Abstract
Aims
To investigate the safety, tolerability and pharmacokinetics of DP-b99 in healthy volunteers. DP-b99 is a newly developed lipophilic, cell permeable derivative of BAPTA (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid), which selectively modulates the distribution of metal ions in hydrophobic milieu, and is in clinical development as a neuroprotectant for cerebral ischaemic stroke. To our knowledge no BAPTA derivative has ever been administered to man. Here we report the first human administration of DP-b99 in a phase I, two-part, double-blind, randomized placebo controlled study, with single IV doses of 0.003–1.0 mg kg−1 day−1 DP-b99 (part 1) or multiple ascending doses of 0.03–1.0 mg kg−1 day−1 DP-b99 over 4 days (part 2).
Methods
A double-blind, dose escalating tolerability study of DP-b99 in normal (young – aged between 18 and 40 years and elderly – aged between 65 and 85 years) healthy adult male volunteers was conducted. Part 1 of the study investigated single administration of ascending intravenous doses, and part 2 examined the effects of ascending doses given repeatedly over 4 days. Twenty-four young volunteers in part 1 received single dose administrations and 26 young volunteers in part 2 received repeated ascending dose administrations of either intravenous DP-b99 or placebo. Subsequently, 10 elderly volunteers received repeated intravenous DP-b99 (1 mg kg−1) or placebo in part 2 over 4 days. Adverse events were identified by either subject self reporting or based upon laboratory parameters (blood chemistry, complete blood cell count, prothrombin time (PT), activated partial thromboplastin (PTT), physical examination, vital signs (blood pressure, pulse rate, respiratory rate, body temperature) and urinalysis. A comprehensive set of cardiovascular parameters was assessed as well (blood pressure, 12 lead-ECG recordings and continuous bedside cardiac monitoring for 6 h on day 1).
Results
The administration of DP-b99 up to the highest dose of 1.0 mg kg−1 was well tolerated and had an acceptable safety profile up to the highest dose of 1.0 mg kg−1 tested in both study parts. No serious or severe adverse events were encountered. Eight mild to moderate adverse events were observed in six of the seven young subjects treated with four repeated doses of 1.0 mg kg−1, with reversible phlebitis being the most frequently reported adverse event. The drug was tolerated better at the injection site by the elderly group compared with the younger subjects. No adverse effects were observed in cardiovascular parameters sensitive to trans-membranous calcium concentrations. The pharmacokinetic parameters were derived by noncompartmental analysis. On day 1 following administration of 1 mg kg−1 the mean half-life of DP-b99 in young volunteers was 3.47 ± 0.90 h and in the elderly was 2.11 ± 0.09 h. On day 4 following the same administration of DP-b99 the mean half-life was 4.36 ± 1.49 and 2.10 ± 1.14 h in the young and elderly, respectively. There was higher systemic exposure in the elderly, for example Cmax, had a mean 1.6-fold higher exposure on day 1 (95% CI Lower 0.90, Upper 2.74) and 2.5-fold on day 4 (95%CI 1.70, 3.68). This increase is in line with the presumed central role of hepatic blood flow in the elimination of DP-b99. No accumulation was observed after repeated dosing with 1 mg kg−1 (mean accumulation calculated by AUC(0, 24 h) (day 4) : AUC(0, 24 h) (day 1) and was observed to be between 0.9 and 1.3 (young, elderly).
Conclusions
This study suggests that DP-b99 is well tolerated in healthy young and elderly volunteers within the dose range evaluated. Studies to investigate further the efficacy of the compound are in progress.
Keywords: BAPTA, calcium, chelator, DP-b99, ischaemia, stroke
Introduction
In the United States, stroke is the third leading cause of death and the leading cause of serious long-term disability [1]. The importance of spatial and temporal changes in neuronal [Ca2+]i during ischaemia has resulted in the development of calcium based therapeutics, such as Ca2+ channel blockers and calcium influx inhibitors (glutamate receptor antagonists), which have so far failed to demonstrate adequate efficacy, and were occasionally associated with safety concerns in clinical trials [2]. Growing evidence suggests that another ion, Zn2+, by its toxicity may also play a significant role in the pathogenesis of neuronal loss after ischaemic insults [3]. A parallel between zinc and calcium has been observed, in that both are divalent metal cations mediating ischaemic neuronal death via excess influx across the plasma membrane [4], and it has been suggested that a reduction in extracellular zinc accumulation [5] may provide an approach towards ameliorating pathological neuronal death.
BAPTA is a high affinity Ca2+ chelator. However, it is extremely hydrophilic and therefore impermeable to cell membranes [6]. BAPTA-AM (BAPTA acetoxymethyl ester), a cell permeant chelator was reported to be neuroprotective in models of ischaemia both in vitro and in vivo [7, 8]. However, its in vivo neuroprotective effects are only observed when BAPTA-AM is administered prior to ischaemia [9]. To the best of our knowledge BAPTA-AM has not been administered to humans, and remains in in vitro biological research due to its superior Ca2+ buffering properties [10].
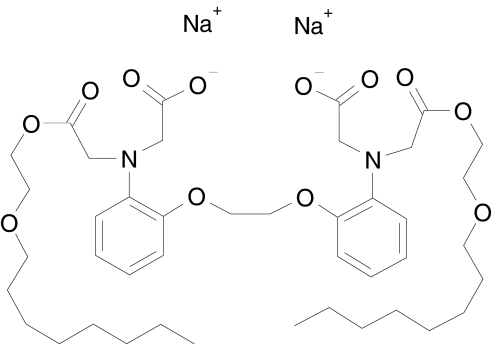
DP-b99, 1,2-bis(2′-aminophenoxy)ethane-N,N,N'N′-tetraacetic acid, N,N′-di(octyloxyethyl ester), N,N′-disodium salt (Figure 1), is a lipophilic, cell permeable derivative of BAPTA currently under development. It is characterized by its preferential binding activity for calcium, zinc and copper ions in cellular hydrophobic milieu, such as cell membranes. In vitro, DP-b99 protects primary cortical neurones from cell death induced by either deprivation of oxygen and glucose or hydrogen peroxide (H2O2), with protection conferred when DP-b99 is added to cells up to 4 h after the addition of H2O2 [11]. It was also shown to attenuate basal activity of MMP-9 as well as TNF-α induced MMP-9 activation in glia and C6 cells [11].
Figure 1.
Chemical structure of DP-b99 (1,2-Bis(2′-aminophenoxy)ethane-N,N′-di(2′-octyloxyethyl acetate)-N,N′-diacetic acid
In a rodent focal ischaemic model, DP-b99 attenuates the extent of cerebral infarction, even when treatment is administered 6 h following ischaemic insult [11]. DP-b99 substantially (by 20–30% over 7 days) increases survival in Mongolian gerbils [12] and in Sprague-Dawley rats in the permanent focal cerebral ischaemic model (10–30%) [11]. In a range of ischaemic animal models DP-b99 significantly reduces neurone-specific enolase (NSE) levels at 24 and 72 h following ischaemia [11][13].
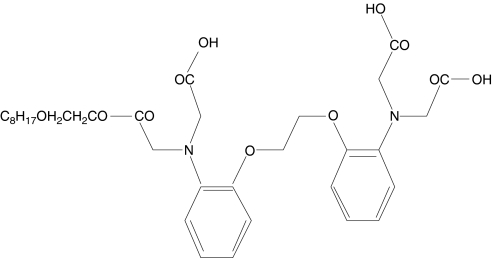
Absorption, distribution, metabolism and excretion (ADME) studies have shown that in human, dog and rat plasma the monoester (Figure 2) is the main metabolite of DP-b99. The hydrolysis to the monoester form is a result of plasma esterases. Additional metabolites were identified in rat bile, namely 17 phase I hepatic metabolism products and negligible amounts of phase II products. Several of the phase I metabolites were also observed in human, rat and dog plasma after intravenous administration of DP-b99, but at lower concentrations than the monester (unpublished data). The process of identifying the CYP450 enzymes involved with the phase I metabolism of DP-b99 is still ongoing. In animals given doses resembling (on a weight basis) the human doses (reported herein), the serum pharmacokinetic parameters were similar to those reported below in humans. It is of interest that distribution data in rat indicate that once the drug has entered the brain following an IV infusion its elimination from the brain is much slower than from serum, an event reflected by an increasing brain to serum ratio over the 24 h interval (data not shown).
Figure 2.
Monoester of DP-b99
The main potential clinical application of DP-b99 would be as a neuroprotectant in cerebral ischaemic stroke or trauma. To our knowledge, there have been no reports of BAPTA or any of its derivatives having been administered to humans previously. This report documents a double-blind, dose escalating, safety profile and tolerability study of DP-b99 in normal (young and elderly) healthy male volunteers. Part 1 of the study investigated single administration of ascending intravenous doses, and part 2 examined the effects of ascending doses given repeatedly over 4 days. This study was designed to provide initial information on the pharmacokinetics, safety profile and tolerability of DP-b99 in man. The primary endpoints of the study were the effects of DP-b99 on ECG, blood pressure, pulse rate, body temperature and changes in baseline laboratory values, adverse events and to determine the maximum tolerated dose in part 1. Secondary endpoints focused upon pharmacokinetics, clearance and plasma drug half-life across both studies.
Methods
Study population
The study was approved by the Ethics Committee at the Medical Board (Artztekammer) of Berlin and was conducted in accordance with the Declaration of Helsinki. All volunteers gave their written informed consent for participation in the study. Sixty-one healthy male volunteers were stratified to either Part 1 to receive a single intravenous single infusion of the test medication or to Part 2 to receive ascending doses repeatedly over a 4 day period. All subjects were male Caucasians, had normal eating habits, were non-smokers or were smokers of less than 10 cigarettes a day and were capable of giving written consent. Young (aged between 18 and 40 years) and elderly males (aged between 65 and 85 years) had to fulfil the following criteria for inclusion in the study: weight within ±10% of the ideal body weight of the scale proposed by the Metropolitan Insurance Company, 1983 [14], normal health 2–8 days prior to first administration of drug, based upon medical history, physical examination, vital signs, ECG and laboratory investigations with values falling within the normal range and also had to have normal blood pressure and heart rate values. In addition, the weight of the elderly subjects had to be within ±15% of their ideal body weight proposed by the Metropolitan Insurance Company 1983 [14]. At screening elderly volunteers were required to have systolic blood pressure within the range of 120–160 mmHg and diastolic blood pressure within 60–95 mmHg and to have a supine pulse rate of <90 beats min−1. The occurrence of the following prevented study enrolment, warranting exclusion: previous history of drug or alcohol abuse (alcohol breath test and urinary drug screening performed before study entry), previous acute or chronic systemic disease or disorder, hypersensitivity to at least one drug, previous use of sedatives, hypnotics, tranquilizers or any other addictive agents, excessive consumption of beverages containing caffeine (>500 mg of caffeine per day), positive for HIV antibodies, hepatitis C antibodies, hepatitis B surface antigen and/or hepatitis B core antibodies, received any drug during the month preceding the start of the study or had received over the counter medication 7 days prior to the study, received treatment which could have led to induction or inhibition of hepatic microsomal enzymes or modified bleeding time within 3 months of the start of the study.
Drugs
The test drug was provided in sterile vials containing DP-b99. Each subject was randomly assigned to receive either active drug or placebo (sterile saline, the drug's vehicle). At each dose level DP-b99 or placebo was clear and colourless in appearance and the solutions were prepared by an unblinded pharmacist according to a prespecified randomization list. The blinded investigators had no access to the dose preparation area. The required dose was prepared extempore as infusion solutions of 0.2 mg ml−1 strength, diluted in normal sterile saline for injection. The test solution was infused over 30 min in an upper extremity vein via an infusion pump. The same volume of normal saline (NaCl 0.9%) alone was provided as a placebo comparator.
In part 1, study subjects were randomized into four groups, two of the groups contained five subjects, four of whom received a single active dose of DP-b99 and one placebo; in the remaining two groups of seven volunteers, six were given an active dose of DP-b99 and one placebo. The repeated dosing study (part 2) divided subjects into four groups, three of these groups included only young male volunteers, and the fourth group elderly subjects. In each of these groups, seven of the subjects received repeated ascending doses of the active drug and two subjects placebo. Dose escalation levels are shown in Table 1. Progression from the lower dose to the next ascending dose was done only if the safety profile and tolerability of DP-b99 was acceptable and was decided by the blinded clinical investigators (physicians). The elderly group was to receive the highest dose tolerated by the young subjects (this was found to be 1 mg kg−1). No concomitant medication was allowed during either part of the study or during the follow up period, except for paracetamol, where symptom relief was judged necessary by the investigator. Water (1500 ml per 24 h) was provided throughout the dosing day and food was permitted after the 4 h observational period that followed cessation of infusion.
Table 1. Dose escalation in part 1 (single dose study) and part 2 (repeated ascending doses).
| Number on DP-b99 | Dose of DP-b99 | Number on placebo | |
|---|---|---|---|
| Part 1 (single administration) | 4 (young) | 0.003 mg kg−1 over 30 min | 1 |
| 4 (young) | 0.03 mg kg−1 over 30 min | 1 | |
| 6 (young) | 0.3 mg kg−1 over 30 min | 1 | |
| 6 (young) | 1 mg kg−1 over 30 min | 1 | |
| Part 2 (repeated administration) | 7 (young) | 0.03 mg kg−1 over 30 min, daily for 4 consecutive days | 2 |
| 7 (young) | 0.3 mg kg−1 over 30 min, daily for 4 consecutive days | 2 | |
| 7 (young) | 1 mg kg−1 over 30 min, daily for 4 consecutive days | 2 | |
| 8 (elderly) (7 subjects planned, one dropped out and was replaced) | 1 mg kg−1 over 30 min, daily for 4 consecutive days | 2 |
Observations
Clinical safety assessments were made at various intervals after infusion and at 24 h and 96 h after dosing in part 1,with assessments including: physical examination, vital signs (blood pressure, pulse rate, oral temperature and respiratory rate), clinical laboratory tests (blood count, blood chemistry, coagulation tests and urinalysis), 12-lead ECG, cardiac monitoring (starting 30 min prior to study drug administration and for 6 h postdose) and recording of adverse events. Similar assessments were made in part 2 at intervals following each infusion and on the third day after the completion of the last administration. Adverse events were categorized by the investigator as mild (i.e. causing no limitation to usual activities), moderate (i.e. causing some limitation of usual activities) or severe (causing inability to carry out usual activities).
Pharmacokinetic analysis
In part 1 of the study, plasma samples for pharmacokinetic (PK) analysis were collected at 0, 0.1, 0.25, 0.5, 0.75 1, 1.5, 2, 3, 6, 9, 12 and 24 h after start of infusion. In part 2, samples for pharmacokinetic analysis were collected on days 1 and 4 at 0, 0.1, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 6, 9 and 12 h after start of infusion, on days 2 and 3 prior to start of infusion and on the morning of day 5. At each sampling point, 9 ml of blood was collected into two separate polystyrene crystal tubes, which contained dry heparin (Lithium Heparin, 9 ml Monovette test tubes). Blood samples were centrifuged at 4°C at 1100 g for 10 min immediately after collection and frozen at −80°C until analyzed.
Analysis
Plasma and urine samples were evaluated by a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) technique using a Sciex API 3000 mass Spectrometer fitted with a turbo ionspray ionization source (PE Applied Biosystems, Warrington, Cheshire UK) and by reversed-phase HPLC at Quintiles (Scotland, UK) [15]. Samples were mixed with 85% orthophosphoric acid, methanol and internal standard and the tubes briefly vortex mixed. They were extracted with chloroform and the chloroform layer was dried with sodium sulphate and then evaporated to dryness under nitrogen. The dried extracts were reconstituted in acetonitrile, filtered and reversed-phase HPLC performed using a Purosphere RP-18, 5 µ, 125 × 3 mm column with a gradient elution with two solvents A: 60 : 40 acetic acid in acetonitrile (1%) : 1% acetic acid (aq), B: 1% acetic acid in acetonitrile. The injection volume was 20–70 µl and the flow rate 1.0 ml min−1. The limit of the assay for DP-b99 quantification in plasma was 10 ng ml−1. Calibration samples were extracted and analyzed for each analytical batch along with quality control samples prepared at three concentrations in duplicate. The measured concentrations were used to determine the between day precision and accuracy of the method and to expose any systematic bias. A full blood chemistry profile (creatinine, glucose, urea, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transpeptidase, total bilirubin, proteins, albumin/globulin ratio, sodium, potassium, chlorides, calcium, bicarbonate, uric acid, CPK, cholesterol, triglycerides, free iron, ferritin) for each patient was determined (W & T Laboratory, Berlin). Three coagulation parameters were also assessed (prothrombin time, activated partial thromboplastin, and fibrinogen estimation). The Abuscreen® test kit (Hoffmann-La Roche) was used to screen for drugs of abuse (amphetamines, barbiturates, benzodiazepines and opiates). Dip-stick urine analyses to detect blood, protein, ketones, bilirubin, nitrite urobilinogen, leucocytes and glucose were performed (Test stick Combur 10®-test Boehringer Mannheim). In addition a microscopic examination of urine sediment was undertaken in order to identify blood cells or cylinders.
Statistical analyses
Pharmacokinetic parameters were derived by noncompartmental analysis using WinNonlin Version 3.0 and summarized as mean ± SD. Assessment of dose-proportionality was performed for AUC(0, 24 h) and Cmax using analysis of variance and linear regression techniques by means of SAS Version 8. Individual PK parameters were fitted to the power model (loge(PK parameter) = µ + β*loge(Dose)).
All demographic and safety variables were listed by dose and summarized by descriptive statistics as appropriate (n, mean, median, SD, SEM, min, max). Analysis of mean post dose effects was performed on absolute changes from baseline using a model of repeated measurements. If relevant, a dose-effect relationship was explored.
Results
All volunteers in part 1 of the study were 22–40 years old, 169–194 cm tall, weighed 63–85 kg and their BMI ranged between 19.9 and 25.6 kg m−2. In part 2 of the study young volunteers were 18–36 years old and elderly volunteers 65–78 years of age, 166–199 cm tall, weighed 61–93 kg and their BMI ranged between 19.0 and 27.8 kg m−3. Infusion of DP-b99 was within 28–36 min in all subjects across both study parts. Only three minor protocol violations occurred in part 1 of the study. An elderly patient was withdrawn in part 2 due to supraventricular tachycardia of moderate severity following the first administration, which was judged unrelated to the study drug. One subject was subsequently enrolled to replace this patient, and therefore 60 subjects completed the study in accordance with the protocol (n = 24 part 1, n = 36 part 2).
Vital signs
Vital signs were generally normal in all subjects, apart from some minor abnormalities, that were all judged by the investigator as unrelated to the treatment or dose. When considering changes in blood pressure, a parameter of particular importance in the setting of acute stroke, there was no significant change over time from pre until 96 h post dose in mean systolic and diastolic blood pressure in any of the dose levels in part 1. Diastolic blood pressure values measured in supine position were all normal except for one value of 91 mmHg from the placebo group, which slightly exceeded the upper limit of 90 mmHg (at follow up). In contrast, in the standing position a total of 12 diastolic blood pressure values above normal were observed in seven subjects originating from all groups except for the 0.003 mg kg−1 group.
Mean changes from baseline values in systolic blood pressure (supine and standing) at any time point post infusion, did not exceed a range of −12–10.8 mmHg. In the same period, changes from baseline values in diastolic blood pressure did not exceed a range of −8.5–7.5 mmHg. In part 2,mean changes in systolic blood pressure (supine and standing) from baseline did not exceed a range of −9.9–11.9 mmHg in young subjects and −16.6–4.0 mmHg in elderly subjects (1 mg kg−1 dose group) at any time point measured after infusion. All mean diastolic blood pressure changes from baseline in part 2 were within −9.4 and 6.1 mmHg (young) and within −10.6 and 1.4 mmHg (elderly, 1.0 mg kg−1) at all post dose measurements. A total of eight young and two elderly subjects showed diastolic blood pressure values (in supine or standing position), slightly exceeding the upper normal of 90 and 95 mmHg, respectively. This occurred after the first infusion on day 1, two in the placebo group (young), three after infusion of 0.03 mg kg−1, one after 0.3 mg kg−1, two after 1.0 mg kg−1 (young) and two following 1.0 mg kg−1 (elderly). There was no tendency towards orthostatic hypotension.
Electrocardiograph and bedside cardiac monitoring
In part 1, there was no evidence for any dose related changes in any ECG parameter. In the QRS interval only minor changes of roughly −6–2 ms were observed. Mean changes in QTc values were also small (−13.3–13.5 ms) and showed no tendency to increase or decrease across all study groups.
A total of 13 abnormal ECG findings, observed after the first administration of the study drug, could have been considered as treatment emergent during part 2 of the study. Of these, three were observed in three placebo treated subjects (of whom one was elderly), and 10 were observed in seven young and two elderly DP-b99 treated volunteers. Most of these findings were sinus arrhythmia or sinus bradycardia (12 out of a total of 16 recorded abnormalities in 11 subjects). All of the abnormalities occurred at varying study days inconsistent with a drug-related pattern and were not clinically significant. Mean changes in the QRS interval did not exceed −3.4–3.0 ms in young subjects and −4.6–0.0 ms (1.0 mg kg−1) in the elderly. In general, in the DP-b99 treated young subjects the mean changes in QTc values ranged from −20.7 to −3.0 ms, in the young placebo group these changes ranged from 8.5 to 12.8 ms, and from −3.7–6.5 ms after infusion of 1.0 mg kg−1 of DP-b99 to the elderly. None of the statistical analyses of mean post dose effects in ECG intervals and heart rate resulted in significant interactions of treatment vs day or/and time, and overall, there was no evidence for any dose-related changes in any ECG parameter in part 2 of the study.
Bedside monitoring from 0.5 h before until 6 h after start of infusion resulted in three patients with abnormal results, of which two were considered clinically insignificant. A clinically significant intermittent supraventricular tachycardia of 20 min was observed in one elderly subject, and was rated as an adverse event and the subject was withdrawn from the trial. This event started 4 h after the end of study drug infusion and was judged by the investigator as unlikely to be related to the study drug.
Laboratory parameters
No clinically significant abnormalities were observed in any laboratory parameter throughout parts 1 and 2 of this study.
Adverse events
In part 1, 16 adverse events were recorded in 10 out of 24 subjects, 12 adverse events were of mild intensity in nine subjects and four adverse events were of moderate intensity in four subjects. There were no withdrawals. All adverse events were reversible. Eleven adverse events recovered spontaneously, while five adverse events in four subjects required remedial drug therapy. These four subjects with concomitant medication due to adverse events had received doses of 0.03 mg kg−1 (one subject) and 1 mg kg−1 (three subjects) of DP-b99.
In part 2 of the study, 20 out of 37 patients reported 27 adverse events. A higher number and incidence of adverse events were observed after the highest dose compared with lower doses. Elderly subjects reported fewer events than the equally sized comparator young 1 mg kg−1 dosing group. No adverse events were reported in the two elderly subjects who received placebo. The most prominent adverse event in both study parts (6 of 16 events in part 1, 17 of 33 events in part 2), which was likely DP-b99 related, was phlebitis at the infusion site, which commonly started 1–3 days after the administration, and lasted for a mean duration of 8 days (range 1–21 days). Phlebitis was an event that was most frequent in the 1 mg kg−1 groups. Small sample size precluded statistical analysis of differences in adverse events between the young and elderly groups. Serious adverse events did not occur in either study. All symptoms resolved without sequelae. Table 2 presents all the adverse events that occurred in parts 1 and 2 in more than a single subject.
Table 2. Table of adverse events reported in the single and repeated dosing study in more than a single subject.
| Single dosing study Part 1 | Repeated dosing study Part 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg kg−1) | 0.03 Single | 0.3 Single | 1 Single | Placebo Young | 0.03 Young | 0.3 Young | 1 Young | 1 Elderly |
| Number of subjects | 1 | 3 | 6 | 4 | 3 | 4 | 6 | 2 |
| Symptoms | ||||||||
| Phlebitis | 1 | 5 | 2 | 2 | 2 | 5 | 1 | |
| Nausea | 2 | 1 | 1 | |||||
| Dizziness | 1 | 1 | ||||||
| Headache | 1 | 2 | 1 | 2 | ||||
| Injection site pain | 1 | 1 | 1 | |||||
| Tiredness | 1 | 1 | ||||||
Pharmacokinetics
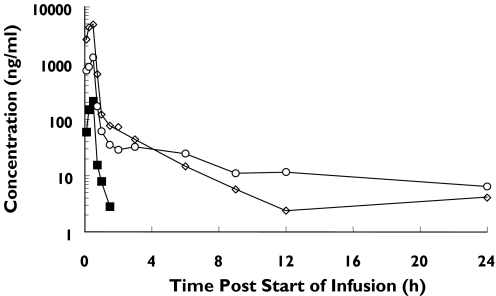
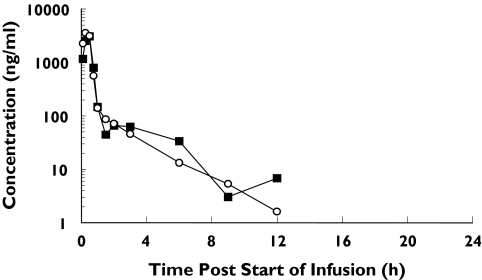
Pharmacokinetic parameters from parts 1 and 2 are given in Tables 3, 4 and 5. The pharmacokinetic parameters were derived by noncompartmental analysis (Win Nonlin, Vers 3.0). The mean concentration-time profiles in subjects in part 1 and for the elderly in part 2 are presented (Figures 3 and 4). In parts 1 and 2 of the study, the maximum plasma concentrations were in general observed 30 min after the start of infusion period (i.e. at the end of the infusion period). Thereafter the serum concentration-time profile was multiphasic. Plasma concentrations were quantifiable up to 1.5, 24 and 24 h in part 1, and up to 12, 6 and 12 h in part 2,for doses of 0.03, 0.3 and 1 mg kg−1, respectively. At the lowest dose of 0.003 mg kg−1 plasma concentrations of DP-b99 were below the limit of quantification of the assay at all time points post dose. Overall, in part 1 a dose-adequate increase in plasma concentrations with ascending doses of 0.03–1 mg kg−1 was observed.
Table 3. Pharmacokinetic parameters (mean ± SD) of DP-b99 in healthy volunteers in Part 1.
| Dose (mg kg−1) | Cinf (ng ml−1) | Cmax (ng ml−1) | tmax (h) | AUC(0, 24 h) (ng ml−1 h) |
|---|---|---|---|---|
| 0.003 | BLQ | BLQ | BLQ | BLQ |
| 0.03 | 211 ± 84.8 | 211 ± 84.8 | 0.50 (0.50–0.50*) | 98.3 ± 41.4 |
| 0.3 | 1258 ± 393 | 1278 ± 376 | 0.46 (0.25–0.50*) | 995 ± 387 |
| 1 | 4824 ± 1201 | 5463 ± 1108 | 0.33 (0.25–0.50*) | 2902 ± 474 |
tmax values represent the range (mean). Plasma concentrations obtained for the lowest dose of 0.003 mg kg−1 had DP-b99 concentrations below the limit of quantification (BLQ), so data for this dose group was unavailable.
Table 4. Pharmacokinetics of DP-b99 (mean ± SD) in healthy volunteers in part 2.
| Cinf (ng ml−1) | Cmax (ng ml−1) | tmax (h) | AUC(0, 24 h) (ng ml−1 h) | ||
|---|---|---|---|---|---|
| 0.03 mg kg−1 dose | Day 1 | 111 ± 50.3 | 111 ± 50.3 | 0.50 ± 0 | 44.0 ± 20.8 |
| (Young) | Day 4 | 89.6 ± 34.4 | 356 ± 312 | 0.214 ± 0.195 | 131 ± 168 |
| 0.3 mg kg−1 dose | Day 1 | 1253 ± 286 | 1520 ± 542 | 0.50 ± 0.144 | 837 ± 288 |
| (Young) | Day 4 | 1112 ± 195 | 2111 ± 1377 | 0.329 ± 0.214 | 898 ± 327 |
| 1 mg kg−1 dose | Day 1 | 1616 ± 296 | 1905 ± 675 | 0.357 ± 0.134 | 1218 ± 277 |
| Young | Day 4 | 1480 ± 281 | 1552 ± 281 | 0.336 ± 0.163 | 1136 ± 284 |
| Elderly | Day 1 | 3084 ± 1725 | 3295* ± 1670 | 0.469 ± 0.160 | 2078* ± 1038 |
| Day 4 | 3116 ± 1564 | 4103** ± 1449 | 0.393 ± 0.134 | 2219** ± 984 |
Borderline significant in the elderly on day 1 (0.05 < P < 0.11) compared with the young.
Significant in the elderly on day 4 (P < 0.05) compared with the young.
Table 5. t1/2, CL and Vss (mean(SEM)) in elderly and young subjects following IV administration of 1 mg kg−1 DP-b99 (Part 2).
| Parameter | Day | Young | Elderly |
|---|---|---|---|
| t1/2 (h) | 1 | 3.47 (0.52) | 2.11 (0.06) |
| 4 | 4.36 (0.86) | 2.10 (0.57) | |
| CL (ml min−1) | 1 | 960 (65) | 1002 (663) |
| 4 | 1042 (115) | 825 (254) | |
| Vss (l) | 1 | 80.1 (3.94) | 64.1 (42.1) |
| 4 | 96.5 (30) | 38.8 (21.5) |
Figure 3.
Mean plasma DP-b99 concentration-time profiles of young subjects following IV. Administration of 0.03, 0.3 and 1 mg kg−1 DP-b99 (part 1). 0.03 mg/kg (▪), 0.3 mg/kg (○), 1 mg/kg (◊)
Figure 4.
Mean plasma DP-b99 concentration-time profiles following repeated IV administration of 1.0 mg kg−1 DP-b99 to elderly subjects (part 2). Day 1 (▪), Day 4 (○)
Area under the plasma concentration vs time curve from time zero to the last sampling time at which concentrations were at or above the limit of quantification (AUC(0, ∞)), was calculated by the linear trapezoidal rule, and AUC(0, 24 h) was calculated from time zero to 24 h post start of infusion. In part 1 an approximate dose-proportional increase in AUC(0, 24 h) and Cmax was observed over the DP-b99 dose range of 0.03 mg to 1 mg (Table 3).
After repeated dosing, the mean AUC(0, 24 h) values on day 1 increased 27.7-fold over the dose range of 0.03 mg to 1 mg, which was consistent with that observed in the single dose study (Table 4). However, the increase in Cmax was less than dose proportional. On day 4 the increase in Cmax and AUC(0, 24 h) was less than dose-proportional over the entire dose range of 0.03–1 mg kg−1. On day 1 following administration of 1 mg kg−1 the mean half-life of DP-b99 in young volunteers was 3.47 ± 0.90 and in the elderly was 2.11 ± 0.09. On day 4 following the same administration of DP-b99, mean half-life was 4.36 ± 1.49 h and 2.10 ± 1.14 h in the young and elderly, respectively. No drug accumulation in plasma was observed for repeated doses of 1 mg kg−1 (mean accumulation calculated by AUC(0, 24 h) (day 4) : AUC(0, 24 h) (day 1) and was observed to be between 0.9 and 1.3 (young, elderly).
The DP-b99 Cmax values observed in elderly subjects at the 1 mg kg−1 dose were 1.6 (day 1) to 2.5-fold (day 4) greater than those observed in young subjects following single and repeated intravenous administration, respectively (95% CI for the mean difference between elderly and young for Cmax were 0.90–2.74 day 1 and 1.70 and 3.68 day 4). AUC(0, 24 h) in the elderly was 1.6 (day 1) and 1.8 (day 4) times greater than that observed in the young following single and repeated administrations of 1 mg kg−1 (95% CI for the mean difference between elderly and young for AUC(0, 24 h) were 0.98, 2.48 day 1 and 1.08, 2.97 day 4).
There was no apparent age related alteration in the total plasma clearance of DP-b99 (Table 5). In the light of the much higher AUC (P < 0.05, day 4) observed in the elderly we would have expected a lower clearance in this group compared with the young, but such a difference in clearance between the groups was probably masked in the calculations due to the limited number of AUC(0, 8 h) estimates that could be determined, and the higher variability observed in elderly subjects (coefficient of variation associated with AUC(0, 8 h) in the elderly subjects was 96.9% compared with 6.5% in the young).
The volume of distribution of DP-b99 appeared to be smaller in elderly subjects compared with young subjects (Table 5). This resulted in an apparent shorter half-life in elderly subjects. Investigation of urine samples from the highest dose group found no detectable DP-b99 in urine and indicated that unchanged DP-b99 is eliminated from plasma by a non-renal route in man.
Discussion and conclusions
The results of this study describe the clinical pharmacology of DP-b99 in healthy volunteers. DP-b99 was well tolerated and had an acceptable safety profile up to the highest dose of 1.0 mg kg−1 tested in both study parts. Eight mild to moderate AEs, at least possibly related to the study drug, were observed in the six subjects treated with a single dose of 1.0 mg kg−1. Also, eight mild to moderate AEs at least possibly related to study drug were observed in six of the seven young subjects treated with four repeated doses of 1.0 mg kg−1. These AEs were most frequently phlebitis. Even though the elderly group was exposed to DP-b99 to a greater extent than the young, the drug was better tolerated at the injection site in the former group (one phlebitis event in the elderly volunteers) than in the younger subjects (five events of phlebitis in the young volunteers). Rates of phlebitis did not differ between the elderly placebo and DP-b99 treated groups. In light of the lower rate of phlebitis in the elderly group, and considering that this age range is typical of stroke patients [1], phlebitis will probably not pose a problem in future studies with DP-b99 in this patient population. Also, whatever the future rate of phlebitis in this population may be, if prospective studies find DP-b99 to improve the prognosis after acute stroke, this will likely outweigh any temporary inconvenience secondary to an injection site irritation.
Despite the calcium binding activity of DP-b99, no adverse effects were seen in cardiovascular parameters sensitive to alterations in extra-cellular calcium levels in either the young or the elderly group.
Systemic exposure was consistent with the assumption of dose proportionality after single doses of DP-b99 and after repeated DP-b99 dosing with respect to AUC(0, 24 h), but not after repeated DP-b99 dosing with respect to Cmax. No accumulation was found after four consecutive daily doses of DP-b99. No measurable concentrations of unchanged DP-b99 in urine could be found, suggesting a principal hepatic route of elimination.
The higher systemic exposure to DP-b99 in the elderly compared to young volunteers is also consistent with the presumed central role of hepatic elimination of this drug: with ageing, hepatic mass and blood flow decrease, which significantly affects the elimination of drugs with high hepatic clearance administered intravenously, e.g. lidocaine [16]. Alternatively, the urine might have contained DP-b99 metabolites that were not detected.
The apparent terminal half-life of DP-b99 seems to be short (in the range of 2–4 h). Stroke patients, who often suffer from concomitant conditions that compromise hepatic perfusion such as congestive heart failure [17, 18], may need lower doses of this apparently hepatically eliminated drug to achieve the same exposure to DP-b99 as healthy subjects. This pharmacokinetic assumption was subsequently confirmed in a study in stroke patients (unpublished data). Currently DP-699 is in its second Phase II study in stroke patients.
Acknowledgments
The study was supported by D-Pharm Ltd, Israel. The authors would like to thank Mrs Adina Bar for her assistance in preparing this manuscript.
References
- 1.American Heart Association (AHA) Dallas, Texas: AHA; 2004. Heart Disease and Stroke Statistics 2003 Update. [Google Scholar]
- 2.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 3.Lee JM, Zipfel GJ, Park KH, He YY, Hsu CY, Choi DW. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–8. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- 4.Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–75. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JH, Sensi SL, Koh JY. Zn (2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 6.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 7.Tymianski M, Spigelman I, Zhang L, Carlen PL, Tator CH, Charlton MP, Wallace MC. Mechanism of action and persistence of neuroprotection by cell-permanent Ca 2+ chelators. J Cereb Blood Flow Metab. 1994;14:911–23. doi: 10.1038/jcbfm.1994.122. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Hamid KM, Tymianski M. Mechanisms and effects of intracellular calcium buffering on neuronal survival in organotypic hippocampal cultures exposed to anoxia/aglycemia or to excitotoxins. J Neurosci. 1997;17:3538–53. doi: 10.1523/JNEUROSCI.17-10-03538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tymianski M, Wallace MC, Spigelman I, Uno M, Carlen PL, Tator CH, Charlton MP. Cell-permeant Ca2+ chelators reduce early excitotoxic and ischemic neuronal injury in vitro and in vivo. Neuron. 1993;11:221–35. doi: 10.1016/0896-6273(93)90180-y. [DOI] [PubMed] [Google Scholar]
- 10.Lomeo RS, Gomez RS, Prado MA, Romano Silva MA, Massensini AR, Gomez MV. Excitotoxic release of [3H]-acetylcholine by ouabain involves intracellular Ca2+ stores in rat brain cortical slices. Cell Mol Neurolbiol. 2003;23:917–27. doi: 10.1023/b:cemn.0000005320.06215.80. [DOI] [PubMed] [Google Scholar]
- 11.Angel I, Bar A, Horovitz T, Taler G, Krakovsky M, Resnitsky D, Rosenberg G, Striem S, Friedman JE, Kozak A. Metal ion chelation in neurodegenerative disorders. Drug Dev Res. 2002;56:300–9. [Google Scholar]
- 12.Angel I, Krakovsky M, Shemesh T, Beit-Yannai E, Friedman JE, Kozak A. DP-b99, a neuroprotective agent for cerebral ischemia. Bio Tech Int. 2000;12:20–2. [Google Scholar]
- 13.Krakovsky M, Polyak M, Angel I, Kozak A. A novel membrane targeted compound active against global and focal ischemia. In: Gjedde A SH, Knudsen GM, Paulson OB, editors. Physiological Imaging of the brain with PET. Academic Press; 2001. pp. 347–52. [Google Scholar]
- 14.Knapp TR. A methodological critique of the ‘ideal weight’ concept. JAMA. 1983;250:506–10. [PubMed] [Google Scholar]
- 15.Ioffe V, Kalendarev T, Rubinstein I, Mizerikhin E, Tsibulsky E. Reversed-phase liquid chromatography of biologically active lipophillic chelators. II. Improvement of chromatographic performance and selected applications in biochemical analysis. J Chromatogr A. 2003;987:169–80. doi: 10.1016/s0021-9673(02)01450-4. [DOI] [PubMed] [Google Scholar]
- 16.Parker BM, Cusack BJ, Vestal RE. Pharmacokinetic optimisation of drug therapy in elderly patients. Drugs Aging. 1995;7:10–8. doi: 10.2165/00002512-199507010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke. 2003;34:122–6. doi: 10.1161/01.str.0000047852.05842.3c. [DOI] [PubMed] [Google Scholar]
- 18.Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction. a medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–49. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]