Abstract
Aims
To compare the onset and duration of action of the new antihistamine levocetirizine with that of the second-generation antihistamine fexofenadine using the Vienna Challenge Chamber (VCC). The latter is an environment where subjects can be exposed to specific aeroallergens in controlled and reproducible conditions allowing for precise comparisons of anti-allergic drugs.
Methods
Ninety-four subjects received a single dose of levocetirizine 5 mg, fexofenadine 120 mg or placebo in a random order using a three-way cross-over design. On day 1, subjects were exposed to grass pollens (1500 grains/m3) in the VCC over a period of 4 h. Treatment was given 2 h after the start of challenge. On day 2, 22 h after drug intake, subjects were again exposed to pollens for 6 h. Specified symptoms were assessed by the subjects every 15 min using 5-point scales. The main efficacy parameter was the change from baseline in the Major Symptom Complex Score (MSCS = sum of rhinorrhea, sneezing, itchy nose and eyes).
Results
Baseline characteristics, including symptoms scores, were similar in the three study groups. During the first 2 h after drug intake both antihistamines achieved clinically relevant and significant (P < 0.001) improvements in symptom scores. Twenty-two to 24 h after drug intake, mean (SEM) MSCS reductions were: 1.9 (0.3) after placebo (baseline of 9.7), 3.8 (0.3) after fexofenadine (baseline of 9.9), and 5.1 (0.3) after levocetirizine (baseline of 9.8). Levocetirizine was significantly (P < 0.001) more effective than fexofenadine with a score difference of 1.3 (95% CI 0.7, 1.9). This was maintained until the end of the study (up to 28 h).
Conclusions
A rapid onset of action in alleviating seasonal allergic rhinitis (SAR) symptoms of subjects exposed to grass pollens in the VCC was observed after levocetirizine and fexofenadine. Levocetirizine was more effective than fexofenadine at and later than 22 h after drug intake, an indication of the longer-duration of action of levocetirizine.
Keywords: antihistamine, fexofenadine, levocetirizine, SAR, seasonal allergic rhinitis, VCC
Introduction
Seasonal allergic rhinitis (SAR) is a disease sometimes ignored either because it is undiagnosed or misdiagnosed as other disorders [1], e.g. recurrent colds, or because it is not perceived as serious enough to be treated promptly. Various studies have shown that it may impair patients’ quality of life (QoL) [2] and can be a costly disease [3], which is partly due to absenteeism from work or due to reduced productivity at work. Controlling symptoms quickly and efficiently could be decisive not only in reducing the number of lost working days due to rhinitis but could also improve the patient's QoL. Fast and sustained control of SAR symptoms is a necessity while, at the same time, treatment should not interfere with daily activities. A potent antihistamine can achieve this goal if its fast onset and long duration of action with once daily administration is clearly and consistently demonstrated in SAR subjects.
Antihistamines have been successfully used for years in the treatment of SAR and differences between first and second-generation molecules are well established. Recently, several new antihistamines, e.g. desloratadine and levocetirizine, have been launched but the increased choice of medications makes it more difficult for the clinician to differentiate between the clinical efficacy of the various medications. They are all safe, with negligible sedative effects, excellent tolerability and have no influence on cardiac parameters [4].
Although differences between the antihistamines at the receptor level, e.g. affinity and selectivity, have been reported [5], the usual clinical trials conducted with patients during the normal allergic seasons may fail to detect differences in efficacy [6]. The latter could be important not only for the patient's well-being and QoL but could also indicate the antihistamine which provides better control of the invisible underlying process of minimal persistent inflammation (MPI) [7].
There could be various reasons for the difficulty in detecting differences between the various medications. A possible explanation could be the high variability of the pollen count between the various seasons and the difficulties in measuring treatment compliance (trial subjects go home and the measurement of whether they have taken their medication is indirect). Thus, there is a need for controlled environments, e.g. the Vienna Challenge Chamber (VCC), where differences in efficacy can be detected much more reliably and precisely.
Levocetirizine is a new, potent [8] and highly selective H1-antihistamine [5], with a fast onset of action [9]. It has consistently been shown to have superior antihistamine activity in the skin of adult volunteers compared with a number of other available antihistamines including fexofenadine [8]. The latter has been shown in numerous studies to be effective in relieving the symptoms of SAR, including nasal congestion [10]. Since data comparing the newer with older antihistamines in patients are limited, we compared the effectiveness of single doses of the second-generation antihistamine fexofenadine 120 mg and the new antihistamine levocetirizine 5 mg, in alleviating, over a 28 h period, the symptoms of SAR in subjects exposed to grass pollens.
Methods
Study design and population
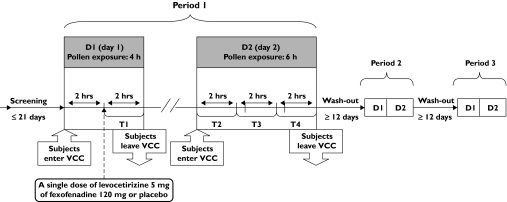
This was a double-blind, randomized, placebo controlled, three treatment, three-period cross-over study (Figure 1) performed outside of the pollen season in subjects known to suffer from SAR. In each study period, over 2 consecutive days, the subjects were exposed to a controlled grass pollen concentration in the VCC as described elsewhere [11].
Figure 1.
Study design flowchart. T1 = Time interval 1 (0–2 hrs after medication intake), T2 = Time interval 2 (22–24 hrs after medication intake), T3 = Time interval 3 (24–26 hrs after medication intake), T4 = Time interval 4 (26–28 hrs after medication intake)
Subjects with documented allergy to grass pollens were enrolled in the study. Allergy was documented by a (+) RAST (= class 3 or = 3.5 IU ml−1) and/or positive skin prick test (wheal = 3 mm larger than the diluent control) to grass pollen allergens performed within the previous year. Women of childbearing potential were eligible if they were not sexually active or if they were following a medically accepted contraceptive method. Subjects were excluded from the study if they used the following medications (number of days before start of study): systemic or topical corticosteroids (30); ketotifen, nedocromil or cromoglicate (14); loratadine, oxatomide or desloratadine (10); systemic theophylline or any other antihistamines (7); systemic or topical decongestants (3); or any sympathomimetics, including nasal and eye drops (1). Subjects with an ENT infection (previous 30 days), allergic bronchial asthma, known cardiac, renal or hepatic dysfunction were not allowed in the study.
No rescue therapy was allowed for the alleviation of allergic rhinitis symptoms. However, during the time spent in the VCC and thereafter, the use of a local β2-sympathomimetic inhaler for immediate relief of asthmatic symptoms was allowed. The following treatments were prohibited between the first and last visits: corticosteroids (systemic and topical), ketotifen, nedocromil or cromoglicate, theophylline (systemic), all other antihistamines, decongestants (systemic, local), all sympathomimetics (including nose and eye drops), ongoing desensitization.
Subjects were randomized to receive a single dose of levocetirizine 5 mg, fexofenadine 120 mg or placebo in a random order using a three-way cross-over design with at least a 12 day washout period between drugs. The tablets of levocetirizine, fexofenadine and placebo were placed inside opaque capsules identical in shape, size and colour in order to allow a double-blind administration. On day 1, subjects were exposed to grass pollens (1500 grains/m3) in the VCC for 4 h. Drugs were given 2 h after the start of the challenge. On day 2, they were again exposed to pollens for 6 h (22–28 h after drug intake).
Efficacy and safety assessment
The primary efficacy parameter was the Major Symptoms Complex Score (MSCS = sum of rhinorrhea, sneezing, itchy nose and itchy eyes). Additionally nasal congestion, nasal resistance (NR, measured by active anterior rhinomanometry), amount of nasal secretions (measured by the weight of handkerchiefs), global evaluation of subject satisfaction and readiness to use the same medication were also evaluated. Safety information was collected by continuously monitoring the adverse events (AEs) and was assessed through the recording of vital signs (blood pressure and heart rate) and FEV1 (in case of occurrence of asthmatic symptoms).
Study assessments were performed over four time intervals as summarized in Figure 1. Upon entering the VCC (on both study days), subjects recorded their symptoms every 15 min on a computer using a 5-point scale ranging from 0 (absent) to 4 (severe > 5 sneezes). The MSCS could range from 0 to 16, with higher scores corresponding to larger impairment. Subject satisfaction was assessed every 15 min using a visual analogue scale (VAS) ranging from 0 mm to 100 mm (the higher the score, the greater the satisfaction of the subject). Rhinomanometry and the weighing of handkerchiefs (to quantify nasal secretions) were performed every 30 min.
The primary efficacy variable was the change from baseline in MSCS during time interval 2 (22–24 h after drug intake). Changes in MSCS were calculated using the mean of the 5-point scales for the individual symptoms. Major secondary variables included the change from baseline in MSCS and the individual symptoms during all time intervals and the difference from baseline in the subject's global evaluation of satisfaction and the subject's readiness to use the same medication in the future.
Statistical methods
All the efficacy and safety variables were analyzed on the intent-to-treat population (ITT = all randomized subjects who received at least one dose of study medication). MSCS was also analyzed on the per-protocol population (PPT = the ITT population with no or only minor deviation to the protocol).
All statistical tests were carried out two-tailed at the 5% level of significance. The primary variable was analyzed using an ancova model adapted for crossover design with baseline score as covariate. No carry-over effect was anticipated due to the presence of a sufficient washout period of 12 days between each of the three treatment periods, during which the subjects received no medications. The treatment comparisons were described using 95% confidence intervals for the difference in least square means.
The safety population included all subjects in the study. All safety data were listed. Adverse events, vital signs, concomitant medications and concomitant procedures were summarized by treatment group.
The calculation of the sample size was based on the results of a study performed at the same centre with cetirizine and fexofenadine [11], which assessed the effects of treatment on SAR symptoms (sneezing, runny nose, itchy nose, nasal obstruction, watery eyes, itchy eyes/ears, itchy throat and cough) in 40 subjects exposed to a controlled grass pollen concentration for 6 h on 2 consecutive days. The study was designed to enrol 84 subjects in order to detect a difference of 0.9 between the mean MSCS of levocetirizine and fexofenadine with a power of 90% at the 5% level of significance.
The study was performed in accordance with the Declaration of Helsinki and the relevant ethics committee approvals were obtained before the start of the study. Informed consent was obtained from each subject.
Results
Demographic and baseline data
A total of 94 subjects (age 25.8 ± 4.5 (SD) years; 56 F and 38 M) who had a mean disease duration of 12.7 ± 7.2 years, were randomized to receive study treatments. Eighty-four subjects completed the study.
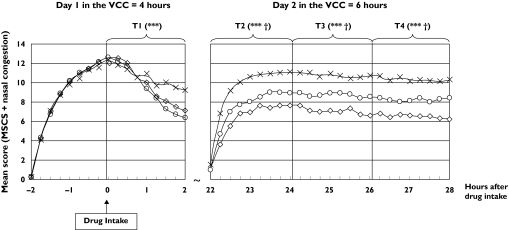
All the subjects had a 4 h exposure to allergen during day 1. After 2 h in the VCC, they received one dose of treatment. Symptom severity reached a peak 2 h after exposure, just before medication intake (baseline) with mean MSCS reaching 9.7, 9.8 and 9.9 in the placebo, levocetirizine and fexofenadine groups, respectively. There was no significant difference between the three treatment groups at baseline. Rhinorrhea was the most severe symptom whereas ocular itching was the least severe symptom at baseline in all groups.
Total and individual symptom scores
On average, 22–24 h after drug intake, the largest improvement in MSCS was achieved with levocetirizine where the adjusted mean reduction from baseline reached −5.10 as shown in Table 1. The difference between levocetirizine and fexofenadine was −1.27 and was significantly in favour of levocetirizine (P < 0.001). When compared with placebo, the difference was significantly in favour of both active medications.
Table 1. MSCS mean scores (SD) during time interval 2 (primary efficacy endpoint). Negative values indicate improvements.
| Treatment | Baseline | Mean change from baseline | Difference vs placebo (95% CI) | Difference vs fexofenadine (95% CI) |
|---|---|---|---|---|
| Placebo | 9.69 (2.59) | −1.87 (0.26) | ||
| Levocetirizine 5 mg | 9.83 (2.99) | −5.10 (0.27) | −3.23 (−3.83, −2.63)*** | −1.27 (−1.87, −0.67)*** |
| Fexofenadine 120 mg | 9.91 (2.71) | −3.84 (0.26) | −1.96 (−2.56, −1.37)*** |
P < 0.001.
PPT analysis confirmed the ITT findings. The difference between levocetirizine and fexofenadine was equal to −1.32 in favour of the levocetirizine group with a 95% confidence interval of −1.94, −0.71. The difference was highly statistically significant (P < 0.001).
Both active treatments improved symptoms within the first 2 h when statistically significant differences vs placebo were achieved (−0.89, P < 0.001 and −1.30, P < 0.001 in favour of levocetirizine and fexofenadine, respectively). These differences from placebo became even larger during day 2 when, in addition, differences between the two active treatment groups also emerged. During time interval 3 (24–26 h post dose) levocetirizine treatment was significantly (P < 0.001) better than fexofenadine with a difference favouring levocetirizine of −1.62 (−2.26, −0.98). This significant superiority (P < 0.001) was maintained until the end of the study (28 h post dose) with a difference favouring levocetirizine of −1.66 (−2.33, −1.00).
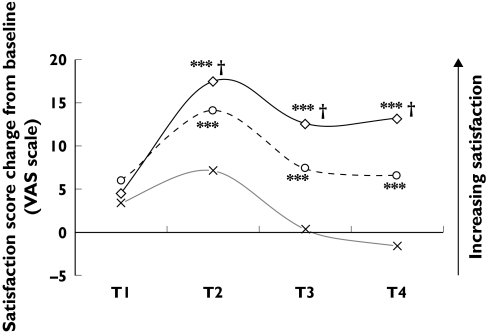
When nasal congestion, a very important and disturbing rhinitis symptom, was added to the MSCS similar significant differences were observed in this study. Among the three treatment groups, levocetirizine was the best performer achieving statistically significant superiority not only against placebo but also against fexofenadine (Figure 2).
Figure 2.
MSCS plus nasal congestion score change from baseline over all time intervals. ***P < 0.001 (difference vs placebo); †P < 0.001 (difference in favour of levocetirizine vs fexofenadine). Levocetirizine (◊), Fexofenadine (○), Placebo (×)
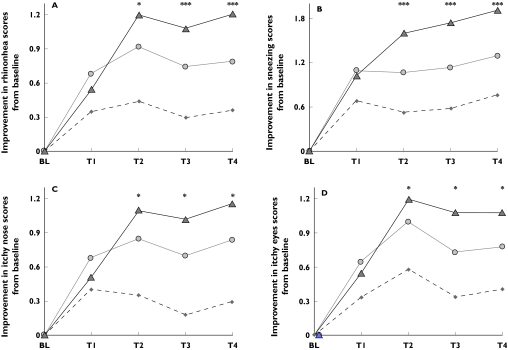
Individual symptoms largely followed the pattern observed for MSCS. In the active treatment groups, there were consistently significant improvements (P < 0.001) from baseline over placebo for all the four major symptoms. In addition, during day 2, improvements in the levocetirizine group reached significant superiority vs fexofenadine (Figure 3). The largest improvements were observed for sneezing and rhinorrhea. Although ocular itching was of milder severity at baseline, improvements in its score were comparable with nasal itching and rhinorrhea.
Figure 3.
Improvements in the mean change from baseline of the individual symptom scores comprising MSCS. ***P < 0.001; *P < 0.05 (P values are for differences of levocetirizine vs fexofenadine). A = rhinorrhea; B = sneezing; C = Nasal itching; D = Eye itching. T1 = 0–2 hours after drug intake, T2 = 22–24 hours after drug intake, T3 = 24–26 hours after drug intake, T4 = 26–28 hours after drug intake. Placebo (♦), fexofenadine ( ), levocetirizine (▴)
), levocetirizine (▴)
Blocked nose was measured either as the symptom ‘nasal obstruction’ or as an objective sign ‘nasal congestion’ assessed by measuring the nasal airflow (cm3/s) by rhinomanometry. The latter could not reveal any differences between the three treatment groups. Although during t4 the active medications reached statistically significant improvement vs placebo (P = 0.006 for levocetirizine and P = 0.014 for fexofenadine) nasal airflow was not significantly different during the other time intervals. Similar to the other symptoms, however, nasal obstruction was reported by the subjects as being improved vs baseline in both active medication groups. Differences vs placebo ranged from P = 0.001 (t2) to P = 0.002 (t4) for levocetirizine and from P = 0.001 (t2) to P = 0.08 (t3) for fexofenadine. There was no statistically significant difference for nasal obstruction between the two antihistamines.
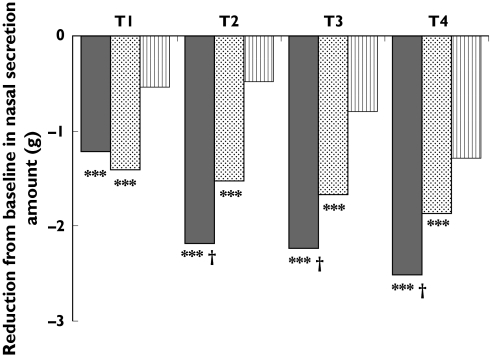
The reduction in nasal secretion, as measured by the difference between the pre and post dose weight of the handkerchiefs, was statistically greater in the levocetirizine group (P < 0.001) as compared with both the fexofenadine and the placebo groups (Figure 4).
Figure 4.
Reduction from baseline in the amount of nasal secretions over all time intervals as measured by the weight of the handkerchiefs. ***P < 0.001 (difference vs placebo); †P < 0.001 (difference in favour of levocetirizine vs fexofenadine). T1 = 0–2 hours after drug intake, T2 = 22–24 hours after drug intake, T3 = 24–26 hours after drug intake, T4 = 26–28 hours after drug intake. Levocetirizine (▪), fexofenadine ( ), placebo (
), placebo ( )
)
In terms of intensity of treatment response, the most frequent category of MSCS reduction in the levocetirizine treatment shifted from the (20%−40%) category over time interval 1 to the (40%−60%) category over time interval 2 and the (60%−80%) category over time interval 4. For comparison, the most frequent category of reduction in the fexofenadine treatment remained the (20%−40%) category over time intervals 1 and 2, and moved down to the (<20%) category for time intervals 3 and 4, indicating that most of the fexofenadine activity was limited to the first hours after medication intake. This is inferior to the longer and stronger effectiveness of the levocetirizine treatment observed during day 2. For the placebo treatment, the most frequent category was the (<20%) reduction in MSCS during the whole study period with slightly more than half the subjects falling into this category.
When either 20% or 50% reduction in MSCS was taken as a threshold, no significant differences between the active medications were observed during day 1. However, during all time intervals on day 2, the differences became statistically significant in favour of levocetirizine vs fexofenadine with more than 90% of the levocetirizine subjects achieving a 20% reduction in MSCS compared with approximately 70% of the fexofenadine subjects. Similarly, about half the subjects achieved at least a 50% reduction with levocetirizine compared with about one-third with fexofenadine.
Scores for subject satisfaction and readiness to use medication
The profiles of the global evaluation of satisfaction by the subject were consistent with the profiles of the symptoms. During the first 2 h after medication intake, there was a slight improvement of satisfaction over baseline value, with no statistically significant difference between the two active treatments (Figure 5). During all the assessment periods on day 2, treatment with levocetirizine was significantly better than that with fexofenadine in improving satisfaction. The differences in favour of levocetirizine grew throughout day 2 with values equal to 5.07 (0.90, 9.24) (P = 0.017) during time interval 3 and increasing to 6.66 (2.56, 10.76) (P = 0.002) during time interval 4.
Figure 5.
Improvement (change from baseline) in subject satisfaction score. ***P < 0.001 (improvement vs placebo); †P < 0.05 (difference in favour of levocetirizine vs fexofenadine). Levocetirizine (◊), fexofenadine (○), placebo (×)
When asked if they were ready to use the same treatment in the future, two thirds of the subjects treated with levocetirizine declared they were against half treated with fexofenadine. A quarter of the subjects treated with placebo were prepared to use it again. Both active treatments were statistically significantly superior to placebo treatment.
Safety
Safety and tolerability were similar between the treatment groups. Twelve subjects (12.8%) reported 13 treatment-emergent AEs (four in the placebo, five in the levocetirizine and four in the fexofenadine groups), all of which were assessed as not related to study medication. Six out of the 13 AEs were attributed to infections, three of them leading to study discontinuation (two after placebo and one after levocetirizine). No clinically relevant changes in vital signs were observed. No deaths or SAEs were reported in the study.
Discussion
This study demonstrates the potent and fast onset of action of the new antihistamine levocetirizine and the second-generation antihistamine fexofenadine. One hour after intake, both medications showed significant reductions in the MSCS as well as improvements in the individual rhinitis symptoms. Their potent efficacy was maintained for a period longer than 24 h with significant differences favouring levocetirizine over fexofenadine observed at and later than 22 h post dose.
In a similarly designed study, we had already compared the efficacy of the older antihistamine cetirizine, the predecessor of levocetirizine, and fexofenadine, in the Vienna Challenge Chamber [11]. Although cetirizine appeared to have a longer duration of action as compared with fexofenadine, the differences between the medications in MSCS were not statistically significant. On the contrary, the results of our current study indicate that the new antihistamine levocetirizine is at least as potent as cetirizine and revealed that levocetirizine is significantly more potent than fexofenadine in improving the symptoms of rhinitis 22–28 h after drug intake. The significantly longer duration of action of levocetirizine was accompanied by a significantly higher satisfaction with the levocetirizine treatment and a higher preference for levocetirizine use in the future.
The better findings with levocetirizine as compared with cetirizine could be attributed partially to differences in study designs. Although very similar, the designs of these two studies were not identical. During day 2 of our previous study comparing cetirizine and fexofenadine, subjects took a second dose of active medication 24 h after the first dose and therefore we could not measure duration of drug efficacy longer than 24 h. The results from both studies during the period 22–24 h, however, indicate a somewhat longer duration of efficacy for levocetirizine as compared with cetirizine. Unfortunately, no other studies have been done with fexofenadine in our chamber that would have allowed comparison of this antihistamine with other drugs of this class.
Experience from an earlier SAR study in a VCC with levocetirizine showed that it performed better than loratadine, another well-established second-generation antihistamine [12]. Levocetirizine was significantly more efficacious during the first 2 h after drug intake (faster onset of action) and reduced the MSCS more than loratadine 24 h after drug intake (longer duration of action).
The findings from our VCC studies discussed above are consistent with studies carried out in another environmental exposure unit (EEU). A study, enrolling subjects suffering from SAR during the pollen season and challenged with ragweed pollen in the EEU, compared desloratadine with levocetirizine [9]. The differences between the two antihistamines in reducing SAR symptoms were significant not only at 24 h but also during the early 1–3 h after medication intake.
The findings of the studies discussed above have been confirmed by other sensitive models of antihistaminic activity. For example, a recently presented facial thermography (FT) study [13], using an infrared camera to detect small changes in skin temperature and nasal mucosal blood flow caused by the pro-inflammatory activity of histamine (after nasal provocation), confirmed the higher anti-H1 activity of levocetirizine vs desloratadine 2 h after intake of study medications. A similar study, in the same FT model, comparing levocetirizine and fexofenadine [14] produced results similar to our findings since it also showed a similar onset of action (2 h after drug intake) for the two antihistamines and a significantly longer duration (24 h after intake) of action for levocetirizine when compared with fexofenadine. Since no facial thermography studies have been performed comparing fexofenadine to other antihistamines, no further comparisons can be made.
In conclusion, the results of this study show that both levocetirizine and fexofenadine control the symptoms of SAR as early as the first 2 h following administration. Levocetirizine was more effective than fexofenadine at or later than 22 h following treatment. The superiority of levocetirizine over fexofenadine in our environmental model is supported by other clinical and non clinical findings. Our results are an important instrument for the treating physician to select the most appropriate antihistamine for their patients based on a sensitive and clinically relevant model.
Acknowledgments
This study was sponsored by UCB Farchim, Bulle, Switzerland
References
- 1.Skoner DP. Allergic rhinitis. definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(1 Suppl):S2–8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 2.Stuck BA, Czajowski J, Hagner AE, Klimek L, Verse T, Hörmann K, Maurer JT. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol. 2004;113:663–8. doi: 10.1016/j.jaci.2003.12.589. [DOI] [PubMed] [Google Scholar]
- 3.Crystal-Peters J, Crown WH, Goetzel RZ, Schutt DC. The cost of productivity losses associated with allergic rhinitis. Am J Manag Care. 2000;6:373–8. [PubMed] [Google Scholar]
- 4.Casale TB, Blaiss MS, Gelfand E, Gilmore T, Harvey PD, Hindmarch I, Simons ER, Spangler DL, Szefler SJ, Terndrup TE, Waldman SA, Weiler J, Wong DF. First do no harm: managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111:S835–42. doi: 10.1067/mai.2003.1550. [DOI] [PubMed] [Google Scholar]
- 5.Gillard M, Christophe B, Wels B, Peck M, Massingham R, Chatelain P. H1 antagonists: receptor affinity versus selectivity. Inflamm Res. 2003;52(Suppl 1):S49–50. doi: 10.1007/s000110300050. [DOI] [PubMed] [Google Scholar]
- 6.Wilson AM, Haggart K, Sims EJ, Lipworth BJ. Effects of fexofenadine and desloratadine on subjective and objective measures of nasal congestion in seasonal allergic rhinitis. Clin Exp Allergy. 2002;32:1504–9. doi: 10.1046/j.1365-2745.2002.01509.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujikura T. New therapeutic approaches to the treatment of nasal allergy. Antiinflammatory effects of H (1) receptor antagonists. Drugs Today (Barc) 2001;37:455–61. doi: 10.1358/dot.2001.37.7.844188. [DOI] [PubMed] [Google Scholar]
- 8.Grant JA, Riethuisen JM, Moulaert B, DeVos C. A double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects. Ann Allergy Asthma Immunol. 2002;88:190–7. doi: 10.1016/S1081-1206(10)61995-3. [DOI] [PubMed] [Google Scholar]
- 9.Day JH, Briscoe MP, Rafeiro E, Ratz JD. Comparative Clinical Efficacy, Onset and Duration of Action of Levocetirizine and Desloratadine for Symptoms of Seasonal Allergic Rhinitis in Subjects Evaluated in the Environmental Exposure Unit (EEU) Int J Clin Pract. 2004;58:109–18. doi: 10.1111/j.1368-5031.2004.0117.x. [DOI] [PubMed] [Google Scholar]
- 10.Meeves SG, Appajosyula S. Efficacy and safety profile of fexofenadine HCl: a unique therapeutic option in H1-receptor antagonist treatment. J Allergy Clin Immunol. 2003;112:S69–77. doi: 10.1016/s0091-6749(03)01879-7. [DOI] [PubMed] [Google Scholar]
- 11.Horak F, Stübner P, Zieglmayer R, Kavina A, Vos CD3, Burtin B, Donnelly F. Controlled comparison of the efficacy and safety of cetirizine 10mg o.d. & fexofenadine 120mg o.d. in reducing symptoms of seasonal allergic rhinitis. Int Arch Allergy Immunol. 2001;125:73–9. doi: 10.1159/000053799. [DOI] [PubMed] [Google Scholar]
- 12.Stübner P, Zieglmayer R, Horak F. A direct comparison of the efficacy of antihistamines in SAR and PAR. randomised, placebo-controlled studies with levocetirizine and loratadine using an environmental exposure unit – the Vienna Challenge Chamber (VCC) Curr Med Res Opinion. 2004;20:891–902. doi: 10.1185/030079904125003700. [DOI] [PubMed] [Google Scholar]
- 13.Larbig M, Stamm H, Hohlfeld J, Krug N. Levocetirizine but not desloratadine inhibits histamine-induced changes of nasal temperature measured by facial thermography: a pilot study. EAACI 2003 Abstract Book pharma service GmbH. 2003:283. [Google Scholar]
- 14.Larbig M, Stamm H, Casper A, Hohlfeld J, Krug K. Twenty-four hour anti-H1 activity of levocetirizine measured by thermography is superior to fexofenadine. EAACI 2004 Abstract Book pharma service documediaS GmbH. 2004:80. p. [Google Scholar]