Abstract
We have combined electrophysiology and imaging to measure the release of neurotransmitter and fluorescent dye at synapses of cultured hippocampal neurons. These experiments have revealed a “kiss and run” mode of exocytosis in which synaptic vesicles release glutamate normally but do not permit dye to enter or escape from the vesicle. During “kiss and run,” the vesicle interior may be exposed very transiently (<6 ms), or a special configuration of the fusion pore may prevent dye exchange. We estimate that about 20% of the vesicles normally use this “kiss and run” pathway, and that the fraction of “kiss and run” events can be increased to over 80% by superfusing the synapses with hypertonic solution.
During a study of synaptic vesicle trafficking in which we measured the release of the fluorescent dye FM1–43 (1) after stimulation with either action potentials or hypertonic solution, we noticed that, with hypertonic stimulation, the escape of dye from previously loaded vesicles seemed to be partly prevented even though the neurotransmitter glutamate was released normally. Hypertonic solution application produces asynchronous release of synaptic vesicles at a rate that is graded with osmolarity, and this type of stimulation draws on the same pool of vesicles as action potential-evoked release; but hypertonic stimulation circumvents the normal dependence of vesicle fusion on calcium ions, presumably through a mechanical lowering of the energy barrier for membrane fusion (2). The experiments presented here are an analysis of this dissociation between neurotransmitter and dye release.
Our main observation is a simple one: when exocytosis is produced by hypertonic solution, a fraction of the vesicles that release neurotransmitter fail either to release or take up fluorescent dye, and this fraction increases to over 80% with increasing osmolarity. Synaptic vesicles participating in exocytosis normally take up (3) or release (4) the dye in less than about 20 ms, so the failure of dye exchange means that either a fusion pore exists for a very brief time (so brief that dye has insufficient time to enter or leave the vesicle), or the conformation of the fusion pore is one that permits glutamate outflow but excludes dye passage. We identify this special exocytic state of the vesicle with “kiss and run” and, at least under the conditions of our experiments, estimate that “kiss and run” constitutes about 20% of the release events during normal synaptic transmission.
Experimental Procedures
Cell Culture.
Primary cultures of hippocampal neurons were prepared from P0–1 rat pups by using standard methods. We used both conventional and “microdot” cultures with single-cell islands (2, 5).
Solutions.
Cultures were superfused with 50 μM d,l-2-amino-5-phosphonovaleric acid (Research Biochemicals) in physiological saline [132 mM NaCl/2 mM KCl/2.6 mM CaCl2/1.3 mM MgCl2/10 mM glucose/10 mM Hepes, with pH ≈7.2 and osmolarity adjusted to ≈0.3 osmolar (osM) with sorbitol]. In addition, 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (Research Biochemicals) was added to block recurrent activity during experiments that used imaging without simultaneous electrophysiological recording. Solutions were made hypertonic with the addition of sucrose, and values reported give the tonicity in excess of the 0.3 OSG from the saline. Solutions were applied with a rapid-switching local superfusion system controlled by computer. All experiments were carried out at room temperature (≈22°C).
Electrophysiology.
Voltage clamp recordings were obtained by using a standard perforated patch method (amphotericin B, ≈0.1 mg/ml) from cells grown 8–14 days in culture.
Stimulation Parameters.
The amount of neurotransmitter release was estimated from integrals of synaptic current (6, 7). One readily releasable pool (RRP) was defined as the charge transferred with stimulation by 0.5 osM hypertonic solution. Briefly, 0.5 osM hypertonic solution was applied for 4.5 s and the total charge transfer calculated; this value was then corrected for refilling by subtracting the charge transfer measured during the steady-state plateau. The lengths of the action potential train and hypertonic solution applications were selected to release one RRP. The stimulation parameters and amount of release are as follows: 36 action potentials at 20 Hz [after treatment with EGTA–acetoxymethyl ester (see below)], 0.98 ± 0.04 RRPs; 0.5 osM hypertonic solution (2.3 s application), 1.04 ± 0.02 RRPs; 0.6 osM (for 1.8 s), 1.06 ± 0.03 RRPs; 0.8 osM (for 1.2 s), 1.06 ± 0.03 RRPs; 1 osM (for 1 s), 1.05 ± 0.05 RRPs; and 1.5 osM (for 0.7 s), 1.63 ± 0.11 RRPs. Because the 1.5 osM solution is very viscous, we used an application time that released more than one RRP.
Except for experiments where release of neurotransmitter and dye were simultaneously measured (the initial experiment and studies where presynaptic calcium levels were altered by pairing action potential generation and hypertonic solution application), preparations in this study were preincubated with 300 μM EGTA–acetoxymethyl ester for 5 min to minimize asynchronous release caused by accumulated intracellular calcium.
Stimulation Protocols.
All of the dye release experiments followed the same protocol. Dye was initially loaded into vesicles with two trains of 75 action potentials at 20 Hz (designed to release about four RRP of neurotransmitter). Dye was present during the loading stimulation and for the ensuing 1 min at a concentration of 10 μM for FM1–43 or 200 μM for FM2–10; the preparation was washed for 10 min before commencement of three successive stimulation epochs: (i) action potentials (36 at 20 Hz), (ii) hypertonic solution superfusion over the imaged region (duration and hypertonicity of superfusion as indicated above), and (iii) a repeat of i. As an internal control to ensure stability of the preparation, data were accepted only if the fraction of dye released was within 10% for epochs i and iii. A 5-min rest period was interposed between each stimulation epoch to permit equilibration of the docked vesicle pool with the remainder of the recycling pool (8). After stimulation epoch iii, the synapses were stimulated for 90 s at 10 Hz to determine the nonspecific background fluorescence; this final image after extensive stimulation was subtracted from each of the other images. Measurements for dye release experiments were fractional changes in fluorescence in the same synapses; the effects of action potentials and hypertonic solutions were measured at the same punctate spots.
For the dye uptake experiments presented in Fig. 2, the stimulation durations were the same as those used above for dye release (36 action potentials at 10 Hz or hypertonic solution superfusion for the duration indicated in Stimulation Parameters) with 10 μM FM1–43 present throughout the stimulation and for the ensuing 1 min. After dye uptake, the preparation was washed for 10 min before acquiring the image.
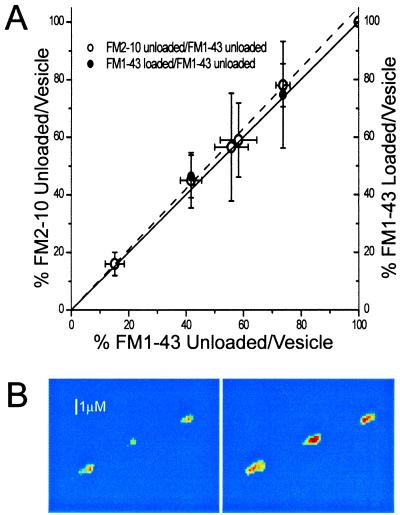
Figure 2.
Two modes of exocytosis exist. (A) Percent FM2–10 and FM1–43 released per quantum released (open circles) and percent FM1–43 taken up per quantum released (filled circles) with hypertonicity of solution as a parameter (Left to Right), the hypertonicities are 1.5, 1.0, 0.8, 0.6, 0.5, and 0.0 (nerve impulse stimulation) osM. For example, the left-most point is derived from experiments in which the release was produced by a 1.5-osM solution; the left ordinate value is the percentage dye release from FM2–10 loaded synapses, and the abscissa value is the percent dye released from FM1–43 loaded synapses. All percentage uptake and release are relative to the dye released or taken up per quantum of glutamate release produced by action potentials. Data for the abscissa is from experiments of Fig. 1. FM2–10 dye release data from 365 synapses in 5 experiments and FM1–43 dye uptake data, from 520 synapses in 5 experiments; error bars indicate one SEM, with the larger of the overlapping error bars associated with the filled circles (dye uptake measurements have more error). The solid line has a slope of one (equality for ordinate and abscissa), and the dotted line, with a slope of 1.05, is the least squares fit to the data. (B) Example of three synapses loaded with FM1–43 by 1 osM hypertonic solution application (Left) and then (after prolonged stimulation to release dye) by action potential stimulation (Right); much less dye was loaded with hypertonic stimulation, although in each case the preparation had been stimulated to release the same amount of neurotransmitter. The images were thresholded and false colored after subtraction of corresponding nonspecific fluorescence remaining after prolonged stimulation. The color map ranges from blue to red for increasing fluorescence and is intended to show the relative difference between dye uptake with hypertonic and action potential stimulation.
Imaging.
Images were obtained by using a cooled CCD camera [Princeton Instruments (Trenton, NJ) “Micromax”] and a Nikon inverted microscope with a 40 × 0.75 n.a. objective. The filter cube was obtained from Molecular Probes (Ex 485/22, DCLP 505, Em LP530). With our configuration, the photobleaching rate was ≈1% per picture. Action potentials in imaging studies were elicited with ≈10 V/cm field stimulation through 2 parallel silver–silver chloride wires. Preparations were imaged through a single layer of glass by using an O-ring chamber.
Imaging data were analyzed with matlab routines [Math Works (Natick, MA)] by using regions of interest to delimit puncta. The average fluorescence of all of the pixels in a 7 × 7-pixel (≈1.2-μm) box surrounding the punctum's intensity center of mass was the basic measurement. To make each stimulation epoch as comparable to others as possible, we used stimulation protocols that had been determined in a series of electrophysiological experiments to release about one RRP (see Stimulation Parameters). In addition, all imaging data were normalized with actual amount of neurotransmitter release.
Simultaneous Imaging and Physiology.
To measure synaptic currents generated by the same synapses that were studied with imaging, the initial experiment and the calcium-loading experiment were carried out on a single neuron island (“autapses”). The design of these experiments was much like the standard imaging experiment (Figs. 1 and 2).
Figure 1.
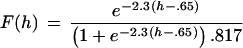 |
Results
Initial Experiment.
The goal of our initial experiment was to confirm the casual observation of a failure of dye escape by comparing dye (FM1–43) and neurotransmitter release produced by two types of stimulation: action potentials and application of hypertonic solution. To ensure that the same population of synapses was studied with both types of stimulation, we patch clamped a hippocampal neuron isolated in culture on a microdot so that this single cell was both the source and the target for all of the synaptic contacts (autapses) (9–11). The vesicles were dye loaded (two 20-Hz trains of 75 action potentials, 10 μM FM1–43), and then dye and glutamate release were measured simultaneously in the same population of synapses; exocytosis was evoked, in turn, with hypertonic solution (0.5 osM for 2.3 seconds) and action potentials (20 Hz, 36 action potentials). Dye release was estimated by the decrease in fluorescence intensity produced by stimulation, and total quantal release was measured by integrating the synaptic currents that flowed during stimulation (12, 13). The amount of dye released, normalized by the quantity of neurotransmitter released, was significantly less when the hypertonic solution rather than the train of action potentials was used to produce exocytosis. With hypertonic stimulation, each exocytotic event on average released 67.6% ± 4.8% (230 synapses, three experiments; P < 0.01 by t test) of the amount of dye that left a vesicle when exocytosis was caused by action potentials.
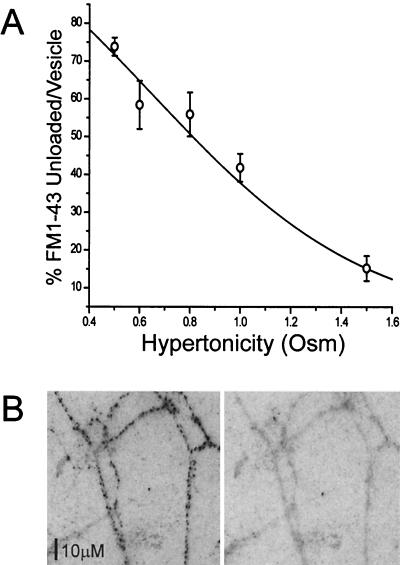
Increased Osmolarities Permit Less Dye Release.
If the failure of dye escape during glutamate release reported in the initial experiment above actually reflects a special mode of exocytosis, the magnitude of the effect should be graded with the osmolarity of the solution used to induce the release. We therefore investigated glutamate and dye release from vesicles over a range of tonicities from 0.5 to 1.5 osM. In the initial experiment described above, we carried out imaging and electrophysiology measurements simultaneously on the same synapses. Because such experiments are technically difficult with a low yield, we adopted a different strategy for the experiments described next. First, electrophysiological experiments were carried out on microdot cultures to determine stimulation parameters that caused the same amount of exocytosis with both action potentials and various hypertonic solutions from 0.5 to 1.5 osM; for each hypertonic solution, we found the duration of superfusion that produced release of one RRP (see Materials and Methods). In separate imaging experiments with microdot and conventional cultures, we then compared the fraction of dye released by solutions of various hypertonicities relative to dye released by a train of action potentials (that also released a single RRP).
Fig. 1 shows that, with increasing hypertonicity, the ratio of dye release to neurotransmitter release progressively decreases. Synaptic vesicles were loaded with FM1–43, and then the synapses were activated with action potentials (train of 36 at 20 Hz) or various tonicities of hypertonic solution. Although the stimulation parameters were selected to always release the same amount of neurotransmitter (≈1 RRP), this constancy in quantity of glutamate released is not reflected in dye release measured optically; increasing hypertonicity permits less dye, on average, to escape per quantum of neurotransmitter released.
Two Modes of Exocytosis Exist.
Do all of the vesicles for the Fig. 1 experiments release a fraction of their dye, or do some of the vesicles permit all of their dye to escape and others, none? To distinguish between these possibilities, we repeated the experiments in Fig. 1 with the dye FM2–10, which has a time constant for departitioning of about 0.6 s (as compared with the approximately 2.5 s for FM1–43) (14). We reasoned that if the decrease in dye released were a consequence of partial dye loss from all of the vesicles (rather than diffusion through the membrane), then a dye with a faster off-rate from the membrane would escape more completely in the brief time available. On the other hand, if there is a special class of release events that permit no dye loss, then the fraction of dye released would be unaffected by the dissociation rate or the lateral diffusion constant.
In Fig. 2, we plot the percent of dye released per exocytotic event and find that it is, within experimental error, the same for FM1–43 (abscissa) and FM2–10 (left ordinate) under all stimulation conditions (hypertonicities 1.5 to 0.5 osM). We conclude that there are two distinct modes of release: the usual one and another that we identify as “kiss and run,” in which neurotransmitter is released but dye is not.
Dye Cannot Enter Some Synaptic Vesicles as They Release Glutamate.
We have demonstrated a distinct “kiss and run” mode of exocytosis, but to address two important concerns, measurements were made of dye uptake (rather than dye release). First, one might argue that the hypertonic solution somehow increases the affinity of the dye for membranes; were this the case, the dye retention described above might be simply an artifact of decreased off-rate of the dye produced by hypertonic conditions or a decreased rate of lateral diffusion. Second, dye uptake is a very rapid process that depends on different kinetic properties of the dye than release so it is a strong test for the existence of two distinct modes of exocytosis.
FM143 partitions into membranes with a rate of 35 μM−1⋅s−1, so exposed vesicle membrane should load with a time constant τ of about 3 ms when the extracellular dye concentration is, as in our experiments, 10 μM (3). We calculated this equilibration time constant τ for a dye concentration of 10 μM from the relation τ = 1/(a + 10b), where a = 1/2.5 s−1 [the off-rate of FM1–43 from membranes (14)], and b = 35 μM−1⋅s−1 [the rate constant for dye partitioning into the membrane (3)].
Fig. 2 (right ordinate) shows that, within experimental error, the average amount of FM1–43 uptake per vesicle is the same as the amount of dye release for hypertonicities of 0.5 and 1.0 osM. This means that our earlier results could not be an artifact of a hypertonicity-produced increase in dye affinity for membranes or a decrease in the lateral diffusion rate. More importantly, these observations demonstrate that dye can neither enter nor leave a fraction of the vesicles that are releasing glutamate. We identify this special release mode with no dye exchange as “kiss and run.” And if we assume that FM1–43 can freely diffuse through the fusion pore, then these results place an upper limit of ≈6 ms (2 time constant of dye uptake) on the time of exposure of the vesicle interior during “kiss and run.”
Neurotransmitter Release Is Unchanged in “Kiss and Run.”
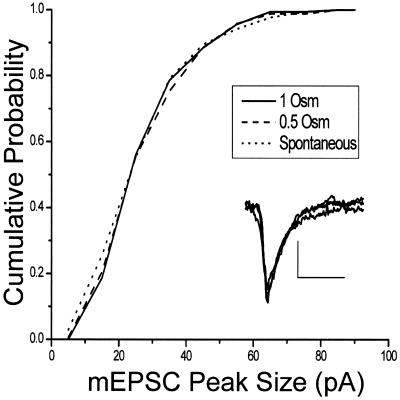
In neuroendocrine cells, transient fusion of the vesicle can occur with only partial release of neurotransmitter [(15); see also ref. 16]. Furthermore, the quantity and time course of dense-core vesicle emptying can depend on the tonicity of the external medium (17). To determine whether modified neurotransmitter release occurs during the “kiss and run” exocytotic mode at hippocampal synapses, we examined the size and time course of miniature excitatory postsynaptic currents (mEPSCs) produced by hypertonic solutions. We reasoned that the existence of a fusion pore that does not permit dye to leave the vesicle might affect glutamate release and produce changes in the amplitude (quantity of transmitter released) and rise times (time course of release) of miniature synaptic currents. We thus compared the amplitudes and rise times of mEPSCs evoked by 0.0 (spontaneous events), 0.5, and 1.0 osM hypertonic solutions. Fig. 3 shows that cumulative amplitude histograms of mEPSCs recorded under these three conditions are not detectably different. Therefore, like some neuroendocrine cells (18), full release of neurotransmitter can occur with “kiss and run” at hippocampal synapses, and tonicity seems not to affect vesicle emptying.
Figure 3.
Neurotransmitter release is unchanged in “kiss and run.” Cumulative histograms of peak mEPSC amplitudes in the presence of bathing medium with hypertonicities of 0.0 osM (spontaneous mEPSCs, dotted line, mean amplitude, 25.67 ± 1.13 pA, n = 172), 0.5 osM (dashed, mean amplitude, 25.8 ± 1.15 pA, n = 166) and 1.0 osM (solid, mean amplitude, 26.09 ± 0.98 pA, n = 182). Inset shows averaged mEPSCs from the three conditions superimposed. (Bar = 10 pA and 10 ms.)
We also found that the mEPSC rise times (20–80%) are unaffected by hypertonicity; the mean rise time is 1.14 ± .07 ms at a hypertonicity of 0.0 osM (spontaneous mEPSCs), 1.2 ± 0.095 ms for 0.5 osM, and 1.19 ± 0.062 ms for 1.0 osM.
The Fraction of “Kiss and Run” Events Is Not Significantly Altered by Changes in Presynaptic Calcium Concentration.
We have presented evidence for “kiss and run” that can be elicited by hypertonic solution in a dose-dependent manner, but we do not know what normally controls the balance between the usual and “kiss and run” modes. Alés et al. (18) have reported that “kiss and run” release by chromaffin cells is made more likely when extracellular calcium concentration is increased, presumably through consequent changes in the intracellular calcium levels. We have therefore examined the effect of manipulations of calcium concentration on the release of FM1–43 from synaptic vesicles.
Rosenmund and Stevens (2) have shown that application of hypertonic solution produces neurotransmitter release at these same synapses by a calcium-independent mechanism and without an increase in intracellular calcium. If increased intracellular calcium concentration facilitated “kiss and run,” then the fraction of dye release per exocytotic event should decrease when hypertonic stimulation is paired with a manipulation that increases intracellular calcium. We achieved this situation by simultaneously superfusing with hypertonic solution (0.5 osM) and generating action potentials (36 at 20 Hz) to increase the intracellular calcium concentration. After normalizing by the quantity of neurotransmitter released, the fraction of dye that escaped during the combined stimulation was 72.7 ± 2.0% (218 synapses in 2 experiments), as compared with 67.6% +/− 4.8% (230 synapses, 3 experiments, the “initial experiment”). Although the terminal was loaded with calcium by action potential stimulation, the proportion of “kiss and run” events elicited in the presence of hypertonic solution remained, within experimental error (P > 0.1 by t test), unchanged.
An alternative approach to this question is to decrease the calcium concentration produced by action potentials (rather than increase the calcium concentration during hypertonic stimulation). To do this, we preincubated the preparation with the membrane-permeable calcium buffer EGTA–acetoxymethyl ester (see Materials and Methods). This procedure is sufficient to prevent the calcium-produced increase in the rate at which empty sites in the RRP are refilled (6) and to inhibit the increased rate of asynchronous release that occurs with repetitive action potential stimulations (data not shown). After normalizing for the amount of transmitter release, each exocytotic event with 0.5 osM hypertonic solution released on average 67.6% +/− 4.8% (230 synapses, 3 experiments, the “initial experiment”) of the amount of dye that left a vesicle when exocytosis was caused by action potentials with the usual accumulation of intracellular calcium. When intracellular calcium was buffered with EGTA, superfusion with 0.5 osM hypertonic solution caused each exocytotic event to release on average 73.8 +/− 2.4% (279 synapses in 2 experiments, from Fig. 1) of the dye released with action potentials. If increased intracellular calcium concentration shifted exocytotic events to the “kiss and run” mode, this should have partially prevented dye release caused by action potentials and therefore should have produced an apparent increase in dye release with hypertonic solution (because dye released per vesicle with hypertonic stimulation is normalized by the dye release with action potentials). Dye release in the two conditions is within experimental error the same (P > 0.1 by t test), so calcium buffering in the presynaptic terminal with EGTA does not seem to alter significantly the relative fraction of “kiss and run” events.
The “Kiss and Run” Mode of Release Could Be Simply the Reversal of the First Step in the Exocytotic Processes.
Ceccarelli and collaborators (19) originally proposed that transmitter release can occur in a way that leaves the vesicle intact, and they called this mode of release “kiss and run.” The opening of a fusion pore can permit neurotransmitter to leave the vesicle, and the subsequent pore closure can return the vesicle to its initial state (but without transmitter). This picture has proved to be accurate for some secretory events by large dense-core vesicles. Capacitance and amperometry measurements from neuroendocrine cells have shown that the intermediate state (fusion pore open) is relatively stable, with a lifetime of many milliseconds (15, 20), and that the closed-to-open fusion pore transition is, at least sometimes, reversible. The obvious postulate is that our “kiss and run” mode of exocytosis represents the reversible opening and then closing of the fusion pore as envisioned by Ceccarelli and collaborators (19). Vesicle fusion is, at least in part, a mechanical process and hypertonic solutions presumably act by changing the energy barrier for steps in the fusion reaction by altering membrane tension and curvature. To test this notion, we have formalized the picture as a simple quantitative theory.
Suppose that a vesicle with an open fusion pore (a vesicle in the intermediate state) can either return to its closed (but now empty) state at a rate α or proceed on, with a rate β, to the next step in the fusion process. The probability P that a vesicle in the intermediate state will close its fusion pore, then, is P = 1/(1 + β/α); this is, in terms of our picture, the fraction of exocytotic events that occur in the “kiss and run” mode. According to absolute rate theory, the transition rates depend exponentially on the energy barriers that must be surmounted to pass out of the intermediate state, so that β/α = exp(−E/kT), where E is the difference in energy barriers for the two transitions (height of the barrier for the β transition minus the barrier for the α transition), k is the Boltzmann constant, and T the absolute temperature. The energy barrier E depends, according to our theory, on the hypertonicity h; we do not know the functional form of this dependence, but the slow variation of p(h) with h provides justification for expanding E(h) in a power series and retaining only the two lowest order terms. With this simplest approximation, the fraction of “kiss and run” events becomes
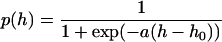 |
with two constants a and h0.
To compare this theory with the data in Fig. 1, we must examine more closely how the Fig. 1 data are presented. We measured the average quantity of dye loss per exocytotic event produced by hypertonic solution, and divided this quantity by the dye loss per exocytotic event produced by action potential stimulation. The fraction of dye lost f(h) is f(h) = 1 − p(h), where p(h) gives the fraction of events for which no dye is lost (“kiss and run” events). What is plotted in Fig. 1, however, is not f(h) but rather F(h) = f(h)/f(0) because f(0) is the fraction of dye that escapes from the vesicle with action potentials where hypertonicity is h = 0; F(h) is the dye released with hypertonic stimulation normalized to the release with action potentials. The equation to compare with the data in Fig. 1, then, is
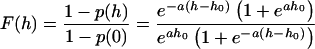 |
which still depends on two parameters, a and h0.
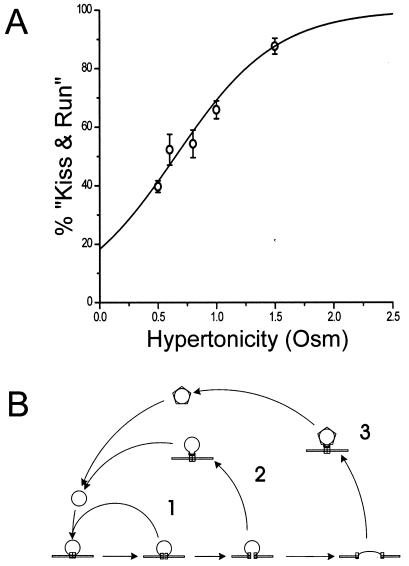
We have fitted the data in Fig. 1 with the equation for F(h) and estimate that a = 2.3 per osM and h0 = 0.65 osM. With these constants, the fraction of “kiss and run” events at h = 0 [that is, with action potentials in isotonic medium) is p(0) = 0.18. To display the fraction of “kiss and run” events as a function of hypertonicity, we estimated this fraction from the observed values F(h) in Fig. 1 by using the relation p(h) = 1 − F(h)(1 − p(0)], with p(0) = 0.18. The values of p(h) obtained are plotted in Fig. 4A with the theoretical curve [p(h) with a = 2.3 and h0 = 0.65]. The agreement between theory and experiment is clearly adequate. We conclude that the “kiss and run” interpretation can account for our data and that, according to this interpretation, about 18% of the exocytotic events would normally be of this sort under the conditions of our experiments.
Figure 4.
“Kiss and run” in normal synaptic transmission.
(A) Predicted and observed percentage of “kiss and
run” events as a function of hypertonicity. Theoretical function is
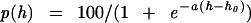 ,
where h is the hypertonicity in osmolars, with a
= 2.3 and h0 = 0.65. Experimental
points are derived from those in Fig. 1. Note that p(0)
= 18.3%, which corresponds to the percentage of action
potential-evoked exocytotic events that are “kiss and run.”
(B) Cartoon of three pathways. (1) “Kiss and run,” in
which the fusion pore opens and then closes to return the vesicle to
its docked state intact; (2) rapid endocytosis that requires dynamin to
pinch the vesicle membrane off from the surface membrane, but not
clathrin; and (3) the classical endocytic pathway that requires both
dynamin and a clathrin coat that is removed after endocytosis.
,
where h is the hypertonicity in osmolars, with a
= 2.3 and h0 = 0.65. Experimental
points are derived from those in Fig. 1. Note that p(0)
= 18.3%, which corresponds to the percentage of action
potential-evoked exocytotic events that are “kiss and run.”
(B) Cartoon of three pathways. (1) “Kiss and run,” in
which the fusion pore opens and then closes to return the vesicle to
its docked state intact; (2) rapid endocytosis that requires dynamin to
pinch the vesicle membrane off from the surface membrane, but not
clathrin; and (3) the classical endocytic pathway that requires both
dynamin and a clathrin coat that is removed after endocytosis.
Discussion
Our main conclusion is that a fraction of the exocytic events occur in a “kiss and run” mode at hippocampal synapses. We estimate that this fraction is normally 10 to 20% and can account for our data with a theory that views “kiss and run” as simply the reversal of the first step in the usual release process. This “kiss and run” mode of release seems not, however, to be affected by manipulations of the presynaptic calcium, unlike what has been reported for chromaffin cells (18).
During “kiss and run,” how could neurotransmitter be released without dye exchange? Zenisek et al. (4) report that FM1–43 escapes from synaptic ribbon vesicles undergoing exocytosis by rapidly diffusing (< about 20 ms) into the surrounding surface membrane. Therefore, “kiss and run” may represent a special configuration of the fusion pore such that dye has no access to the surface membrane. Another possibility is that “kiss and run” represents a very rapid transient opening of the fusion pore for <6 ms such that glutamate can escape but dye does not have time to exchange. Finally, because FM1–43 is about four times larger than glutamate, the dye may not be able to pass through the fusion pore, although glutamate can. Although we conclude “kiss and run” is a distinct mode of exocytosis, we cannot distinguish whether it is a consequence of a special fusion pore configuration or extremely brief time of exposure of the vesicle interior.
An alternative explanation for our data is possible although, we believe, unlikely. Suppose a few vesicles release (or take up) dye, and then these same vesicles rapidly recycle to repeatedly release neurotransmitter. Effectively, then, a smaller pool of vesicles would be releasing (or taking up) dye so that other vesicles would have no opportunity to release their dye (or to become stained). Although this is a logical possibility, it would mean that some vesicles would have to release, refill, dock, prime, and release again within about a second. For example, the 1-osM solution is applied for one second, and dye release is less than one-half the amount with action potentials; each vesicle would have to be able to release twice per second, much faster than reported previously. We would view this rapid cycling as a type of “kiss and run.”
Henkel and Betz (21) have previously reported that, after staurosporine treatment, FM1–43 release is prevented at the frog neuromuscular junction while acetylcholine release is intact. Because staining was not prevented—dye uptake was 1/2 to 2/3 of normal—staurosporine treatment appears to modify the vesicle trafficking in such a way that the vesicle interior is exposed for several milliseconds (to permit some vesicle staining) but not long enough for any appreciable amount of dye to escape. This staurosporine-induced process may be very similar, if not identical, to the “kiss and run” mode of exocytosis described here.
Although “kiss and run” exocytosis is accepted for neuroendocrine cells, the exact sequence of steps that constitutes this mode of release is unclear. The original view of “kiss and run” seemed to involve simply the closure of a fusion pore (19) without specific requirements for what happened next. Some authors have interpreted a fast mode of endocytosis that is reported to use dynamin, but not clathrin, as constituting “kiss and run” (20, 22, 23). Because not all vesicles need pass through an endosomal compartment to be reconstituted (24), and because vesicles docked to the active zone can undock (8), presumably without a need for dynamin, one can imagine an alternative pathway for terminating exocytosis that is very close to the original Ceccarelli view in which the fusion pore simply closes. In any case, we believe that a distinction must be drawn between possible mechanisms (Fig. 4B) in which: (i) an open fusion pore just closes, returning the vesicle to its prior, but now empty, state (this seems to be the mechanism studied here); (ii) a rapid endocytic pathway that uses dynamin but not clathrin [this would be the mode described by Artalejo et al. (22)]; and (iii) a classical Heuser–Reese endocytic mode for which clathrin is required (25).
Evidence for “kiss and run” of small vesicles at synapses is scant. Klingauf et al. (26) have argued for a fast mode of endocytosis at hippocampal synapses based on a difference in the rate at which FM1–43 and FM2–10 are lost from stained vesicles. These authors interpret their kinetic data as indicating the existence of two endocytic processes: one with a time constant of 8 seconds and the other with a time constant of 1.7 seconds. They were, therefore, presumably studying a rapid clathrin-independent process (mechanism 2 in Fig. 4B) or an accelerated classical Heuser–Reese endocytic pathway (mechanism 3 in Fig. 4B).
Our data, taken with other information, indicate that the trafficking of small synaptic vesicles at central synapses is rather complicated. After a vesicle has formed a fusion pore, it has the choice of retracing its path (what has been identified as “kiss and run” exocytosis, pathway 1 in Fig. 4B) or proceeding on to a dissociation of the fusion pore and a “flattened” state. Flattened vesicles seem to have at least two choices of how to return to the vesicle pool (pathways 2 and 3 in Fig. 4B), both of which require dynamin, and one that is clathrin mediated. The challenge for the future is to discover what regulatory mechanisms govern these various choices, under what conditions they are used, and for what molecules are responsible.
Acknowledgments
We thank Mary Anne Pilla, Richard Jacobs, Dinah Misner, Venki Murthy, Jane Sullivan, and John Wesseling for their assistance. This work was supported by the Howard Hughes Medical Institute and by a grant from National Institutes of Health (to C.F.S.). J.H.W. was supported by the medical scientist training program at University of California San Diego.
Abbreviations
- osM
osmolar
- RRP
readily releasable pool
- mEPSC
miniature excitatory postsynaptic current
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230438697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230438697
References
- 1.Cochilla A J, Angleson J K, Betz W J. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Rosenmund C, Stevens C F. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 3.Neves G, Lagnado L. J Physiol. 1999;515.1:181–202. doi: 10.1111/j.1469-7793.1999.181ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenisek D, Steyer J A, Almers W. Nature (London) 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- 5.Stevens C F, Wesseling J F. Neuron. 1999;22:139–146. doi: 10.1016/s0896-6273(00)80685-6. [DOI] [PubMed] [Google Scholar]
- 6.Stevens C F, Wesseling J F. Neuron. 1998;21:415–424. doi: 10.1016/s0896-6273(00)80550-4. [DOI] [PubMed] [Google Scholar]
- 7.Stevens C F, Tsujimoto T. Proc Natl Acad Sci USA. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy V N, Stevens C F. Nat Neurosci. 1999;2:503–507. doi: 10.1038/9149. [DOI] [PubMed] [Google Scholar]
- 9.Furshpan E J, MacLeish P R, O'Lague P H, Potter D D. Proc Natl Acad Sci USA. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal M M, Furshpan E J. J Neurophysiol. 1990;64:1390–1399. doi: 10.1152/jn.1990.64.5.1390. [DOI] [PubMed] [Google Scholar]
- 11.Bekkers J M, Stevens C F. Proc Natl Acad Sci USA. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekkers J M, Stevens C F. J Neurophysiol. 1995;73:1145–1156. doi: 10.1152/jn.1995.73.3.1145. [DOI] [PubMed] [Google Scholar]
- 13.Stevens C F, Tsujimoto T. Proc Natl Acad Sci USA. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan T A, Smith S J, Reuter H. Proc Natl Acad Sci USA. 1996;93:5567–5571. doi: 10.1073/pnas.93.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez de Toledo G, Fernandez-Chacon R, Fernandez J M. Nature (London) 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 16.Fesce R, Meldolesi J. Nat Cell Biol. 1999;1:E3–E4. doi: 10.1038/8950. [DOI] [PubMed] [Google Scholar]
- 17.Borges R, Travis E R, Hocstetler S E, Wightman R M. J Biol Chem. 1997;272:8325–8331. doi: 10.1074/jbc.272.13.8325. [DOI] [PubMed] [Google Scholar]
- 18.Alés E, Tabares L, Poyato J, Valero V, Lindau M, Alvarez de Toledo G. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- 19.Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 20.Henkel A W, Almers W. Curr Opin Neurobiol. 1996;6:350–357. doi: 10.1016/s0959-4388(96)80119-x. [DOI] [PubMed] [Google Scholar]
- 21.Henkel A W, Betz W J. J Neurosci. 1995;15:8246–8258. doi: 10.1523/JNEUROSCI.15-12-08246.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artalejo C R, Henley J R, McNiven M A, Palfrey H C. Proc Natl Acad Sci USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palfrey H C, Artalejo C R. Neuroscience. 1998;83:969–989. doi: 10.1016/s0306-4522(97)00453-3. [DOI] [PubMed] [Google Scholar]
- 24.Murthy V N, Stevens C F. Nature (London) 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- 25.Heuser J E, Reese T S. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingauf J, Kavalali E T, Tsien R W. Nature (London) 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]