switchers and continuers. Note that for continuers and new users/switchers different Y-axes are used
Discussion
Our results based on population data of HRT use showed a modest decline in HRT prescribing after the publication of the WHI study, whereas the release of results from the MWS was followed by a dramatic fall in the prescribing of HRT. The decreasing prevalence was especially evident among the younger age groups and was most pronounced among users of oestrogen/progestagen combinations. However, the proportion of long-term users (at least 3 years) did not decrease at all after publication of the MWS in August 2003.
The overall prescribing of HRT in women aged 45–69 in the Netherlands, being 10% in 2000–2002, is far below the prevalence of HRT use reported in the USA and UK. We found the highest prevalence among women in the age group 50–54 years [18]. In the US, data from 1995 show that approximately 38% of postmenopausal women were taking HRT [19] and a recent publication with data from 2002 (before publication of the WHI) showed that 50% of white American women reported to take HRT [12]. In the MWS a cumulative prevalence is presented: 50% of UK postmenopausal women reported they had ever used HRT [4]. Over a period of 6 years we found in our database a cumulative prevalence of 32%.
From January 2000 through the release of results of the WHI study in July 2002, the prevalence of HRT prescribing per quarter remained stable in the defined age groups. Following publication of the WHI study, which did not receive much media attention in the Netherlands, a modest decline was visible in the 45–49 and 50–54 age groups. In contrast, the publication of the British Million Women Study was followed by a sharp decline in HRT prescribing. How can we explain this substantial change in the prescribing of hormone therapy in response to the MWS? Although our data cannot directly address this question, several explanations are possible. First, the publication of the MWS in the Lancet was accompanied by an editorial written by two Dutch GPs, both professors in Community Health. They stated very clearly that the use of HRT should be discouraged and that guidelines need to be rewritten. Women who already use HRT should be advised about stopping and if in case HRT was needed it should not be used for longer than 3–6 months [20]. Second, the Dutch association of gynaecologists as well as the association of general practitioners reacted promptly to the MWS with recommendations to prescribe HRT for as short a period as possible [8, 9]. This change in guidelines was much discussed in several professional journals as well as well covered in women's magazines. Third, 40% of Dutch pharmacists supported GPs by tracing long-term HRT users in their pharmacy computer system and subsequently these women were proactively approached and informed about the benefits and risks and advised to stop hormone therapy [21]. Fourth, in contrast to the WHI study, the MWS was accompanied by excessive media attention in the Netherlands directly after publication. On the day of the Lancet publication, six national daily newspapers discussed this issue on their front page. The WHI study received attention only in scientific supplements of two national newspapers. Fifth, the modest effect of the WHI study on Dutch prescribing of HRT can also be explained by the fact that the discussion at that time was focused on the type of oestrogens. In the WHI study only conjugated oestrogens were used, while in the Netherlands 30% of HRT users received conjugated oestrogens [22]. Comments in journals on the WHI study emphasized the difference in the type of oestrogens used and concluded that risks of conjugated oestrogens could not simply be extrapolated to HRT strategies with estradiol. Sixth, the results of the WHI could have had an unseen impact on Dutch professional opinion about HRT use, which was not translated into action until the release of the MWS. Despite the recommendations by the associations of gynaecologists and general practitioners that especially long-term HRT use should be discouraged, the proportion of long-term users did not change after the publication of the MWS.
Limitations
A shortcoming of our study is that at the time of this analysis, data were available for only 4 months after the publication of the MWS, which implied no time series analysis could be performed to confirm trends statistically in time. Longer follow-up data may be needed to establish whether the decline shown is sustainable or only temporary. Although it may not be clear whether any observed changes in prescribing trends are due to prescribing guidelines for health providers or patient pressure for a reduction in prescribing as a result of media campaigns, nonetheless, we think our study demonstrates the overall extent to which prescribing trends have changed.
Conclusions
In contrast to publication of the WHI results, the release of new safety information on HRT in the MWS in 2003 was followed by a dramatic fall in HRT prescribing in the Netherlands.
Despite the new recommendation that long-term HRT use should be discouraged, the proportion of long-term users did not change after the publication of the MWS. The decreased prescribing of HRT can be attributed mainly to fewer patients initiating treatment and stopping of patients who recently initiated therapy.
Contributions of authors
All authors had full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis. Conception and design: A.F., M.B., T.E. and L.J.B. Acquisition of data: A.F., L.L., P.B. and L.J.B. Analysis and interpretation of data: A.F., M.B., L.L., P.B. and L.J.B. Drafting of the manuscript: A.F., M.B., T.E. and L.J.B. Critical revision of the manuscript for important intellectual content and final approval: A.F., M.B., L.L., P.B., T.E. and L.J.B. Guarantor: L.J.B.
Acknowledgments
Competing interests: None declared.
References
- 1.Writing group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results form the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women's Health Initiative Memory study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 3.Writing group for the Women's Health Initiative Investigators. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 4.Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 5.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–61. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 6.Pan CX. Hormone replacement therapy for secondary prevention of coronary heart disease. JAMA. 1999;281:794. [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Dutch College of General Practitioners. [on 8 November 2004]; http://nhg.artsennet.nl.
- 9.Dutch Society of Obstetrics and Gynaecology. [on 8 November 2004]; Press release on website: http://www.nvog.nl.
- 10.Wilkinson JJ, Force RW, Cady PS. Impact of safety warnings on drug utilization: marketplace life span of cisapride and troglitazone. Pharmacotherapy. 2004;24:978–86. doi: 10.1592/phco.24.11.978.36136. [DOI] [PubMed] [Google Scholar]
- 11.Maclure M, Dormuth C, Naumann T, McCormack J, Rangno Whiteside C, Wright JM. Influences of educational interventions and adverse news about calcium-channel blockers on first-line prescribing of antihypertensive drugs to elderly people in British Columbia. Lancet. 1998;352:943–8. doi: 10.1016/S0140-6736(97)11390-3. [DOI] [PubMed] [Google Scholar]
- 12.Hersh AL, Ste MJ, Stafford RS. National use of postmenopausal hormone therapy. Annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trials results. Ann Intern Med. 2004;140:184–8. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lawton B, Rose S, McLeod D, Dowell A. Changes in use of hormone replacement therapy after the report from the Women's Health Initiative: cross sectional survey of users. BMJ. 2003;327:845–6. doi: 10.1136/bmj.327.7419.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobi H, van den Berg PB, de Jong-van den Berg LTW. The Interaction Database: synergy of science and practice in pharmacy. In: Brause RW, Hanisch E, editors. Medical Data Analysis. Berlin: Springerverlag; 2000. pp. 206–11. [Google Scholar]
- 16.Schirm E, Monster TBM, de Vries R, van den Berg PB, de Jong-van den Berg LTW, Tobi H. How to estimate the population that is covered by community pharmacies? An evaluation of two methods using drug utilisation information. Pharmacoepidemiol Drug Saf. 2004;13:173–9. doi: 10.1002/pds.882. [DOI] [PubMed] [Google Scholar]
- 17.Leufkens HGM, Urquhart J. Automated pharmacy record linkage in the Netherlands. In: Strom BL, editor. Pharmacoepidemiology. 3. Chichester: John Wiley & Sons Ltd; 2000. pp. 347–60. [Google Scholar]
- 18.Tobi H, van den Berg PB, Brouwers JRBJ, de Jong-van den Berg LTW. Hormone replacement therapy in peri- and postmenopausal women: more than half use HRT longer than 1 year (in Dutch) Ned Tijdschr Geneeskd. 2003;147:1853–5. [PubMed] [Google Scholar]
- 19.Keating NL, Clearly PD, Rossi AS, Zaslavsky AM, Aynian JZ. Use of hormone replacement therapy by postmenopausal women in the United States. Ann Intern Med. 1999;130:545–53. doi: 10.7326/0003-4819-130-7-199904060-00002. [DOI] [PubMed] [Google Scholar]
- 20.Lagro-Janssen T, Rosser WW, van Weel C. Breast cancer and hormone-replacement therapy: up to general practice to pick up the pieces. Lancet. 2003;362:414–5. doi: 10.1016/S0140-6736(03)14095-0. (Editorial). [DOI] [PubMed] [Google Scholar]
- 21.Teichert M, de Smet PAGM. Hormone use during menopause [Hormoongebruik in de overgang] Pharm Weekbl. 2004;139:634–5. [Google Scholar]
- 22.Monster TBM, de Jong-van den Berg LTW. No longer place for long-term hormone replacement therapy [Geen plaats meer voor langdurige hormoonsubstitutie therapie] Pharm Weekbl. 2002;137:1132–4. [Google Scholar]